Liver Transplantation from Elderly Donors (≥85 Years Old)
Abstract
Simple Summary
Abstract
1. Introduction
2. Patients and Methods
2.1. Study Design and Study Population
2.2. Donor Evaluation
2.3. Graft Allocation Policy
2.4. Data Collection and Definitions
2.5. Statistical Analysis
3. Results
3.1. Characteristics of the Whole Donor Cohort (before PSM)
3.1.1. Elderly Donor Cohort (n = 76)
3.1.2. Young Donor Cohort (n = 349)
3.2. Comparison of Recipient and Donor Characteristics after Propensity Score Matching (PSM)
3.3. Comparison of Perioperative Outcomes after LT in Groups after PSM
3.4. Intraoperative Features Influencing Outcomes after LT with Elderly Donor Liver Grafts
3.5. Outcomes after LT in Both Groups Compared with First-Year Benchmark Cutoffs
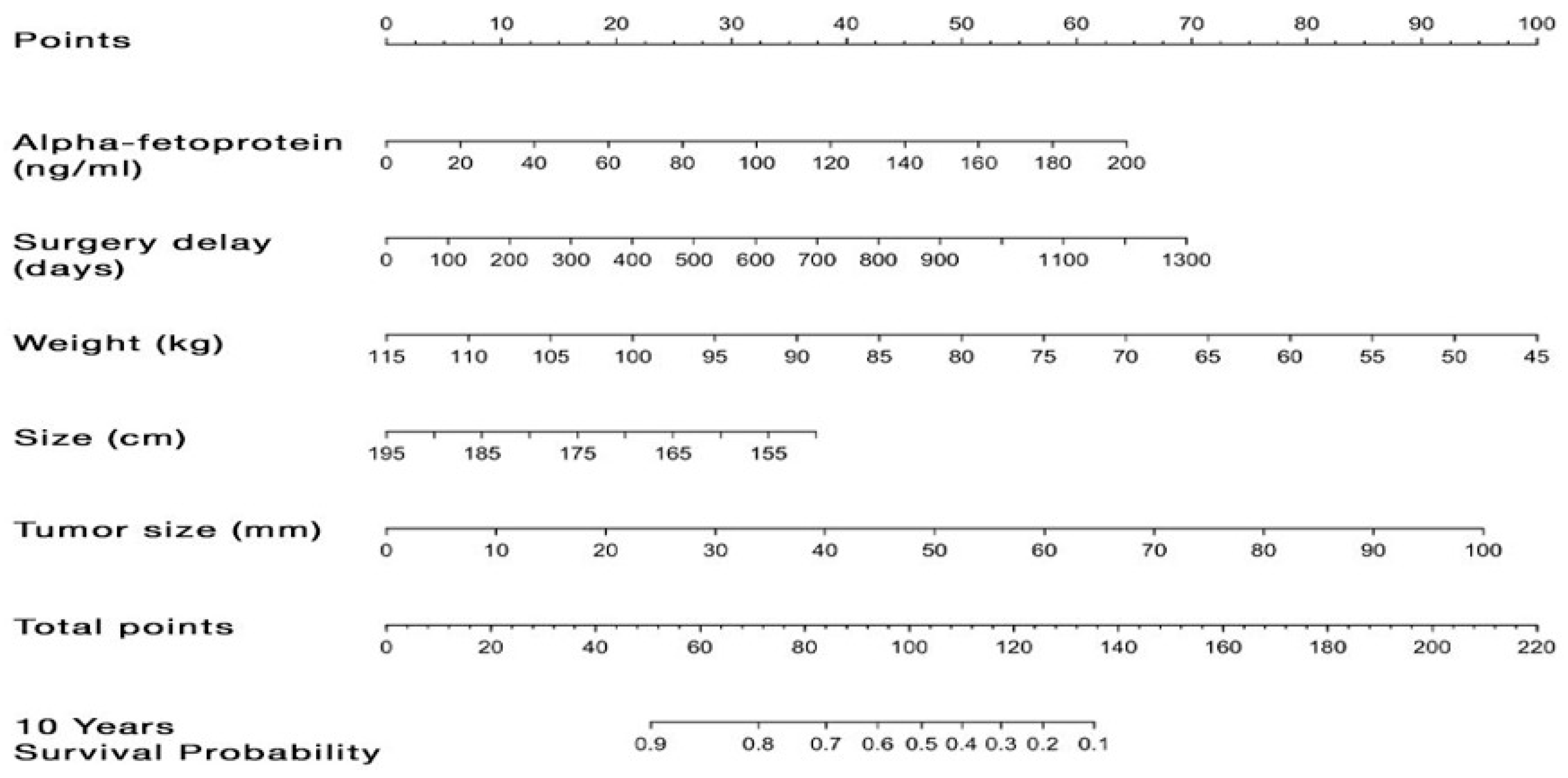
3.6. Preoperative Predictors of Graft and Patient Survivals
4. Discussion
4.1. Statement of Principal Findings
4.2. Strengths and Weaknesses of the Study
4.3. Interpretation with Reference to Other Studies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yao, F.Y. Liver Transplantation for Hepatocellular Carcinoma: Beyond the Milan Criteria. Am. J. Transplant. 2008, 8, 1982–1989. [Google Scholar] [CrossRef]
- Kulkarni, S.; Cronin, D.C. Ethical tensions in solid organ transplantation: The price of success. World J. Gastroenterol. 2006, 12, 3259–3264. Available online: www.wjgnet.com (accessed on 28 May 2006). [CrossRef][Green Version]
- Soubrane, O.; Scatton, O. The Development of Transplant Oncology May Worsen the Liver Gap and Needs New Technical Options in Liver Transplantation. Ann. Surg. 2023, 279, 226–227. [Google Scholar] [CrossRef]
- Houben, P.; Döhler, B.; Weiß, K.H.; Mieth, M.; Mehrabi, A.; Süsal, C. Differential Influence of Donor Age Depending on the Indication for Liver Transplantation—A Collaborative Transplant Study Report. Transplantation 2020, 104, 779–787. [Google Scholar] [CrossRef]
- Lucendo, A.P.; Sáez, F.S.; Carmona, S.A. Is Age an Exclusive Factor for Accepting Liver Donors? Transplant. Proc. 2021, 53, 2663–2665. [Google Scholar] [CrossRef]
- Schneider, S.; Jaime, F.D.; Mara, K.; Dierkhising, R.; Heimbach, J.; Watt, K.D.; Berenguer, M. Long-term outcomes of the octogenarian donor liver recipient: The era of the new centurion. Clin. Transplant. 2019, 33, e13629. [Google Scholar] [CrossRef]
- Roullet, S.; Defaye, M.; Quinart, A.; Adam, J.-P.; Chiche, L.; Laurent, C.; Neau-Cransac, M. Liver Transplantation with Old Grafts: A Ten-Year Experience. Transplant. Proc. 2017, 49, 2135–2143. [Google Scholar] [CrossRef]
- Pratschke, S.; Bender, A.; Boesch, F.; Andrassy, J.; van Rosmalen, M.; Samuel, U.; Rogiers, X.; Meiser, B.; Küchenhoff, H.; Driesslein, D.; et al. Association between donor age and risk of graft failure after liver transplantation: An analysis of the Eurotransplant database. Transpl. Int. 2019, 32, 270–279. [Google Scholar] [CrossRef]
- Ghinolfi, D.; Pezzati, D.; Rreka, E.; Balzano, E.; Catalano, G.; Coletti, L.; Tincani, G.; Carrai, P.; Petruccelli, S.; Martinelli, C.; et al. Nonagenarian Grafts for Liver Transplantation. Liver Transplant. 2019, 25, 1439–1444. [Google Scholar] [CrossRef]
- Domagala, P.; Takagi, K.; Ijzermans, J.N.; Polak, W.G. Grafts from selected deceased donors over 80 years old can safely expand the number of liver transplants: A systematic review and meta-analysis. Transplant. Rev. 2019, 33, 209–218. [Google Scholar] [CrossRef]
- Petridis, I.; Gruttadauria, S.; Nadalin, S.; Viganò, J.; di Francesco, F.; Pietrosi, G.; Fili’, D.; Montalbano, M.; D’Antoni, A.; Volpes, R.; et al. Liver Transplantation Using Donors Older Than 80 Years: A Single-Center Experience. Transplant. Proc. 2008, 40, 1976–1978. [Google Scholar] [CrossRef]
- Rabelo, A.; Alvarez, M.; Méndez, C.; Villegas, M.; Mgraneroa, K.; Becerra, A.; Dominguez, M.; Raya, A.; Exposito, M.; Suárez, Y. Liver Transplantation Outcomes Using Grafts from Donors Older Than the Age of 80 Years. Transplant. Proc. 2015, 47, 2645–2646. [Google Scholar] [CrossRef]
- Cascales-Campos, P.; Ramírez, P.; González-Sánchez, M.; Alconchel, F.; Martínez-Insfran, L.; Sánchez-Bueno, F.; Robles, R.; Pons, J.; Vargas, Á.; Sanmartín, J.; et al. Orthotopic Liver Transplantation with Elderly Donors (Over 80 Years of Age): A Prospective Evaluation. Transplant. Proc. 2018, 50, 3594–3600. [Google Scholar] [CrossRef]
- Martín, L.G.; Grande, A.M.; Roux, D.P.; Cibrián, C.G.; Martín, C.F.; Gandía, M.R.; Buenadicha, A.L. Short-term Results of Liver Transplantation with Octogenarian Donors. Transplant. Proc. 2018, 50, 184–191. [Google Scholar] [CrossRef]
- Haugen, C.E.; Holscher, C.M.; Luo, X.; Bowring, M.G.; Orandi, B.J.; Thomas, A.G.; Garonzik-Wang, J.; Massie, A.B.; Philosophe, B.; McAdams-DeMarco, M.; et al. Assessment of Trends in Transplantation of Liver Grafts from Older Donors and Outcomes in Recipients of Liver Grafts from Older Donors, 2003–2016. JAMA Surg. 2019, 154, 441–449. [Google Scholar] [CrossRef]
- Dutkowski, P.; Oberkofler, C.E.; Slankamenac, K.; Puhan, M.A.; Schadde, E.; Müllhaupt, B.; Geier, A.; Clavien, P.A. Are There Better Guidelines for Allocation in Liver Transplantation? Ann. Surg. 2011, 254, 745–754. [Google Scholar] [CrossRef]
- Duvoux, C.; Roudot–Thoraval, F.; Decaens, T.; Pessione, F.; Badran, H.; Piardi, T.; Francoz, C.; Compagnon, P.; Vanlemmens, C.; Dumortier, J.; et al. Liver Transplantation for Hepatocellular Carcinoma: A Model Including α-Fetoprotein Improves the Performance of Milan Criteria. Gastroenterology 2012, 143, 986–994.e3. [Google Scholar] [CrossRef]
- Halldorson, J.; Roberts, J.P. Decadal analysis of deceased organ donation in Spain and the United States linking an increased donation rate and the utilization of older donors. Liver Transplant. 2013, 19, 981–986. [Google Scholar] [CrossRef]
- Gastaca, M.; Guerra, M.; Martinez, L.A.; Ruiz, P.; Ventoso, A.; Palomares, I.; Prieto, M.; Matarranz, A.; Valdivieso, A.; de Urbina, J.O. Octogenarian Donors in Liver Transplantation. Transplant. Proc. 2016, 48, 2856–2858. [Google Scholar] [CrossRef]
- Ghinolfi, D.; De Simone, P.; Lai, Q.; Pezzati, D.; Coletti, L.; Balzano, E.; Arenga, G.; Carrai, P.; Grande, G.; Pollina, L.; et al. Risk analysis of ischemic-type biliary lesions after liver transplant using octogenarian donors. Liver Transplant. 2016, 22, 588–598. [Google Scholar] [CrossRef]
- Biancofiore, G.; Bindi, M.; Ghinolfi, D.; Lai, Q.; Bisa, M.; Esposito, M.; Meacci, L.; Mozzo, R.; Spelta, A.; Filipponi, F. Octogenarian donors in liver transplantation grant an equivalent perioperative course to ideal young donors. Dig. Liver Dis. 2017, 49, 676–682. [Google Scholar] [CrossRef]
- Verhelst, X.; Geerts, A.; Jochmans, I.; Vanderschaeghe, D.; Paradissis, A.; Vanlander, A.; Berrevoet, F.; Dahlqvist, G.; Nevens, F.; Pirenne, J.; et al. Glycome Patterns of Perfusate in Livers Before Transplantation Associate with Primary Nonfunction. Gastroenterology 2018, 154, 1361–1368. [Google Scholar] [CrossRef]
- Olthoff, K.M.; Kulik, L.; Samstein, B.; Kaminski, M.; Abecassis, M.; Emond, J.; Shaked, A.; Christie, J.D. Validation of a current definition of early allograft dysfunction in liver transplant recipients and analysis of risk factors. Liver Transplant. 2010, 16, 943–949. [Google Scholar] [CrossRef]
- Demetris, A.J.; Batts, K.P.; Dhillon, A.P.; Ferrell, L.; Fung, J.; Geller, S.A.; Hart, J.; Hayry, P.; Hofmann, W.J.; Hubscher, S.; et al. Banff schema for grading liver allograft rejection: An international consensus document. J. Hepatol. 1997, 25, 658–663. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. Ann. Intern. Med. 2007, 147, 573–577. [Google Scholar] [CrossRef]
- Muller, X.; Marcon, F.; Sapisochin, G.; Marquez, M.; Dondero, F.; Rayar, M.; Doyle, M.M.B.; Callans, L.; Li, J.; Nowak, G.; et al. Defining Benchmarks in Liver Transplantation. Ann. Surg. 2018, 267, 419–425. [Google Scholar] [CrossRef]
- Maestro, O.C.; Alonso, I.J.; Quinto, A.M.; Municio, A.M.; Pulido, J.C.; García-Sesma, A.; Jiménez-Romero, C. Expanding donor age in liver transplantation using liver grafts from nonagenarian donors. Clin. Transplant. 2022, 36, e14684. [Google Scholar] [CrossRef]
- Jiménez-Romero, C.; Cambra, F.; Caso, O.; Manrique, A.; Calvo, J.; Marcacuzco, A.; Rioja, P.; Lora, D.; Justo, I. Octogenarian liver grafts: Is their use for transplant currently justified? World J. Gastroenterol. 2017, 23, 3099–3110. [Google Scholar] [CrossRef]
- Nardo, B.; Masetti, M.; Urbani, L.; Caraceni, P.; Montalti, R.; Filipponi, F.; Mosca, F.; Martinelli, G.; Bernardi, M.; Pinna, A.D.; et al. Liver Transplantation from Donors Aged 80 Years and Over: Pushing the Limit. Am. J. Transplant. 2004, 4, 1139–1147. [Google Scholar] [CrossRef]
- Barbier, L.; Cesaretti, M.; Dondero, F.; Cauchy, F.; Khoy-Ear, L.; Aoyagi, T.; Weiss, E.; Roux, O.; Dokmak, S.; Francoz, C.; et al. Liver Transplantation with Older Donors. Transplantation 2016, 100, 2410–2415. [Google Scholar] [CrossRef]
- Bertuzzo, V.R.; Cescon, M.; Odaldi, F.; Di Laudo, M.; Cucchetti, A.; Ravaioli, M.; Del Gaudio, M.; Ercolani, G.; D’errico, A.; Pinna, A.D. Actual Risk of Using Very Aged Donors for Unselected Liver Transplant Candidates. Ann. Surg. 2017, 265, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Kitano, Y.; Pietrasz, D.; Fernandez-Sevilla, E.; Golse, N.; Vibert, E.; Cunha, A.S.; Azoulay, D.; Cherqui, D.; Baba, H.; Adam, R.; et al. Subjective Difficulty Scale in Liver Transplantation: A Prospective Observational Study. Transpl. Int. 2022, 35, 10308. [Google Scholar] [CrossRef] [PubMed]

| Before PSM | After PSM | |||||
|---|---|---|---|---|---|---|
| Control Study (Donors ≤ 40 yo) n = 349 | Study Group (Donors ≥ 85 yo) n = 76 | p-Value | Control Study (Donors ≤ 40 yo) n = 76 | Study Group (Donors ≥ 85 yo) n = 76 | p-Value | |
| Sex M (n, %) | 212 (60.7%) | 50 (65.8%) | 0.44 | 62 (81.6%) | 50 (65.8%) | 0.04 |
| Age (years, median, IQR) | 44 [31, 59] | 59 [54, 66] | <0.001 | 61 [56, 66] | 59 [54, 66] | 0.49 |
| BMI (kg/m2, median, IQR) | 24,6 [21, 27] | 26.1 [22, 29] | 0.02 | 25.6 [22, 29] | 26.1 [22, 29] | 0.55 |
| MELD (median, IQR) | 17 [12, 29] | 17 [10, 26] | 0.91 | 14.3 [10, 25] | 17 [10, 26] | 0.98 |
| ReLT (n, %) | 83 (23.8%) | 4 (5.3%) | 0.001 | 5 (6.6%) | 4 (5.3%) | 1 |
| HCC (n, %) | 44 (12.6%) | 36 (47.4%) | <0.001 | 39 (51.3%) | 36 (47.4%) | 0.75 |
| Fulminant hepatitis (n, %) | 32 (9.2%) | 6 (7.9%) | 0.53 | 32 (24.3%) | 6 (7.9%) | 0.53 |
| Combined transplant (n, %) | 37 (10.6%) | 0 (0%) | 0.001 | 0 (0%) | 0 (0%) | NA |
| Vascular cause of death (n, %) | 65 (18.6%) | 61 (80.3%) | <0.001 | 10 (13.2%) | 61 (80.3%) | <0.001 |
| Younger Group (n = 76) | Older Group (n = 76) | p-Value | ||
|---|---|---|---|---|
| Recipient demographics | Sex M (n, %) | 62 (81.6%) | 50 (65.8%) | 0.04 |
| Age, y (median, IQR) | 61 [56, 66] | 59 [54, 66] | 0.49 | |
| BMI (median, IQR) | 25.6 [22, 29] | 26.1 [22, 29] | 0.55 | |
| Recipient underlying hepatopathy | MELD (median, IQR) | 16 [11, 27] | 17 [10, 26] | 98 |
| HCC (n, %) | 39 (51.3%) | 36 (47.4%) | 0.75 | |
| Type of LT | ReLT (n, %) | 5 (6.6%) | 4 (5.3%) | 1 |
| Fulminant hepatitis (n, %) | 32 (24.3%) | 6 (7.9%) | 0.53 | |
| Donor characteristics | Sex M (n, %) | 55 (72.4%) | 19 (25%) | <0.001 |
| BMI (median, IQR) | 23.5 [21, 26) | 23.2 [21, 26] | 0.62 | |
| Donor cause of death | Anoxia | 26 (34.2%) | 10 (13.2%) | 0.004 |
| Trauma | 38 (50%) | 15 (19.7%) | <0.001 | |
| Vascular | 12 (15.8%) | 50 (76.6%) | <0.001 | |
| Graft characteristics | Steatosis * ≤ 30% (n, %) | 72 (95%) | 64 (94%) ** | 1 |
| GRWR (mean ± IQR) | 1.94 [1.6, 2.8] | 1.4 [1.2, 1.7] | <0.001 | |
| CIT, min (mean ± IQR) | 442 [376, 521] | 416 [371, 483] | 0.40 | |
| D-MELD score | D-MELD score value | 458 [300, 764] | 1505 [840, 2306] | <0.001 |
| D-MELD score > 1600 (n, %) | 1 (1.3%) | 36 (47.4%) | <0.001 |
| Outcomes | Younger Group (n = 76) | Older Group (n = 76) | p-Value | |
|---|---|---|---|---|
| Perioperative data | Intraoperative RBC transfusions (median, IQR) | 0 [0, 7] | 4 [0, 6] | 0.065 |
| ICU length of stay (median, IQR) | 8 [5, 14] | 5 [4, 9] | <0.001 | |
| Total length of stay (median, IQR) | 15 [9, 21] | 20 [15, 32] | <0.001 | |
| Graft function | EAD (n, %) | 11 (14.4) | 20 (26.3) | 0.07 |
| PNF (n, %) | 0 (0) | 2 (2.6) | 0.50 | |
| Rejection (n, %) | 2 (2.62) | 6 (7.9) | 0.17 | |
| Complications | ≥Grade III Clavien–Dindo score (n, %) | 22 (28.9) | 21 (27.6) | 1 |
| CCI (mean, ±SD) | 16 (23.1) | 22.9 (22.3) | 0.054 | |
| Biliary complications (n, %) | 7 (9.21) | 7 (9.21) | 0.33 | |
| Vascular complications (n, %) | 14 (18.4) | 8 (10.5) | 0.10 | |
| Patient survival | 1 y survival rates (95% CI) | 85.2% | 90.2% | 0.1 |
| 3 y survival rates (95% CI) | 80.8% | 74.7% | ||
| 5 y survival rates (95% CI) | 73.3% | 63.3% | ||
| Graft survival | 1 y survival rates (95% CI) | 82.6% | 87.6% | |
| 3 y survival rates (95% CI) | 78.2% | 70.4% | ||
| 5 y survival rates (95% CI) | 72.7% | 61% |
| Case (>85 yo) | Benchmark Cutoffs (at 12 Months) | Control (<40 yo) | |
|---|---|---|---|
| MELD | 17 [10, 26] | 12 [9, 16] | 14 [10, 25] |
| OP duration (h), (median, IQR) | 7.1 (6.4, 8.1] | ≤6 | 7 [6, 7.9] |
| Intraoperative RBC transfusions (median, IQR) | 4 (0, 6) | ≤3 | 0 (0, 7) |
| ICU stays (d), (median, IQR) | 5 (4, 9) | ≤4 | 8 (5, 14) |
| Hospital stays (d), (median, IQR) | 20 (15, 32) | ≤18 | 15 (9, 21) |
| ≥Grade III Clavien–Dindo score (%) | 27.6 | ≤59 | 28.9 |
| Biliary complications, (%) | 9.2 | ≤28 | 9.21 |
| Re-transplantations, (%) | 5.3 | ≤4 | 2.8 |
| CCI (mean, ±SD) | 22.9 (22.3) | ≤29.6 | 16 (23.1) |
| 1-year mortality, (%) | 10.5 | ≤9 | 14.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romano, P.; Cano, L.; Pietrasz, D.; Beghdadi, N.; Allard, M.-A.; Salloum, C.; Blandin, F.; Ciacio, O.; Pittau, G.; Adam, R.; et al. Liver Transplantation from Elderly Donors (≥85 Years Old). Cancers 2024, 16, 1803. https://doi.org/10.3390/cancers16101803
Romano P, Cano L, Pietrasz D, Beghdadi N, Allard M-A, Salloum C, Blandin F, Ciacio O, Pittau G, Adam R, et al. Liver Transplantation from Elderly Donors (≥85 Years Old). Cancers. 2024; 16(10):1803. https://doi.org/10.3390/cancers16101803
Chicago/Turabian StyleRomano, Pierluigi, Luis Cano, Daniel Pietrasz, Nassiba Beghdadi, Marc-Antoine Allard, Chady Salloum, Frédérique Blandin, Oriana Ciacio, Gabriella Pittau, René Adam, and et al. 2024. "Liver Transplantation from Elderly Donors (≥85 Years Old)" Cancers 16, no. 10: 1803. https://doi.org/10.3390/cancers16101803
APA StyleRomano, P., Cano, L., Pietrasz, D., Beghdadi, N., Allard, M.-A., Salloum, C., Blandin, F., Ciacio, O., Pittau, G., Adam, R., Azoulay, D., Sa Cunha, A., Vibert, E., De Carlis, L., Vitale, A., Cillo, U., Cherqui, D., & Golse, N. (2024). Liver Transplantation from Elderly Donors (≥85 Years Old). Cancers, 16(10), 1803. https://doi.org/10.3390/cancers16101803









