Assessment of the Psychosocial Impact of Pancreatic Cancer Surveillance in High-Risk Individuals
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
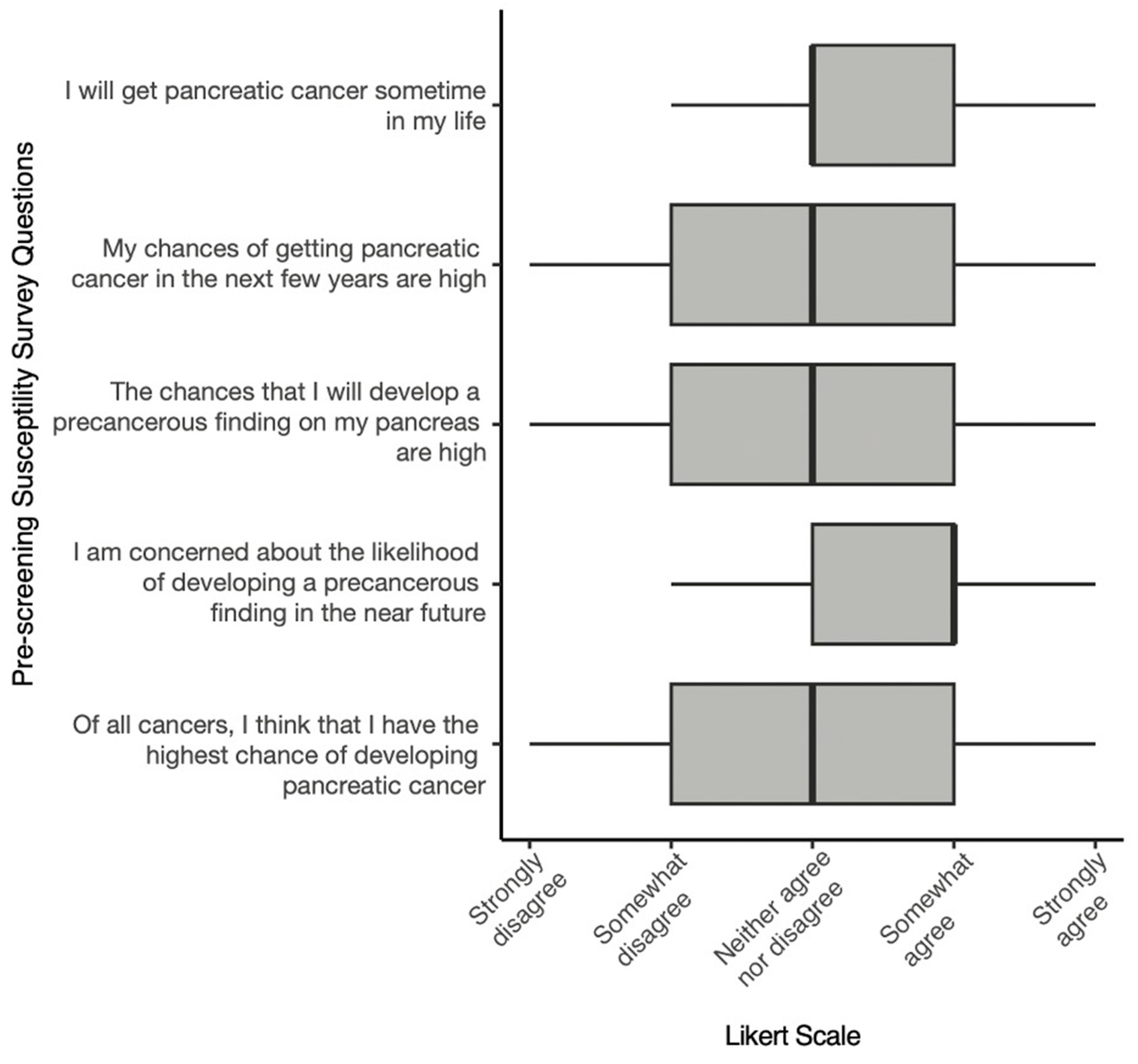
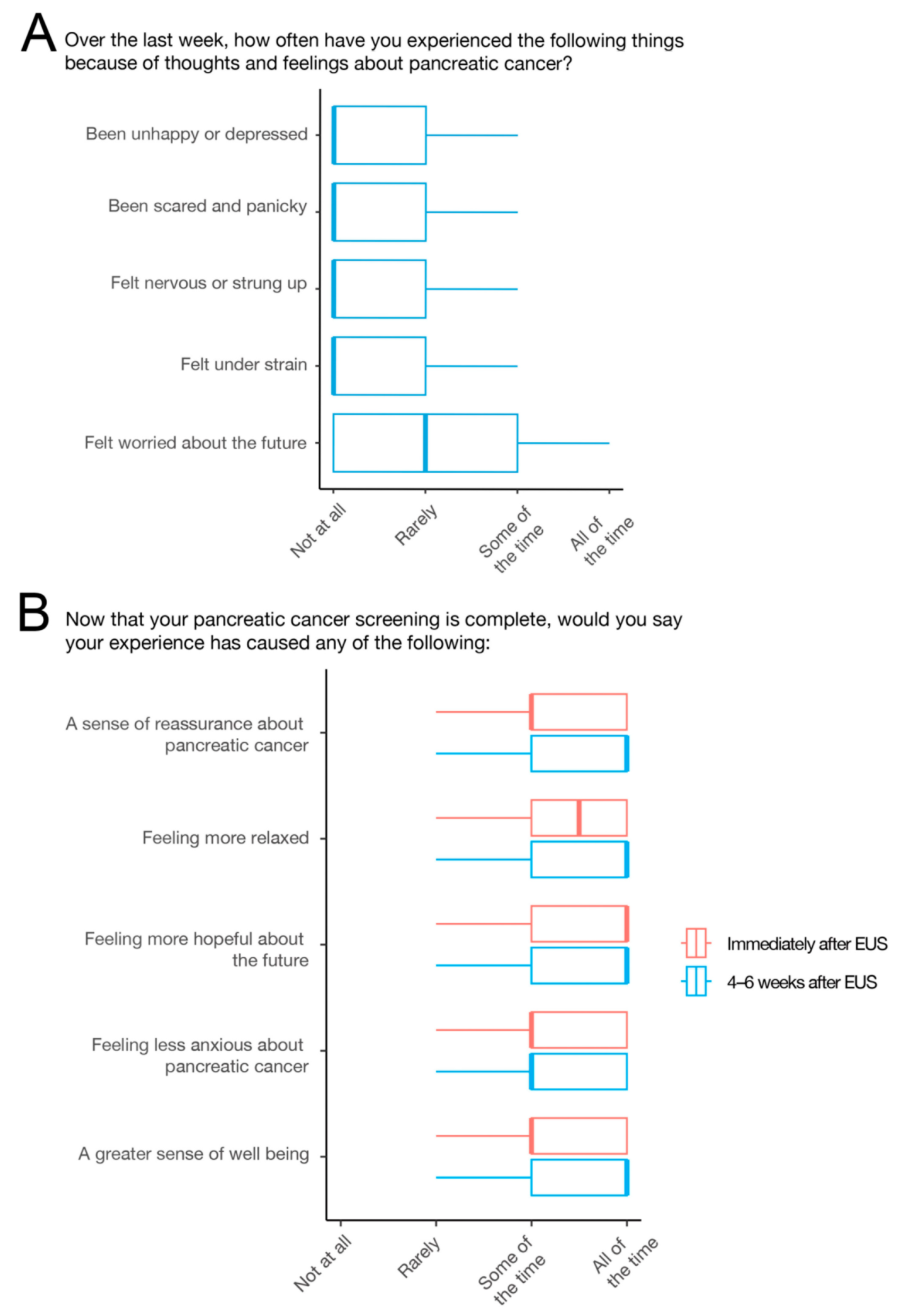
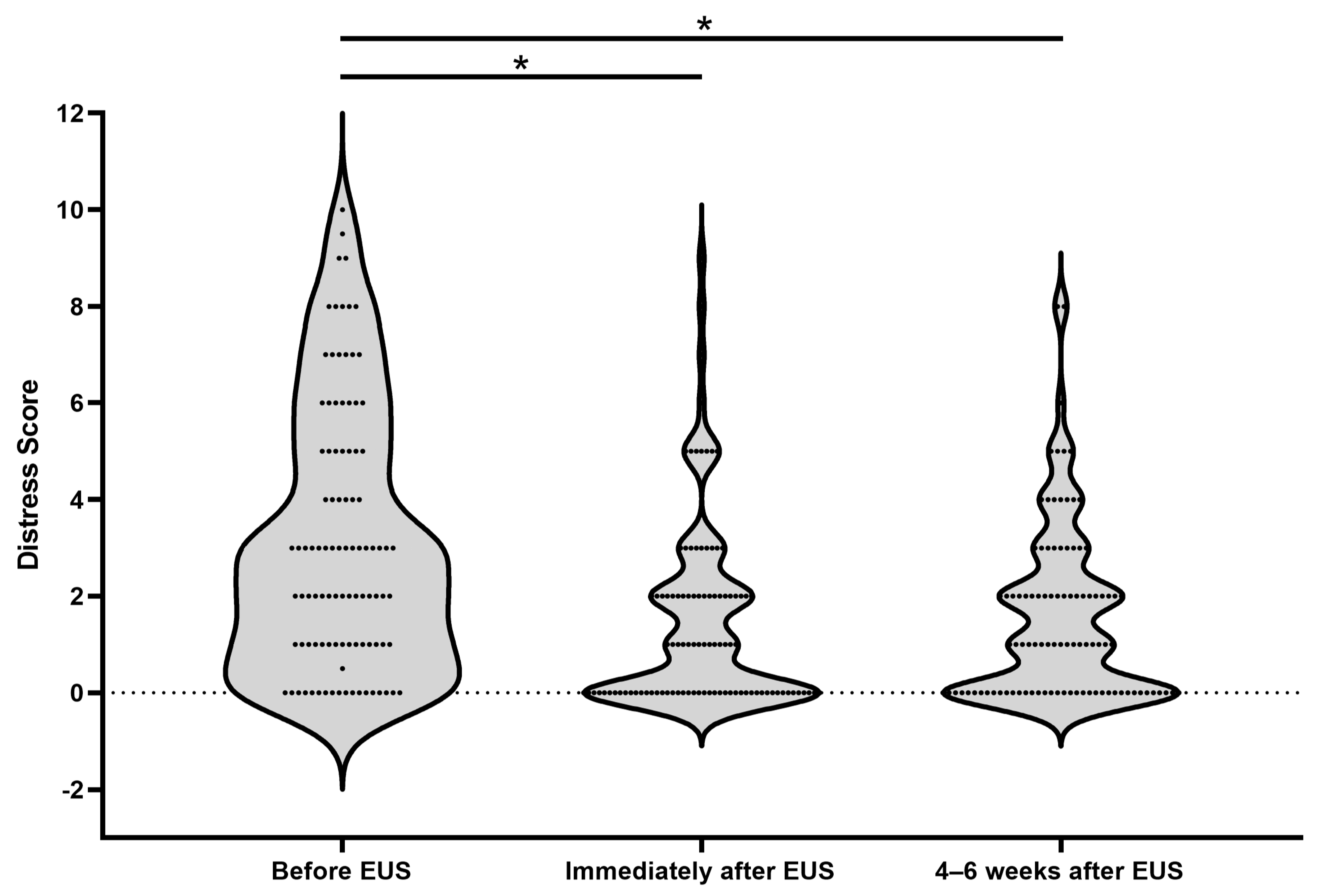
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- NCI. SEER Cancer Stat Facts: Pancreatic Cancer; NCI: Bethesda, MD, USA, 2022. [Google Scholar]
- Blackford, A.L.; Canto, M.I.; Klein, A.P.; Hruban, R.H.; Goggins, M. Recent Trends in the Incidence and Survival of Stage 1A Pancreatic Cancer: A Surveillance, Epidemiology, and End Results Analysis. J. Natl. Cancer Inst. 2020, 112, 1162–1169. [Google Scholar] [CrossRef] [PubMed]
- NCCN. Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic (Version 3.2023). Available online: https://www.nccn.org/professionals/physician_gls/pdf/genetics_bop.pdf (accessed on 11 April 2023).
- Canto, M.I.; Hruban, R.H.; Fishman, E.K.; Kamel, I.R.; Schulick, R.; Zhang, Z.; Topazian, M.; Takahashi, N.; Fletcher, J.; Petersen, G.; et al. Frequent detection of pancreatic lesions in asymptomatic high-risk individuals. Gastroenterology 2012, 142, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Dbouk, M.; Katona, B.W.; Brand, R.E.; Chak, A.; Syngal, S.; Farrell, J.J.; Kastrinos, F.; Stoffel, E.M.; Blackford, A.L.; Rustgi, A.K.; et al. The Multicenter Cancer of Pancreas Screening Study: Impact on Stage and Survival. J. Clin. Oncol. 2022, 40, 3257–3266. [Google Scholar] [CrossRef] [PubMed]
- Vasen, H.; Ibrahim, I.; Ponce, C.G.; Slater, E.P.; Matthai, E.; Carrato, A.; Earl, J.; Robbers, K.; van Mil, A.M.; Potjer, T.; et al. Benefit of Surveillance for Pancreatic Cancer in High-Risk Individuals: Outcome of Long-Term Prospective Follow-Up Studies From Three European Expert Centers. J. Clin. Oncol. 2016, 34, 2010–2019. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, A.M.; Buchain, P.C.; Vizzotto, A.D.B.; Elkis, H.; Cordeiro, Q. Psychosocial Impact. In Encyclopedia of Behavioral Medicine; Gellman, M.D., Turner, J.R., Eds.; Springer: New York, NY, USA, 2013; pp. 1583–1584. [Google Scholar]
- Sohler, N.L.; Jerant, A.; Franks, P. Socio-psychological factors in the Expanded Health Belief Model and subsequent colorectal cancer screening. Patient Educ. Couns. 2015, 98, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Watson, E.K.; Henderson, B.J.; Brett, J.; Bankhead, C.; Austoker, J. The psychological impact of mammographic screening on women with a family history of breast cancer--a systematic review. Psychooncology 2005, 14, 939–948. [Google Scholar] [CrossRef]
- Benito, L.; Farre, A.; Binefa, G.; Vidal, C.; Cardona, A.; Pla, M.; Garcia, M. Factors related to longitudinal adherence in colorectal cancer screening: Qualitative research findings. Cancer Causes Control 2018, 29, 103–114. [Google Scholar] [CrossRef]
- Brown Sofair, J.; Lehlbach, M. The role of anxiety in a mammography screening program. Psychosomatics 2008, 49, 49–55. [Google Scholar] [CrossRef]
- Cazacu, I.M.; Luzuriaga Chavez, A.A.; Saftoiu, A.; Bhutani, M.S. Psychological impact of pancreatic cancer screening by EUS or magnetic resonance imaging in high-risk individuals: A systematic review. Endosc. Ultrasound 2019, 8, 17–24. [Google Scholar] [CrossRef]
- Paiella, S.; Marinelli, V.; Secchettin, E.; Mazzi, M.A.; Ferretto, F.; Casolino, R.; Bassi, C.; Salvia, R. The emotional impact of surveillance programs for pancreatic cancer on high-risk individuals: A prospective analysis. Psychooncology 2020, 29, 1004–1011. [Google Scholar] [CrossRef] [PubMed]
- Che Mohamed, N.; Moey, S.F.; Lim, B.C. Validity and Reliability of Health Belief Model Questionnaire for Promoting Breast Self-examination and Screening Mammogram for Early Cancer Detection. Asian Pac. J. Cancer Prev. 2019, 20, 2865–2873. [Google Scholar] [CrossRef] [PubMed]
- Ghodsbin, F.; Zare, M.; Jahanbin, I.; Ariafar, A.; Keshavarzi, S. A Survey of the Knowledge and Beliefs of Retired Men about Prostate Cancer Screening Based on Health Belief Model. Int. J. Community Based Nurs. Midwifery 2014, 2, 279–285. [Google Scholar] [PubMed]
- Lau, J.; Lim, T.Z.; Jianlin Wong, G.; Tan, K.K. The health belief model and colorectal cancer screening in the general population: A systematic review. Prev. Med. Rep. 2020, 20, 101223. [Google Scholar] [CrossRef] [PubMed]
- Underhill-Blazey, M.; Blonquist, T.; Lawrence, J.; Hong, F.; Yurgelun, M.B.; Syngal, S. Health behaviours and beliefs in individuals with familial pancreatic cancer. Fam. Cancer 2019, 18, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Cockburn, J.; De Luise, T.; Hurley, S.; Clover, K. Development and validation of the PCQ: A questionnaire to measure the psychological consequences of screening mammography. Soc. Sci. Med. 1992, 34, 1129–1134. [Google Scholar] [CrossRef] [PubMed]
- Konings, I.C.; Sidharta, G.N.; Harinck, F.; Aalfs, C.M.; Poley, J.W.; Kieffer, J.M.; Kuenen, M.A.; Smets, E.M.; Wagner, A.; van Hooft, J.E.; et al. Repeated participation in pancreatic cancer surveillance by high-risk individuals imposes low psychological burden. Psychooncology 2016, 25, 971–978. [Google Scholar] [CrossRef]
- Ownby, K.K. Use of the Distress Thermometer in Clinical Practice. J. Adv. Pract. Oncol. 2019, 10, 175–179. [Google Scholar]
- Vernon, S.W.; Myers, R.E.; Tilley, B.C. Development and validation of an instrument to measure factors related to colorectal cancer screening adherence. Cancer Epidemiol. Biomark. Prev. 1997, 6, 825–832. [Google Scholar]
- Hays, R.D.; Schalet, B.D.; Spritzer, K.L.; Cella, D. Two-item PROMIS(R) global physical and mental health scales. J Patient Rep Outcomes 2017, 1, 2. [Google Scholar] [CrossRef]
- Smith, J.; Dodd, R.H.; Hersch, J.; McCaffery, K.J.; Naganathan, V.; Cvejic, E.; Jansen, J. Psychosocial and clinical predictors of continued cancer screening in older adults. Patient Educ. Couns. 2021, 104, 3093–3096. [Google Scholar] [CrossRef] [PubMed]
- Gilfoyle, M.; Garcia, J.; Chaurasia, A.; Oremus, M. Perceived susceptibility to developing cancer and mammography screening behaviour: A cross-sectional analysis of Alberta’s Tomorrow Project. Public Health 2019, 177, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Ait Ouakrim, D.; Lockett, T.; Boussioutas, A.; Keogh, L.; Flander, L.B.; Hopper, J.L.; Jenkins, M.A. Screening participation predictors for people at familial risk of colorectal cancer: A systematic review. Am. J. Prev. Med. 2013, 44, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Peterson, E.B.; Ostroff, J.S.; DuHamel, K.N.; D’Agostino, T.A.; Hernandez, M.; Canzona, M.R.; Bylund, C.L. Impact of provider-patient communication on cancer screening adherence: A systematic review. Prev. Med. 2016, 93, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Roman, L.; Meghea, C.; Ford, S.; Penner, L.; Hamade, H.; Estes, T.; Williams, K.P. Individual, provider, and system risk factors for breast and cervical cancer screening among underserved Black, Latina, and Arab women. J. Womens Health (Larchmt) 2014, 23, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Hatamian, S.; Etesam, S.; Mazidimoradi, A.; Momenimovahed, Z.; Salehiniya, H. The Barriers and Facilitators of Gastric Cancer Screening: A Systematic Review. J. Gastrointest. Cancer 2021, 52, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Castelo, M.; Brown, Z.; D’Abbondanza, J.A.; Wasilewski, N.V.; Eisen, A.; Muradali, D.; Hansen, B.E.; Grunfeld, E.; Scheer, A.S. Psychological consequences of MRI-based screening among women with strong family histories of breast cancer. Breast Cancer Res. Treat. 2021, 189, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.K.; Hershberger, P.E.; Kim, S.; Collins, E.; Quinn, L.T.; Park, C.G.; Ferrans, C.E. Factors Influencing Surveillance Mammography Adherence Among Breast Cancer Survivors. Oncol. Nurs. Forum 2019, 46, 701–714. [Google Scholar] [CrossRef]
- Maheu, C.; Vodermaier, A.; Rothenmund, H.; Gallinger, S.; Ardiles, P.; Semotiuk, K.; Holter, S.; Thayalan, S.; Esplen, M.J. Pancreatic cancer risk counselling and screening: Impact on perceived risk and psychological functioning. Fam. Cancer 2010, 9, 617–624. [Google Scholar] [CrossRef]
- Underhill, M.; Hong, F.; Lawrence, J.; Blonquist, T.; Syngal, S. Relationship between individual and family characteristics and psychosocial factors in persons with familial pancreatic cancer. Psychooncology 2018, 27, 1711–1718. [Google Scholar] [CrossRef]
- Jacobsen, P.B.; Lamonde, L.A.; Honour, M.; Kash, K.; Hudson, P.B.; Pow-Sang, J. Relation of family history of prostate cancer to perceived vulnerability and screening behavior. Psychooncology 2004, 13, 80–85. [Google Scholar] [CrossRef] [PubMed]
- McQueen, A.; Swank, P.R.; Bastian, L.A.; Vernon, S.W. Predictors of perceived susceptibility of breast cancer and changes over time: A mixed modeling approach. Health Psychol. 2008, 27, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Katona, B.W.; Klute, K.; Brand, R.E.; Everett, J.N.; Farrell, J.J.; Hawthorne, K.; Kaul, V.; Kupfer, S.S.; Paiella, S.; Simeone, D.M.; et al. Racial, Ethnic, and Sex-based Disparities among High-risk Individuals Undergoing Pancreatic Cancer Surveillance. Cancer Prev. Res. (Phila) 2023, 16, 343–352. [Google Scholar] [CrossRef] [PubMed]

| Participated in Survey | Declined Survey Participation | p-Value | |
|---|---|---|---|
| n = 100 | n = 34 | ||
| Age at surveillance (mean, SD) | 59.0 (53.5–64.5) | 66.0 (57.0–71.0) | 0.01 |
| Sex | 0.34 | ||
| Male | 29.0% (29) | 20.6% (7) | |
| Female | 71.0% (71) | 79.4% (27) | |
| Race | 0.50 | ||
| White | 96.0% (96) | 100.0% (34) | |
| Black | 3.0% (3) | 0.0% (0) | |
| Asian | 1.0% (1) | 0.0% (0) | |
| Ethnicity | 0.41 | ||
| Hispanic or Latino | 2.0% (2) | 0.0% (0) | |
| Not Hispanic or Latino | 98.0% (98) | 100.0% (34) | |
| Personal history of cancer | 42.0% (42) | 47.1% (16) | 0.61 |
| Family history of pancreatic cancer | 76.0% (76) | 64.7% (22) | 0.20 |
| Prior genetic testing | 96.0% (96) | 87.9% (29) | 0.09 |
| Presence of a PGV in a PC risk gene | 75.0% (72) | 79.3% (23) | 0.63 |
| ATM | 4.0% (4) | 14.7% (5) | 0.03 |
| BRCA1 | 15.0% (15) | 11.8% (4) | 0.64 |
| BRCA2 | 39.0% (39) | 35.3% (12) | 0.70 |
| CDKN2A | 1.0% (1) | 2.9% (1) | 0.42 |
| PALB2 | 6.0% (6) | 2.9% (1) | 0.49 |
| STK11 | 2.0% (2) | 0.0% (0) | 0.41 |
| Lynch syndrome-associated PGV | 6.0% (6) | 2.9% (1) | 0.49 |
| Other | 1% (1) | 5.9% (2) | 0.10 |
| Subject undergoing their baseline pancreatic cancer surveillance study | 25.0% (25) | 26.5% (9) | 0.86 |
| Univariate Linear Regression Analysis | Multivariate Linear Regression Analysis | |||||
|---|---|---|---|---|---|---|
| Coefficient | p-Value | [95% Confidence Interval] | Coefficient | p-Value | [95% Confidence Interval] | |
| Change in Distress | ||||||
| Age | −0.03 | 0.27 | −0.08 to 0.02 | −0.01 | 0.66 | −0.07 to 0.04 |
| Female sex | 1.22 | 0.02 | 0.2 to 2.22 | 1.10 | 0.05 | 0.01 to 2.19 |
| Race | ||||||
| Black * | −1.06 | 0.44 | −3.80 to 1.68 | −1.23 | 0.38 | −4.04 to 1.57 |
| Asian * | −1.73 | 0.47 | −6.42 to 2.97 | −1.71 | 0.49 | −6.63 to 3.20 |
| Ethnicity | ||||||
| Not Hispanic or Latino ** | −1.35 | 0.42 | −4.67 to 1.98 | −0.72 | 0.68 | −4.25 to 2.80 |
| Personal history of cancer | 0.22 | 0.64 | −0.72 to 1.17 | −0.07 | 0.90 | −1.10 to 0.97 |
| Pathogenic gene variant | 0.07 | 0.90 | −1.05 to 1.19 | −0.05 | 0.94 | −1.27 to 1.18 |
| First surveillance study | 0.48 | 0.38 | −0.59 to 1.55 | 0.54 | 0.36 | −0.62 to 1.71 |
| Family history of pancreatic cancer | 0.40 | 0.47 | −0.69 to 1.49 | 0.62 | 0.33 | −0.64 to 1.87 |
| Reported harm to mental health | 0.71 | <0.01 | 0.23 to 1.18 | 0.57 | 0.04 | 0.03 to 1.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anez-Bruzual, I.; Coughlin, S.; Clay, D.; Heiman, J.; Dungan, M.; Weber, M.; Almario, C.V.; Leung, G.; Ahmad, N.A.; Ginsberg, G.G.; et al. Assessment of the Psychosocial Impact of Pancreatic Cancer Surveillance in High-Risk Individuals. Cancers 2024, 16, 86. https://doi.org/10.3390/cancers16010086
Anez-Bruzual I, Coughlin S, Clay D, Heiman J, Dungan M, Weber M, Almario CV, Leung G, Ahmad NA, Ginsberg GG, et al. Assessment of the Psychosocial Impact of Pancreatic Cancer Surveillance in High-Risk Individuals. Cancers. 2024; 16(1):86. https://doi.org/10.3390/cancers16010086
Chicago/Turabian StyleAnez-Bruzual, Isabel, Sarah Coughlin, Daniel Clay, Jordan Heiman, Michaela Dungan, Marina Weber, Christopher V. Almario, Galen Leung, Nuzhat A. Ahmad, Gregory G. Ginsberg, and et al. 2024. "Assessment of the Psychosocial Impact of Pancreatic Cancer Surveillance in High-Risk Individuals" Cancers 16, no. 1: 86. https://doi.org/10.3390/cancers16010086
APA StyleAnez-Bruzual, I., Coughlin, S., Clay, D., Heiman, J., Dungan, M., Weber, M., Almario, C. V., Leung, G., Ahmad, N. A., Ginsberg, G. G., Kochman, M. L., Valverde, K. D., Long, J. M., & Katona, B. W. (2024). Assessment of the Psychosocial Impact of Pancreatic Cancer Surveillance in High-Risk Individuals. Cancers, 16(1), 86. https://doi.org/10.3390/cancers16010086








