The Role of tRNA-Centered Translational Regulatory Mechanisms in Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
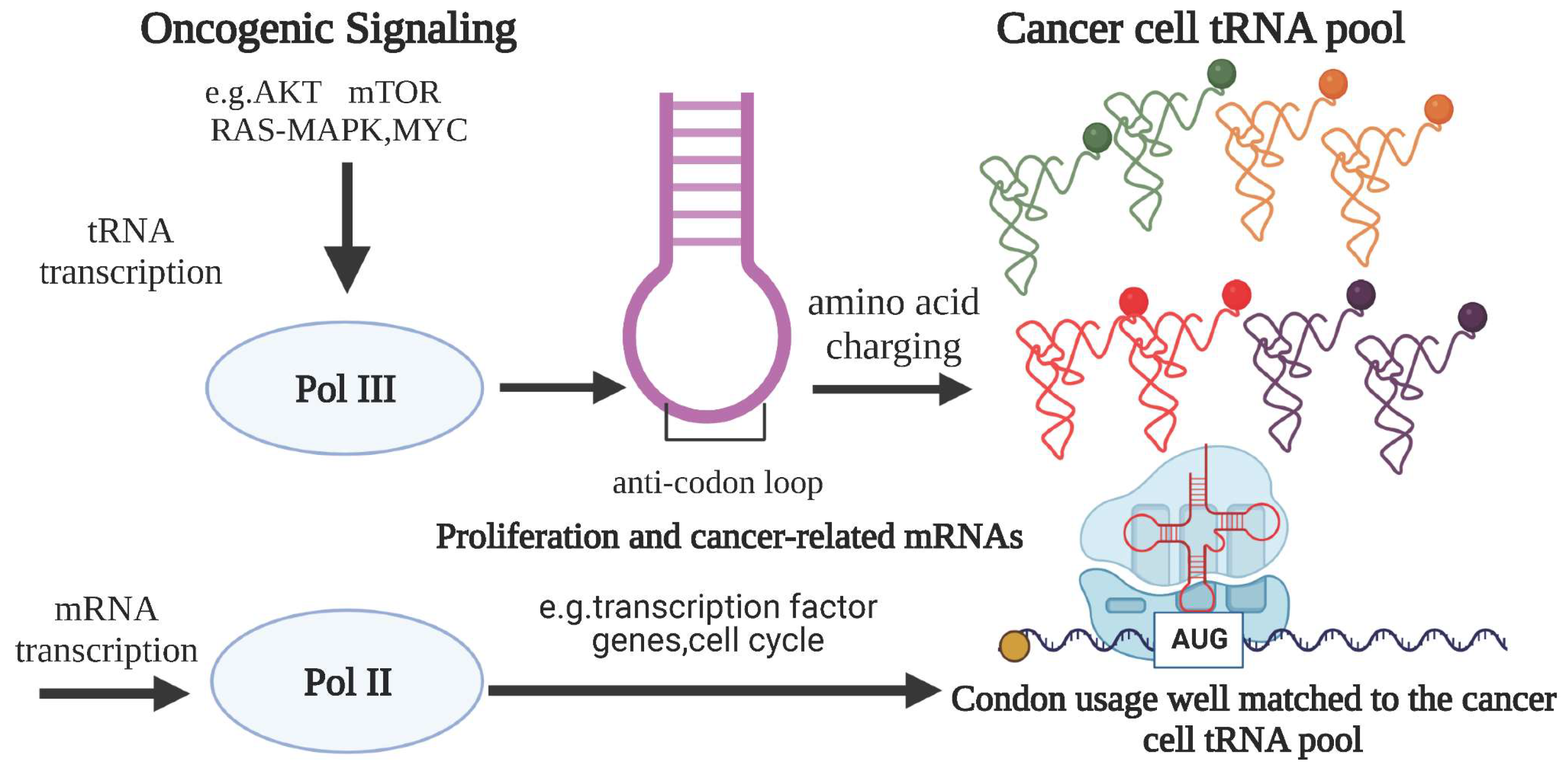
2. tRNA Influences Translational Regulation of Tumors at Multiple Levels
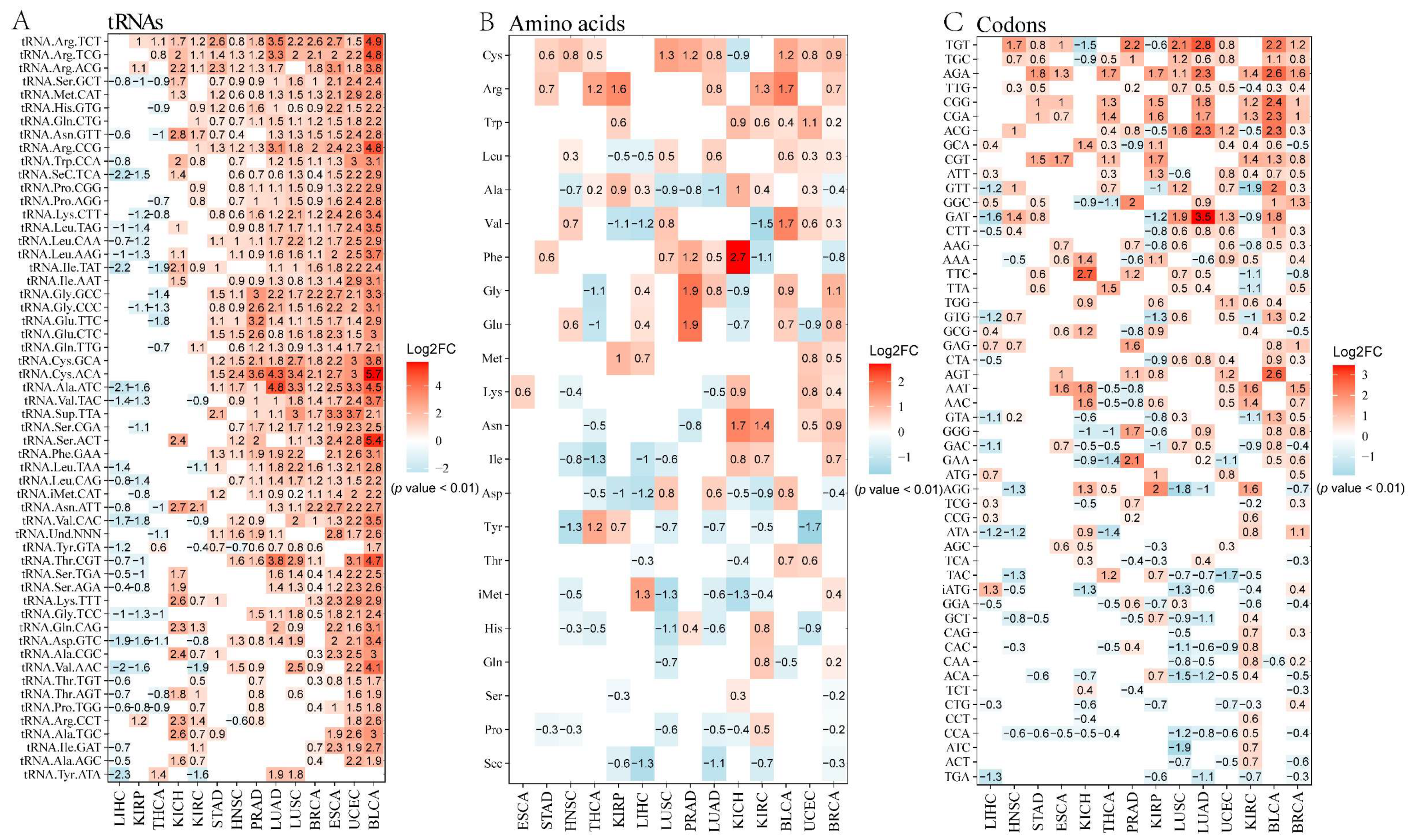
2.1. The Effect of tRNA Level (Defined as tRNAamino acidanticodon) on Translational Regulation in Tumor
2.2. The Effect of Amino Acid Level (Defined as tRNAamino acid) on Tumor Translation Regulation
2.3. The Effect of Codon Level (Defined as tRNAamino acid(codon)) on Tumor Translational Regulation
2.4. Unequal Alterations at the tRNA Copy Number, tRNAamino acid, and tRNAamino acid(codon) Levels
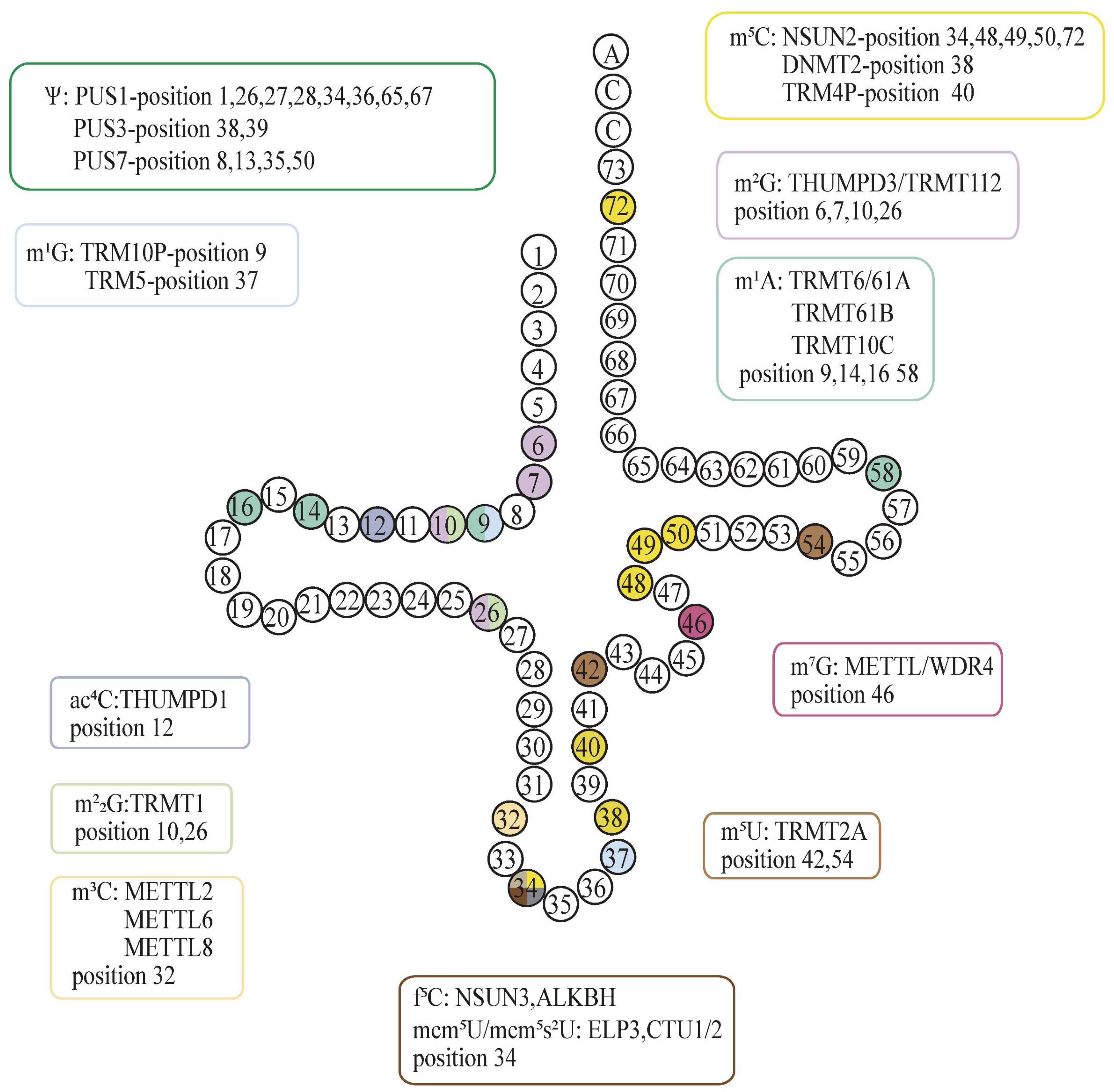
3. Regulation of Protein Translation by tRNA Modification
3.1. Regulation of Protein Translation by Correct Anticodon Modification of tRNA
3.2. Several Classical Abnormal tRNA Modifications and Cancer
3.2.1. Abnormal 5-Methylcytosine (m5C) Modification in Cancer
3.2.2. Abnormal 7-Methylguanosine (m7G) Modification in Cancer
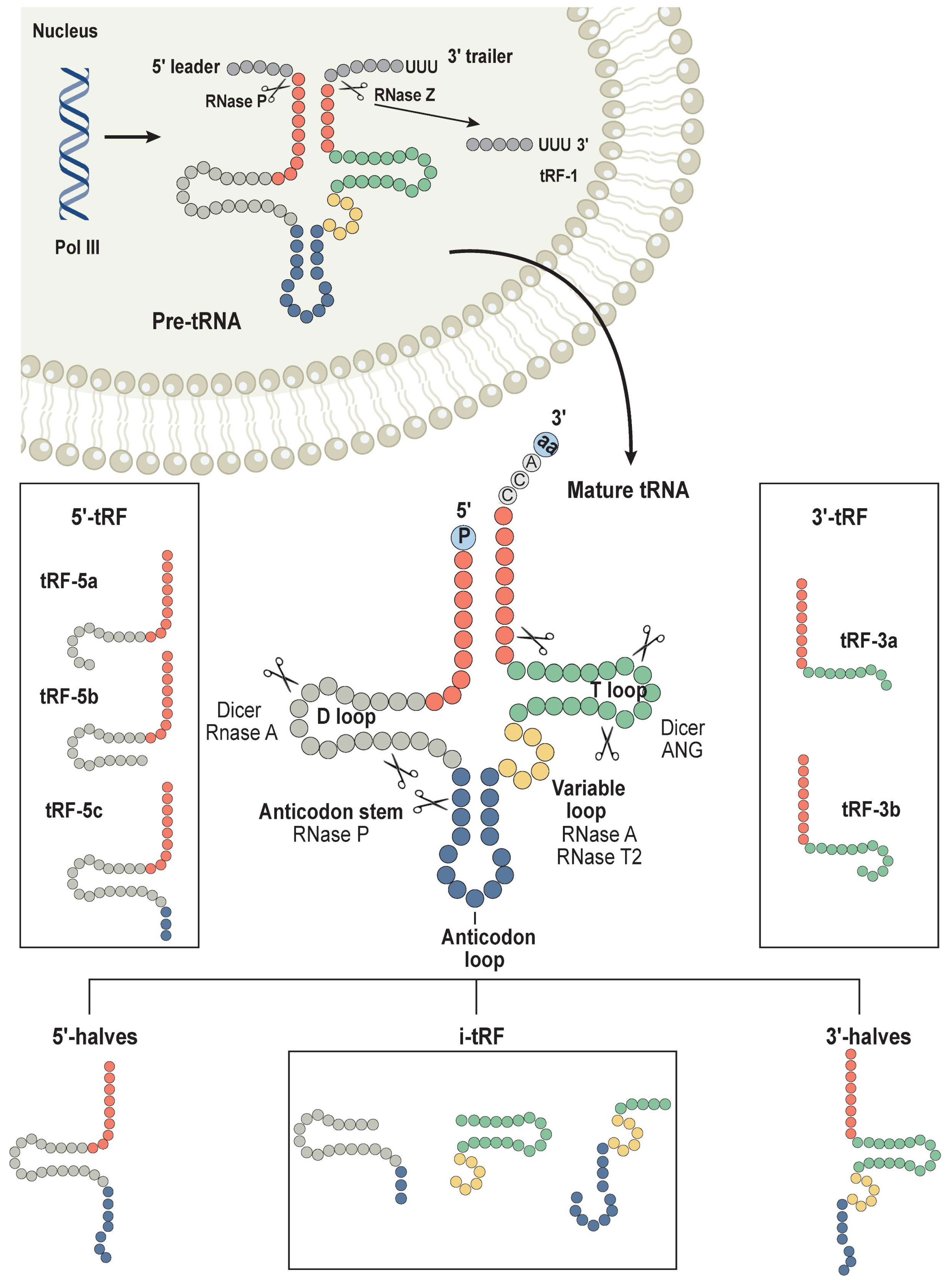
4. Regulation of tRNA Fragmentation on Translation in Cancer
4.1. Sources and Types of tRFs
4.2. Global Inhibition of Translation Regulation by tRFs
4.3. tRFs Mediate Translational Activation through Ribosomes
4.4. tRFs Mediates Translation Regulation by Regulating mRNA Stability
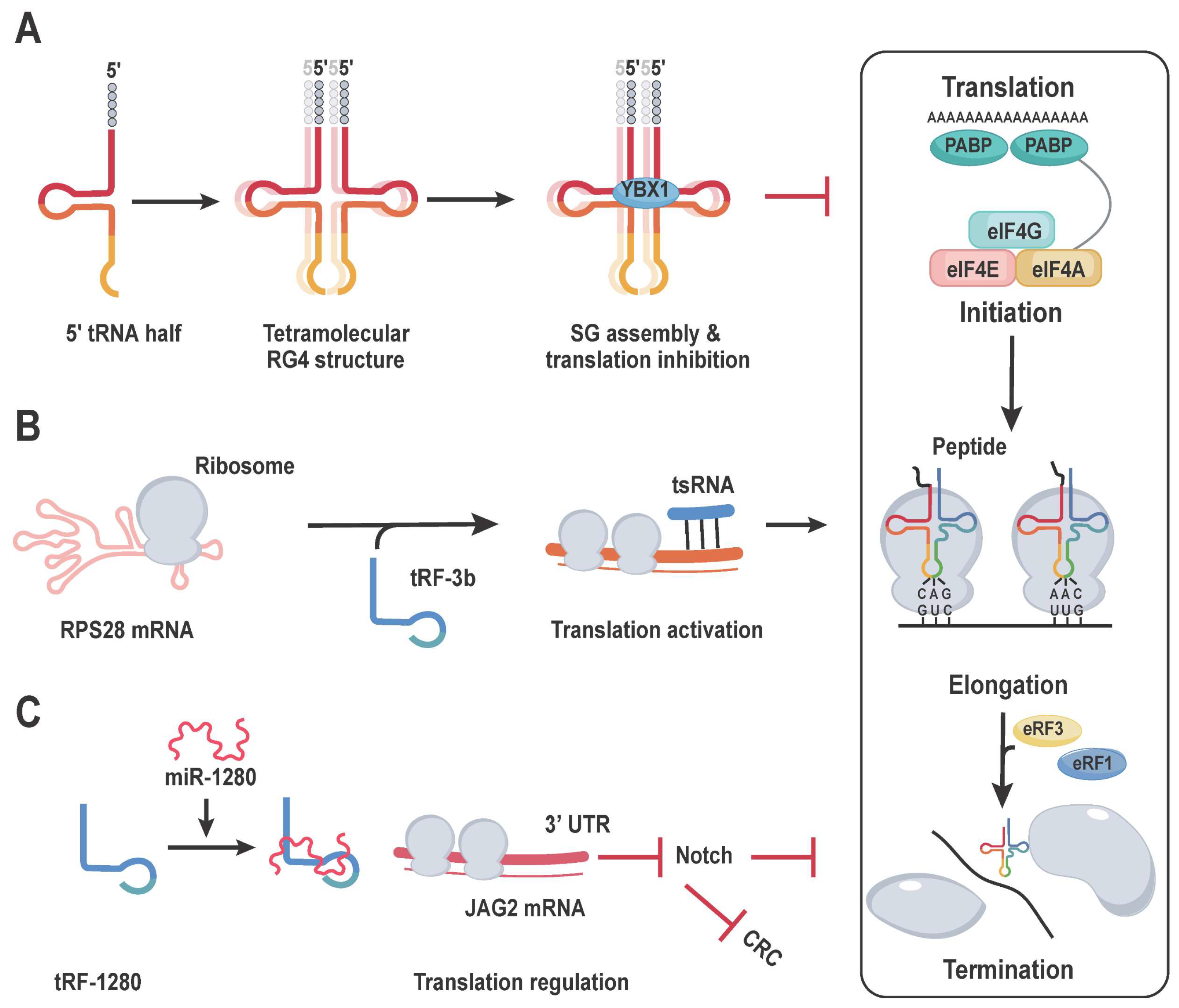
5. tRNA-Mediated Translation Models under Stress
6. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Truitt, M.L.; Ruggero, D. New Frontiers in Translational Control of the Cancer Genome. Nat. Rev. Cancer 2016, 16, 288–304, Erratum in Nat. Rev. Cancer 2017, 17, 332. https://doi.org/10.1038/nrc.2017.30. [Google Scholar] [CrossRef] [PubMed]
- Blanco, S.; Dietmann, S.; Flores, J.V.; Hussain, S.; Kutter, C.; Humphreys, P.; Lukk, M.; Lombard, P.; Treps, L.; Popis, M.; et al. Aberrant Methylation of tRNAs Links Cellular Stress to Neuro-Developmental Disorders. EMBO J. 2014, 33, 2020–2039. [Google Scholar] [CrossRef] [PubMed]
- Tuorto, F.; Liebers, R.; Musch, T.; Schaefer, M.; Hofmann, S.; Kellner, S.; Frye, M.; Helm, M.; Stoecklin, G.; Lyko, F. RNA Cytosine Methylation by Dnmt2 and NSun2 Promotes tRNA Stability and Protein Synthesis. Nat. Struct. Mol. Biol. 2012, 19, 900–905. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Ye, Y.; Gong, J.; Ruan, H.; Liu, C.-J.; Xiang, Y.; Cai, C.; Guo, A.-Y.; Ling, J.; Diao, L.; et al. Global Analysis of tRNA and Translation Factor Expression Reveals a Dynamic Landscape of Translational Regulation in Human Cancers. Commun. Biol. 2018, 1, 234. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Arroyo, V.M.; Raj, R.; Babu, K.; Onolbaatar, O.; Roberts, P.H.; Nam, Y. Structures and Mechanisms of tRNA Methylation by METTL1-WDR4. Nature 2023, 613, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Long, J.; Yao, Z.; Zhao, Y.; Zhao, Y.; Liao, J.; Lei, K.; Xiao, H.; Dai, Z.; Peng, S.; et al. METTL1-Mediated m7G tRNA Modification Promotes Lenvatinib Resistance in Hepatocellular Carcinoma. Cancer Res. 2023, 83, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wang, W.; Zhou, R.; Ding, Y.; Li, X. The M5 C Methyltransferase NSUN2 Promotes Codon-Dependent Oncogenic Translation by Stabilising tRNA in Anaplastic Thyroid Cancer. Clin. Transl. Med. 2023, 13, e1466. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, H.; Song, X.; Wang, J.; Zhao, S.; Deng, H.; He, L.; Zhou, X.; Yin, X.; Zhang, K.; et al. Transcription Factor GATA4 Drives RNA Polymerase III-Directed Transcription and Transformed Cell Proliferation through a Filamin A/GATA4/SP1 Pathway. J. Biol. Chem. 2022, 298, 101581. [Google Scholar] [CrossRef]
- Sun, Y.; Fang, Q.; Liu, W.; Liu, Y.; Zhang, C. GANT-61 Induces Cell Cycle Resting and Autophagy by down-Regulating RNAP III Signal Pathway and tRNA-Gly-CCC Synthesis to Combate Chondrosarcoma. Cell Death Dis. 2023, 14, 461. [Google Scholar] [CrossRef]
- Endres, T.; Solvie, D.; Heidelberger, J.B.; Andrioletti, V.; Baluapuri, A.; Ade, C.P.; Muhar, M.; Eilers, U.; Vos, S.M.; Cramer, P.; et al. Ubiquitylation of MYC Couples Transcription Elongation with Double-Strand Break Repair at Active Promoters. Mol. Cell 2021, 81, 830–844.e13. [Google Scholar] [CrossRef]
- Bild, A.H.; Yao, G.; Chang, J.T.; Wang, Q.; Potti, A.; Chasse, D.; Joshi, M.-B.; Harpole, D.; Lancaster, J.M.; Berchuck, A.; et al. Oncogenic Pathway Signatures in Human Cancers as a Guide to Targeted Therapies. Nature 2006, 439, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Felton-Edkins, Z.A.; Fairley, J.A.; Graham, E.L.; Johnston, I.M.; White, R.J.; Scott, P.H. The Mitogen-Activated Protein (MAP) Kinase ERK Induces tRNA Synthesis by Phosphorylating TFIIIB. EMBO J. 2003, 22, 2422–2432. [Google Scholar] [CrossRef] [PubMed]
- Kawai, H.; Kanegae, T.; Christensen, S.; Kiyosue, T.; Sato, Y.; Imaizumi, T.; Kadota, A.; Wada, M. Responses of Ferns to Red Light Are Mediated by an Unconventional Photoreceptor. Nature 2003, 421, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Tsang, C.K.; Zheng, X.F.S. Mechanisms of Regulation of RNA Polymerase III-Dependent Transcription by TORC1. EMBO J. 2009, 28, 2220–2230. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Yin, K.; Zhang, X.; Ye, P.; Chen, X.; Chao, J.; Meng, H.; Wei, J.; Roeth, D.; Li, L.; et al. Targeting PUS7 Suppresses tRNA Pseudouridylation and Glioblastoma Tumorigenesis. Nat. Cancer 2021, 2, 932–949. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Noordam, L.; Ou, X.; Ma, B.; Li, Y.; Das, P.; Shi, S.; Liu, J.; Wang, L.; Li, P.; et al. The Biological Process of Lysine-tRNA Charging Is Therapeutically Targetable in Liver Cancer. Liver Int. 2021, 41, 206–219. [Google Scholar] [CrossRef]
- Yang, M.; Mo, Y.; Ren, D.; Liu, S.; Zeng, Z.; Xiong, W. Transfer RNA-Derived Small RNAs in Tumor Microenvironment. Mol. Cancer 2023, 22, 32. [Google Scholar] [CrossRef]
- Robichaud, N.; Sonenberg, N.; Ruggero, D.; Schneider, R.J. Translational Control in Cancer. Cold Spring Harb. Perspect. Biol. 2019, 11, a032896. [Google Scholar] [CrossRef]
- Blanco, S.; Bandiera, R.; Popis, M.; Hussain, S.; Lombard, P.; Aleksic, J.; Sajini, A.; Tanna, H.; Cortés-Garrido, R.; Gkatza, N.; et al. Stem Cell Function and Stress Response Are Controlled by Protein Synthesis. Nature 2016, 534, 335–340. [Google Scholar] [CrossRef]
- Pinzaru, A.M.; Tavazoie, S.F. Transfer RNAs as Dynamic and Critical Regulators of Cancer Progression. Nat. Rev. Cancer 2023, 23, 746–761. [Google Scholar] [CrossRef]
- Goodarzi, H.; Nguyen, H.C.B.; Zhang, S.; Dill, B.D.; Molina, H.; Tavazoie, S.F. Modulated Expression of Specific tRNAs Drives Gene Expression and Cancer Progression. Cell 2016, 165, 1416–1427. [Google Scholar] [CrossRef] [PubMed]
- Gingold, H.; Tehler, D.; Christoffersen, N.R.; Nielsen, M.M.; Asmar, F.; Kooistra, S.M.; Christophersen, N.S.; Christensen, L.L.; Borre, M.; Sørensen, K.D.; et al. A Dual Program for Translation Regulation in Cellular Proliferation and Differentiation. Cell 2014, 158, 1281–1292. [Google Scholar] [CrossRef] [PubMed]
- Delaunay, S.; Rapino, F.; Tharun, L.; Zhou, Z.; Heukamp, L.; Termathe, M.; Shostak, K.; Klevernic, I.; Florin, A.; Desmecht, H.; et al. Elp3 Links tRNA Modification to IRES-Dependent Translation of LEF1 to Sustain Metastasis in Breast Cancer. J. Exp. Med. 2016, 213, 2503–2523. [Google Scholar] [CrossRef] [PubMed]
- Pavon-Eternod, M.; Gomes, S.; Geslain, R.; Dai, Q.; Rosner, M.R.; Pan, T. tRNA Over-Expression in Breast Cancer and Functional Consequences. Nucleic Acids Res. 2009, 37, 7268–7280. [Google Scholar] [CrossRef] [PubMed]
- Dos Reis, M.; Savva, R.; Wernisch, L. Solving the Riddle of Codon Usage Preferences: A Test for Translational Selection. Nucleic Acids Res. 2004, 32, 5036–5044. [Google Scholar] [CrossRef] [PubMed]
- Dedon, P.C.; Begley, T.J. Dysfunctional tRNA Reprogramming and Codon-Biased Translation in Cancer. Trends Mol. Med. 2022, 28, 964–978. [Google Scholar] [CrossRef] [PubMed]
- Mitchener, M.M.; Begley, T.J.; Dedon, P.C. Molecular Coping Mechanisms: Reprogramming tRNAs To Regulate Codon-Biased Translation of Stress Response Proteins. Accounts Chem. Res. 2023, 56, 3504–3514. [Google Scholar] [CrossRef] [PubMed]
- Dittmar, K.A.; Goodenbour, J.M.; Pan, T. Tissue-Specific Differences in Human Transfer RNA Expression. PLoS Genet. 2006, 2, e221. [Google Scholar] [CrossRef]
- Butterfield, S.P.; Sizer, R.E.; Rand, E.; White, R.J. Selection of tRNA Genes in Human Breast Tumours Varies Substantially between Individuals. Cancers 2023, 15, 3576. [Google Scholar] [CrossRef]
- Gomez-Roman, N.; Grandori, C.; Eisenman, R.N.; White, R.J. Direct Activation of RNA Polymerase III Transcription by C-Myc. Nature 2003, 421, 290–294. [Google Scholar] [CrossRef]
- Kantidakis, T.; Ramsbottom, B.A.; Birch, J.L.; Dowding, S.N.; White, R.J. mTOR Associates with TFIIIC, Is Found at tRNA and 5S rRNA Genes, and Targets Their Repressor Maf1. Proc. Natl. Acad. Sci. USA 2010, 107, 11823–11828. [Google Scholar] [CrossRef] [PubMed]
- Pavon-Eternod, M.; Gomes, S.; Rosner, M.R.; Pan, T. Overexpression of Initiator Methionine tRNA Leads to Global Reprogramming of tRNA Expression and Increased Proliferation in Human Epithelial Cells. RNA 2013, 19, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.; Fidalgo, A.; Varanda, A.S.; Soares, A.R.; Almeida, G.M.; Martins, D.; Mendes, N.; Oliveira, C.; Santos, M.A.S. Upregulation of tRNA-Ser-AGA-2-1 Promotes Malignant Behavior in Normal Bronchial Cells. Front. Mol. Biosci. 2022, 9, 809985. [Google Scholar] [CrossRef] [PubMed]
- Huh, D.; Passarelli, M.C.; Gao, J.; Dusmatova, S.N.; Goin, C.; Fish, L.; Pinzaru, A.M.; Molina, H.; Ren, Z.; McMillan, E.A.; et al. A Stress-Induced Tyrosine-tRNA Depletion Response Mediates Codon-Based Translational Repression and Growth Suppression. EMBO J. 2021, 40, e106696. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Goodenbour, J.M.; Godley, L.A.; Wickrema, A.; Pan, T. High Levels of tRNA Abundance and Alteration of tRNA Charging by Bortezomib in Multiple Myeloma. Biochem. Biophys. Res. Commun. 2009, 385, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, E.M.; Selenica, P.; Vahdatinia, M.; Pareja, F.; Da Cruz Paula, A.; Ferrando, L.; Gazzo, A.M.; Dopeso, H.; Ross, D.S.; Bakhteri, A.; et al. TERT Promoter Hotspot Mutations and Gene Amplification in Metaplastic Breast Cancer. NPJ Breast Cancer 2021, 7, 43. [Google Scholar] [CrossRef]
- Ducrest, A.-L.; Szutorisz, H.; Lingner, J.; Nabholz, M. Regulation of the Human Telomerase Reverse Transcriptase Gene. Oncogene 2002, 21, 541–552. [Google Scholar] [CrossRef]
- Rapino, F.; Delaunay, S.; Rambow, F.; Zhou, Z.; Tharun, L.; De Tullio, P.; Sin, O.; Shostak, K.; Schmitz, S.; Piepers, J.; et al. Codon-Specific Translation Reprogramming Promotes Resistance to Targeted Therapy. Nature 2018, 558, 605–609. [Google Scholar] [CrossRef]
- Duret, L. tRNA Gene Number and Codon Usage in the C. Elegans Genome Are Co-Adapted for Optimal Translation of Highly Expressed Genes. Trends Genet. 2000, 16, 287–289. [Google Scholar] [CrossRef]
- Man, O.; Pilpel, Y. Differential Translation Efficiency of Orthologous Genes Is Involved in Phenotypic Divergence of Yeast Species. Nat. Genet. 2007, 39, 415–421. [Google Scholar] [CrossRef]
- Lampson, B.L.; Pershing, N.L.K.; Prinz, J.A.; Lacsina, J.R.; Marzluff, W.F.; Nicchitta, C.V.; MacAlpine, D.M.; Counter, C.M. Rare Codons Regulate KRas Oncogenesis. Curr. Biol. 2013, 23, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Thandapani, P.; Kloetgen, A.; Witkowski, M.T.; Glytsou, C.; Lee, A.K.; Wang, E.; Wang, J.; LeBoeuf, S.E.; Avrampou, K.; Papagiannakopoulos, T.; et al. Valine tRNA Levels and Availability Regulate Complex I Assembly in Leukaemia. Nature 2022, 601, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Kuang, M.; Zheng, D.; Tao, X.; Peng, Y.; Pan, Y.; Zheng, S.; Zhang, Y.; Li, H.; Yuan, C.; Zhang, Y.; et al. tRNA-Based Prognostic Score in Predicting Survival Outcomes of Lung Adenocarcinomas. Int. J. Cancer 2019, 145, 1982–1990. [Google Scholar] [CrossRef] [PubMed]
- Earnest-Noble, L.B.; Hsu, D.; Chen, S.; Asgharian, H.; Nandan, M.; Passarelli, M.C.; Goodarzi, H.; Tavazoie, S.F. Two Isoleucyl tRNAs That Decode Synonymous Codons Divergently Regulate Breast Cancer Metastatic Growth by Controlling Translation of Proliferation-Regulating Genes. Nat. Cancer 2022, 3, 1484–1497. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tao, E.-W.; Tan, J.; Gao, Q.-Y.; Chen, Y.-X.; Fang, J.-Y. tRNA Modifications: Insights into Their Role in Human Cancers. Trends Cell Biol. 2023, 33, 1035–1048. [Google Scholar] [CrossRef]
- Barbieri, I.; Kouzarides, T. Role of RNA Modifications in Cancer. Nat. Rev. Cancer 2020, 20, 303–322. [Google Scholar] [CrossRef]
- Schimmel, P. The Emerging Complexity of the tRNA World: Mammalian tRNAs beyond Protein Synthesis. Nat. Rev. Mol. Cell Biol. 2018, 19, 45–58. [Google Scholar] [CrossRef]
- Hanson, G.; Coller, J. Codon Optimality, Bias and Usage in Translation and mRNA Decay. Nat. Rev. Mol. Cell Biol. 2018, 19, 20–30. [Google Scholar] [CrossRef]
- Zhou, J.-B.; Wang, E.-D.; Zhou, X.-L. Modifications of the Human tRNA Anticodon Loop and Their Associations with Genetic Diseases. Cell Mol. Life Sci. 2021, 78, 7087–7105. [Google Scholar] [CrossRef]
- Suzuki, T. The Expanding World of tRNA Modifications and Their Disease Relevance. Nat. Rev. Mol. Cell Biol. 2021, 22, 375–392. [Google Scholar] [CrossRef]
- Stuart, J.W.; Koshlap, K.M.; Guenther, R.; Agris, P.F. Naturally-Occurring Modification Restricts the Anticodon Domain Conformational Space of tRNA(Phe). J. Mol. Biol. 2003, 334, 901–918. [Google Scholar] [CrossRef] [PubMed]
- Nedialkova, D.D.; Leidel, S.A. Optimization of Codon Translation Rates via tRNA Modifications Maintains Proteome Integrity. Cell 2015, 161, 1606–1618. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Yashiro, Y.; Kikuchi, I.; Ishigami, Y.; Saito, H.; Matsuzawa, I.; Okada, S.; Mito, M.; Iwasaki, S.; Ma, D.; et al. Complete Chemical Structures of Human Mitochondrial tRNAs. Nat. Commun. 2020, 11, 4269. [Google Scholar] [CrossRef] [PubMed]
- Kumbhar, B.V.; Kamble, A.D.; Sonawane, K.D. Conformational Preferences of Modified Nucleoside N(4)-Acetylcytidine, ac4C Occur at “Wobble” 34th Position in the Anticodon Loop of tRNA. Cell Biochem. Biophys. 2013, 66, 797–816. [Google Scholar] [CrossRef] [PubMed]
- Nakano, S.; Suzuki, T.; Kawarada, L.; Iwata, H.; Asano, K.; Suzuki, T. NSUN3 Methylase Initiates 5-Formylcytidine Biogenesis in Human Mitochondrial tRNA(Met). Nat. Chem. Biol. 2016, 12, 546–551. [Google Scholar] [CrossRef]
- Song, H.; Zhang, J.; Liu, B.; Xu, J.; Cai, B.; Yang, H.; Straube, J.; Yu, X.; Ma, T. Biological Roles of RNA m5C Modification and Its Implications in Cancer Immunotherapy. Biomark. Res. 2022, 10, 15. [Google Scholar] [CrossRef]
- Lin, S.; Liu, Q.; Lelyveld, V.S.; Choe, J.; Szostak, J.W.; Gregory, R.I. Mettl1/Wdr4-Mediated m7G tRNA Methylome Is Required for Normal mRNA Translation and Embryonic Stem Cell Self-Renewal and Differentiation. Mol. Cell 2018, 71, 244–255.e5. [Google Scholar] [CrossRef]
- Dai, Z.; Liu, H.; Liao, J.; Huang, C.; Ren, X.; Zhu, W.; Zhu, S.; Peng, B.; Li, S.; Lai, J.; et al. N7-Methylguanosine tRNA Modification Enhances Oncogenic mRNA Translation and Promotes Intrahepatic Cholangiocarcinoma Progression. Mol. Cell 2021, 81, 3339–3355.e8. [Google Scholar] [CrossRef]
- Chen, Z.; Zhu, W.; Zhu, S.; Sun, K.; Liao, J.; Liu, H.; Dai, Z.; Han, H.; Ren, X.; Yang, Q.; et al. METTL1 Promotes Hepatocarcinogenesis via M7 G tRNA Modification-Dependent Translation Control. Clin. Transl. Med. 2021, 11, e661. [Google Scholar] [CrossRef]
- Anton, B.P.; Russell, S.P.; Vertrees, J.; Kasif, S.; Raleigh, E.A.; Limbach, P.A.; Roberts, R.J. Functional Characterization of the YmcB and YqeV tRNA Methylthiotransferases of Bacillus Subtilis. Nucleic. Acids. Res. 2010, 38, 6195–6205. [Google Scholar] [CrossRef]
- Murphy, F.V.; Ramakrishnan, V.; Malkiewicz, A.; Agris, P.F. The Role of Modifications in Codon Discrimination by tRNA(Lys)UUU. Nat. Struct. Mol. Biol. 2004, 11, 1186–1191. [Google Scholar] [CrossRef]
- Durant, P.C.; Bajji, A.C.; Sundaram, M.; Kumar, R.K.; Davis, D.R. Structural Effects of Hypermodified Nucleosides in the Escherichia Coli and Human tRNALys Anticodon Loop: The Effect of Nucleosides s2U, mcm5U, mcm5s2U, mnm5s2U, t6A, and ms2t6A. Biochemistry 2005, 44, 8078–8089. [Google Scholar] [CrossRef]
- Lin, H.; Miyauchi, K.; Harada, T.; Okita, R.; Takeshita, E.; Komaki, H.; Fujioka, K.; Yagasaki, H.; Goto, Y.-I.; Yanaka, K.; et al. CO2-Sensitive tRNA Modification Associated with Human Mitochondrial Disease. Nat. Commun. 2018, 9, 1875. [Google Scholar] [CrossRef]
- Khonsari, B.; Klassen, R. Impact of Pus1 Pseudouridine Synthase on Specific Decoding Events in Saccharomyces Cerevisiae. Biomolecules 2020, 10, 729. [Google Scholar] [CrossRef]
- Tomikawa, C. 7-Methylguanosine Modifications in Transfer RNA (tRNA). Int. J. Mol. Sci. 2018, 19, 4080. [Google Scholar] [CrossRef]
- Väre, V.Y.P.; Eruysal, E.R.; Narendran, A.; Sarachan, K.L.; Agris, P.F. Chemical and Conformational Diversity of Modified Nucleosides Affects tRNA Structure and Function. Biomolecules 2017, 7, 29. [Google Scholar] [CrossRef]
- Huang, Z.-X.; Li, J.; Xiong, Q.-P.; Li, H.; Wang, E.-D.; Liu, R.-J. Position 34 of tRNA Is a Discriminative Element for m5C38 Modification by Human DNMT2. Nucleic. Acids. Res. 2021, 49, 13045–13061. [Google Scholar] [CrossRef]
- Genenncher, B.; Durdevic, Z.; Hanna, K.; Zinkl, D.; Mobin, M.B.; Senturk, N.; Da Silva, B.; Legrand, C.; Carré, C.; Lyko, F.; et al. Mutations in Cytosine-5 tRNA Methyltransferases Impact Mobile Element Expression and Genome Stability at Specific DNA Repeats. Cell Rep. 2018, 22, 1861–1874. [Google Scholar] [CrossRef]
- Okamoto, M.; Fujiwara, M.; Hori, M.; Okada, K.; Yazama, F.; Konishi, H.; Xiao, Y.; Qi, G.; Shimamoto, F.; Ota, T.; et al. tRNA Modifying Enzymes, NSUN2 and METTL1, Determine Sensitivity to 5-Fluorouracil in HeLa Cells. PLoS Genet. 2014, 10, e1004639. [Google Scholar] [CrossRef]
- Yang, J.-C.; Risch, E.; Zhang, M.; Huang, C.; Huang, H.; Lu, L. Association of tRNA Methyltransferase NSUN2/IGF-II Molecular Signature with Ovarian Cancer Survival. Futur. Oncol. 2017, 13, 1981–1990. [Google Scholar] [CrossRef]
- Awah, C.U.; Winter, J.; Mazdoom, C.M.; Ogunwobi, O.O. NSUN6, an RNA Methyltransferase of 5-mC Controls Glioblastoma Response to Temozolomide (TMZ) via NELFB and RPS6KB2 Interaction. Cancer Biol. Ther. 2021, 22, 587–597. [Google Scholar] [CrossRef]
- Yang, R.; Liang, X.; Wang, H.; Guo, M.; Shen, H.; Shi, Y.; Liu, Q.; Sun, Y.; Yang, L.; Zhan, M. The RNA Methyltransferase NSUN6 Suppresses Pancreatic Cancer Development by Regulating Cell Proliferation. EBioMedicine 2021, 63, 103195. [Google Scholar] [CrossRef]
- Ma, J.; Han, H.; Huang, Y.; Yang, C.; Zheng, S.; Cai, T.; Bi, J.; Huang, X.; Liu, R.; Huang, L.; et al. METTL1/WDR4-Mediated m7G tRNA Modifications and m7G Codon Usage Promote mRNA Translation and Lung Cancer Progression. Mol. Ther. 2021, 29, 3422–3435. [Google Scholar] [CrossRef]
- Ying, X.; Liu, B.; Yuan, Z.; Huang, Y.; Chen, C.; Jiang, X.; Zhang, H.; Qi, D.; Yang, S.; Lin, S.; et al. METTL1-M7 G-EGFR/EFEMP1 Axis Promotes the Bladder Cancer Development. Clin. Transl. Med. 2021, 11, e675. [Google Scholar] [CrossRef]
- Chen, B.; Jiang, W.; Huang, Y.; Zhang, J.; Yu, P.; Wu, L.; Peng, H. N7-Methylguanosine tRNA Modification Promotes Tumorigenesis and Chemoresistance through WNT/β-Catenin Pathway in Nasopharyngeal Carcinoma. Oncogene 2022, 41, 2239–2253. [Google Scholar] [CrossRef]
- Han, H.; Yang, C.; Ma, J.; Zhang, S.; Zheng, S.; Ling, R.; Sun, K.; Guo, S.; Huang, B.; Liang, Y.; et al. N7-Methylguanosine tRNA Modification Promotes Esophageal Squamous Cell Carcinoma Tumorigenesis via the RPTOR/ULK1/Autophagy Axis. Nat. Commun. 2022, 13, 1478. [Google Scholar] [CrossRef]
- Fu, M.; Gu, J.; Wang, M.; Zhang, J.; Chen, Y.; Jiang, P.; Zhu, T.; Zhang, X. Emerging Roles of tRNA-Derived Fragments in Cancer. Mol. Cancer 2023, 22, 30. [Google Scholar] [CrossRef]
- Lee, Y.S.; Shibata, Y.; Malhotra, A.; Dutta, A. A Novel Class of Small RNAs: tRNA-Derived RNA Fragments (tRFs). Genes Dev. 2009, 23, 2639–2649. [Google Scholar] [CrossRef]
- Godoy, P.M.; Bhakta, N.R.; Barczak, A.J.; Cakmak, H.; Fisher, S.; MacKenzie, T.C.; Patel, T.; Price, R.W.; Smith, J.F.; Woodruff, P.G.; et al. Large Differences in Small RNA Composition Between Human Biofluids. Cell Rep. 2018, 25, 1346–1358. [Google Scholar] [CrossRef]
- Wang, J.; Ma, G.; Li, M.; Han, X.; Xu, J.; Liang, M.; Mao, X.; Chen, X.; Xia, T.; Liu, X.; et al. Plasma tRNA Fragments Derived from 5’ Ends as Novel Diagnostic Biomarkers for Early-Stage Breast Cancer. Mol. Ther. Nucleic. Acids. 2020, 21, 954–964. [Google Scholar] [CrossRef]
- Di Fazio, A.; Schlackow, M.; Pong, S.K.; Alagia, A.; Gullerova, M. Dicer Dependent tRNA Derived Small RNAs Promote Nascent RNA Silencing. Nucleic. Acids. Res. 2022, 50, 1734–1752. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, L.; Sun, X.; Zhang, Z.; Liu, J.; Xin, Y.; Yu, J.; Jia, Y.; Sheng, J.; Hu, G.-F.; et al. Angiogenin Mediates Paternal Inflammation-Induced Metabolic Disorders in Offspring through Sperm tsRNAs. Nat. Commun. 2021, 12, 6673. [Google Scholar] [CrossRef]
- Fagan, S.G.; Helm, M.; Prehn, J.H.M. tRNA-Derived Fragments: A New Class of Non-Coding RNA with Key Roles in Nervous System Function and Dysfunction. Prog. Neurobiol. 2021, 205, 102118. [Google Scholar] [CrossRef]
- Tao, E.-W.; Wang, H.-L.; Cheng, W.Y.; Liu, Q.-Q.; Chen, Y.-X.; Gao, Q.-Y. A Specific tRNA Half, 5’tiRNA-His-GTG, Responds to Hypoxia via the HIF1α/ANG Axis and Promotes Colorectal Cancer Progression by Regulating LATS2. J. Exp. Clin. Cancer Res. 2021, 40, 67. [Google Scholar] [CrossRef]
- Pekarsky, Y.; Balatti, V.; Palamarchuk, A.; Rizzotto, L.; Veneziano, D.; Nigita, G.; Rassenti, L.Z.; Pass, H.I.; Kipps, T.J.; Liu, C.-G.; et al. Dysregulation of a Family of Short Noncoding RNAs, tsRNAs, in Human Cancer. Proc. Natl. Acad. Sci. USA 2016, 113, 5071–5076. [Google Scholar] [CrossRef]
- Dong, X.; Fan, X.; He, X.; Chen, S.; Huang, W.; Gao, J.; Huang, Y.; Wang, H. Comprehensively Identifying the Key tRNA-Derived Fragments and Investigating Their Function in Gastric Cancer Processes. OncoTargets Ther. 2020, 13, 10931–10943. [Google Scholar] [CrossRef]
- Keam, S.P.; Sobala, A.; Ten Have, S.; Hutvagner, G. tRNA-Derived RNA Fragments Associate with Human Multisynthetase Complex (MSC) and Modulate Ribosomal Protein Translation. J. Proteome Res. 2017, 16, 413–420. [Google Scholar] [CrossRef]
- Pliatsika, V.; Loher, P.; Telonis, A.G.; Rigoutsos, I. MINTbase: A Framework for the Interactive Exploration of Mitochondrial and Nuclear tRNA Fragments. Bioinformatics 2016, 32, 2481–2489. [Google Scholar] [CrossRef]
- Schimmel, P.; Giegé, R.; Moras, D.; Yokoyama, S. An Operational RNA Code for Amino Acids and Possible Relationship to Genetic Code. Proc. Natl. Acad. Sci. USA 1993, 90, 8763–8768. [Google Scholar] [CrossRef]
- Ivanov, P.; Emara, M.M.; Villen, J.; Gygi, S.P.; Anderson, P. Angiogenin-Induced tRNA Fragments Inhibit Translation Initiation. Mol. Cell 2011, 43, 613–623. [Google Scholar] [CrossRef]
- Ivanov, P.; O’Day, E.; Emara, M.M.; Wagner, G.; Lieberman, J.; Anderson, P. G-Quadruplex Structures Contribute to the Neuroprotective Effects of Angiogenin-Induced tRNA Fragments. Proc. Natl. Acad. Sci. USA 2014, 111, 18201–18206. [Google Scholar] [CrossRef]
- Lyons, S.M.; Gudanis, D.; Coyne, S.M.; Gdaniec, Z.; Ivanov, P. Identification of Functional Tetramolecular RNA G-Quadruplexes Derived from Transfer RNAs. Nat. Commun. 2017, 8, 1127. [Google Scholar] [CrossRef]
- Lyons, S.M.; Kharel, P.; Akiyama, Y.; Ojha, S.; Dave, D.; Tsvetkov, V.; Merrick, W.; Ivanov, P.; Anderson, P. eIF4G Has Intrinsic G-Quadruplex Binding Activity That Is Required for tiRNA Function. Nucleic. Acids. Res. 2020, 48, 6223–6233. [Google Scholar] [CrossRef]
- Guzzi, N.; Cieśla, M.; Ngoc, P.C.T.; Lang, S.; Arora, S.; Dimitriou, M.; Pimková, K.; Sommarin, M.N.E.; Munita, R.; Lubas, M.; et al. Pseudouridylation of tRNA-Derived Fragments Steers Translational Control in Stem Cells. Cell 2018, 173, 1204–1216.e26. [Google Scholar] [CrossRef]
- Kim, H.K.; Xu, J.; Chu, K.; Park, H.; Jang, H.; Li, P.; Valdmanis, P.N.; Zhang, Q.C.; Kay, M.A. A tRNA-Derived Small RNA Regulates Ribosomal Protein S28 Protein Levels after Translation Initiation in Humans and Mice. Cell Rep. 2019, 29, 3816–3824.e4. [Google Scholar] [CrossRef]
- Kim, H.K.; Fuchs, G.; Wang, S.; Wei, W.; Zhang, Y.; Park, H.; Roy-Chaudhuri, B.; Li, P.; Xu, J.; Chu, K.; et al. A Transfer-RNA-Derived Small RNA Regulates Ribosome Biogenesis. Nature 2017, 552, 57–62. [Google Scholar] [CrossRef]
- Cao, J.; Cowan, D.B.; Wang, D.-Z. tRNA-Derived Small RNAs and Their Potential Roles in Cardiac Hypertrophy. Front. Pharmacol. 2020, 11, 572941. [Google Scholar] [CrossRef]
- Zong, T.; Yang, Y.; Zhao, H.; Li, L.; Liu, M.; Fu, X.; Tang, G.; Zhou, H.; Aung, L.H.H.; Li, P.; et al. tsRNAs: Novel Small Molecules from Cell Function and Regulatory Mechanism to Therapeutic Targets. Cell Prolif. 2021, 54, e12977. [Google Scholar] [CrossRef]
- Yu, X.; Xie, Y.; Zhang, S.; Song, X.; Xiao, B.; Yan, Z. tRNA-Derived Fragments: Mechanisms Underlying Their Regulation of Gene Expression and Potential Applications as Therapeutic Targets in Cancers and Virus Infections. Theranostics 2021, 11, 461–469. [Google Scholar] [CrossRef]
- Huang, B.; Yang, H.; Cheng, X.; Wang, D.; Fu, S.; Shen, W.; Zhang, Q.; Zhang, L.; Xue, Z.; Li, Y.; et al. tRF/miR-1280 Suppresses Stem Cell-like Cells and Metastasis in Colorectal Cancer. Cancer Res. 2017, 77, 3194–3206. [Google Scholar] [CrossRef]
- Zeng, W.; Long, X.; Liu, P.-S.; Xie, X. The Interplay of Oncogenic Signaling, Oxidative Stress and Ferroptosis in Cancer. Int. J. Cancer 2023, 153, 918–931. [Google Scholar] [CrossRef]
- Luo, J.; Solimini, N.L.; Elledge, S.J. Principles of Cancer Therapy: Oncogene and Non-Oncogene Addiction. Cell 2009, 136, 823–837. [Google Scholar] [CrossRef]
- Begley, U.; Dyavaiah, M.; Patil, A.; Rooney, J.P.; DiRenzo, D.; Young, C.M.; Conklin, D.S.; Zitomer, R.S.; Begley, T.J. Trm9-Catalyzed tRNA Modifications Link Translation to the DNA Damage Response. Mol. Cell 2007, 28, 860–870. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Y.; Feng, Y.; Wang, Q.; Dong, G.; Xia, W.; Jiang, F. The Role of tRNA-Centered Translational Regulatory Mechanisms in Cancer. Cancers 2024, 16, 77. https://doi.org/10.3390/cancers16010077
Shi Y, Feng Y, Wang Q, Dong G, Xia W, Jiang F. The Role of tRNA-Centered Translational Regulatory Mechanisms in Cancer. Cancers. 2024; 16(1):77. https://doi.org/10.3390/cancers16010077
Chicago/Turabian StyleShi, Yuanjian, Yipeng Feng, Qinglin Wang, Gaochao Dong, Wenjie Xia, and Feng Jiang. 2024. "The Role of tRNA-Centered Translational Regulatory Mechanisms in Cancer" Cancers 16, no. 1: 77. https://doi.org/10.3390/cancers16010077
APA StyleShi, Y., Feng, Y., Wang, Q., Dong, G., Xia, W., & Jiang, F. (2024). The Role of tRNA-Centered Translational Regulatory Mechanisms in Cancer. Cancers, 16(1), 77. https://doi.org/10.3390/cancers16010077



