miR-410 Is a Key Regulator of Epithelial-to-Mesenchymal Transition with Biphasic Role in Prostate Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Cell Culture
2.2. miRNA Transfections
2.3. Cell Viability Assays
2.4. Cell Cycle Analyses
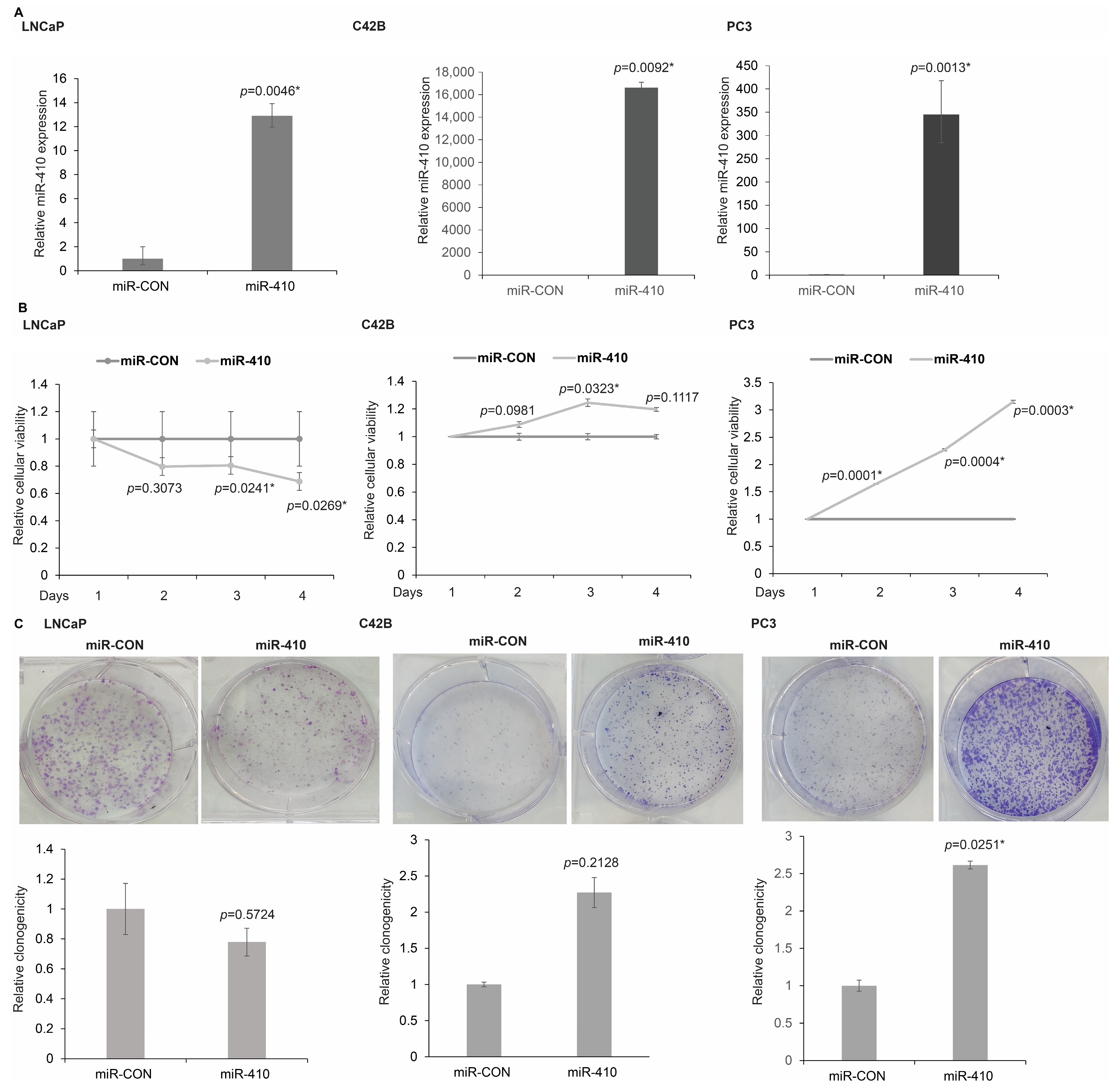
2.5. Clonogenicity Assay
2.6. Migration and Invasion Assays
2.7. Western Blotting
2.8. Xenograft Tumors
2.9. RNA and miRNA Extraction
2.10. Quantitative Real-Time PCR
2.11. Luciferase Assays
2.12. Microarray Analyses
2.13. Statistics
3. Results
3.1. miR-410 Expression Is Biphasic in Prostate Cancer
3.2. miR-410 Regulates Proliferation of PCa Cells in a Context Dependent Manner
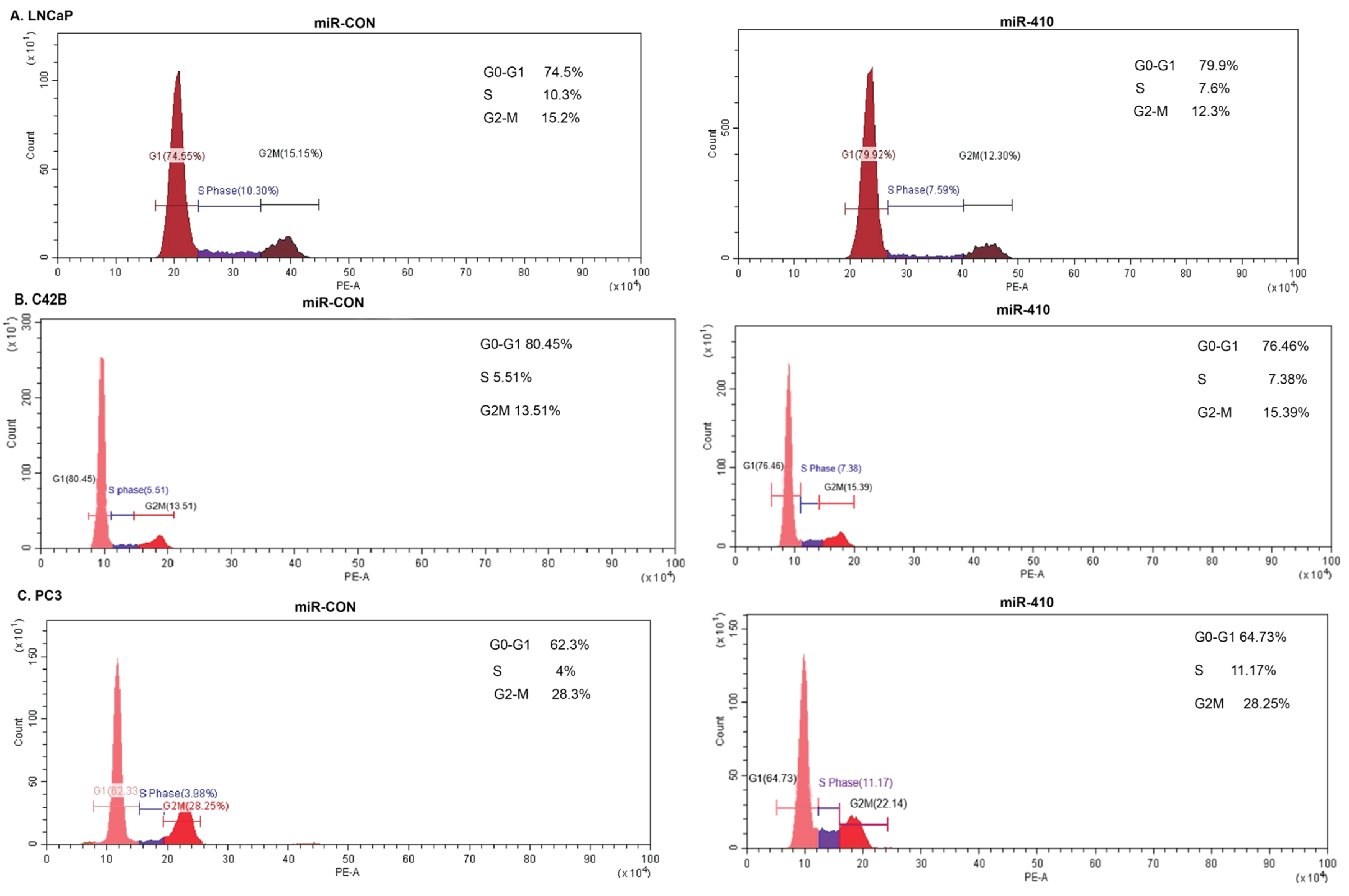
3.3. miR-410 Regulates Cell Cycle Progression of Prostate Cancer Cells in a Context-Dependent Manner
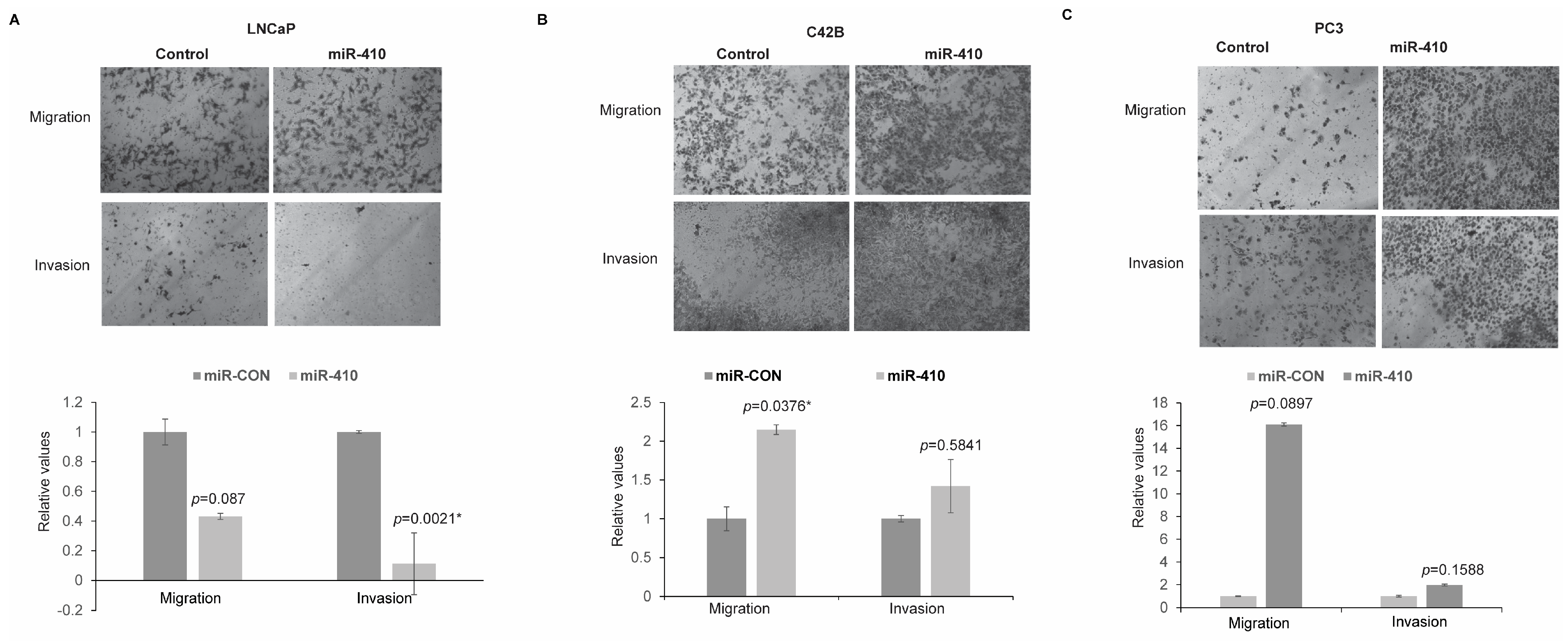
3.4. miR-410 Overexpression Influences Migratory and Invasive Properties of PCa Cell Lines
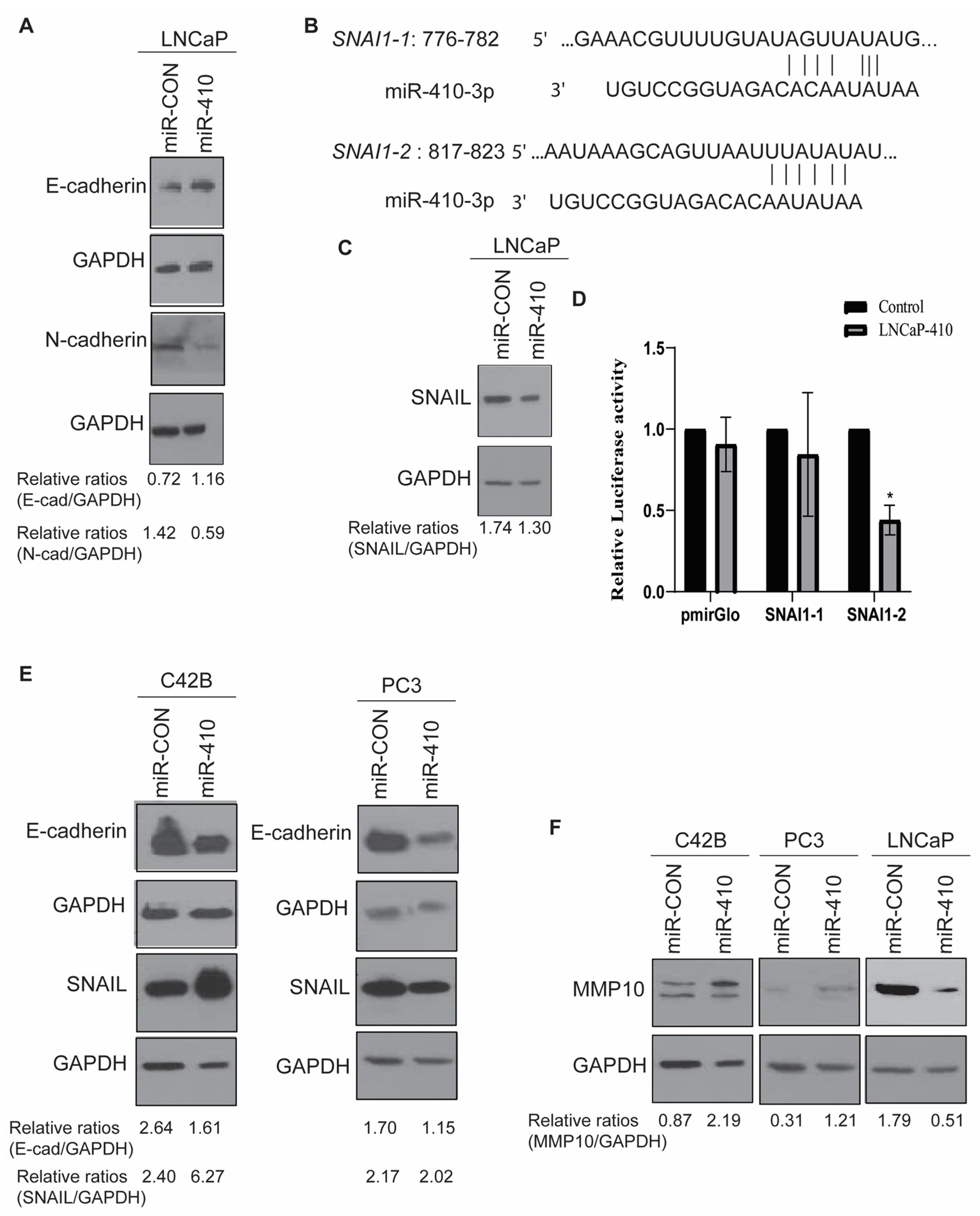
3.5. miR-410 Regulates Epithelial-to-Mesenchymal Transition in Prostate Cancer
3.6. Microarray Analyses of miR-410 Target Genes Reveals Its Important Role in Critical Cellular Processes
3.7. Protein Kinase D1 Is a Potential miR-410 Target in Prostate Cancer
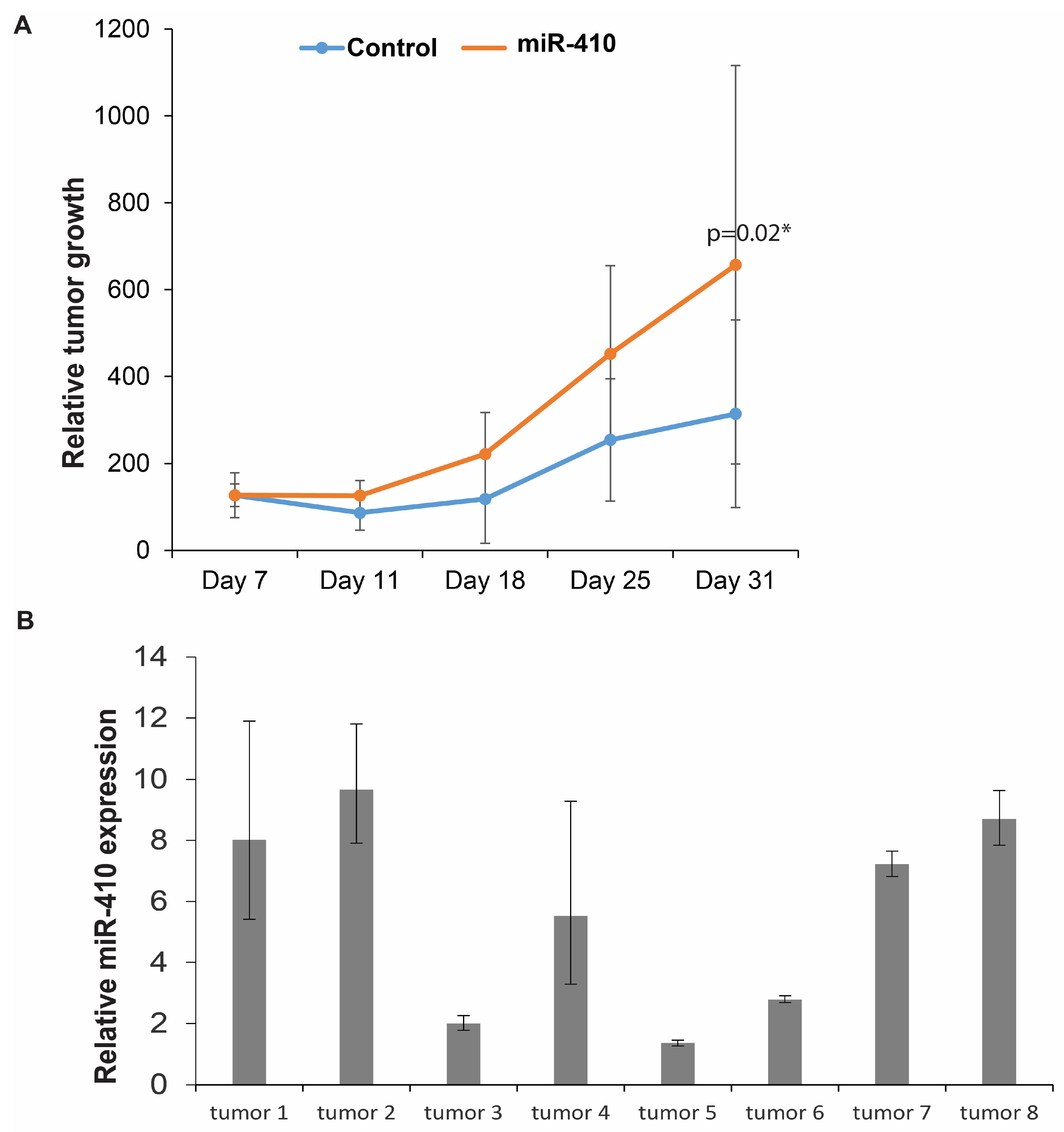
3.8. Effects of miR-410 Overexpression In Vivo
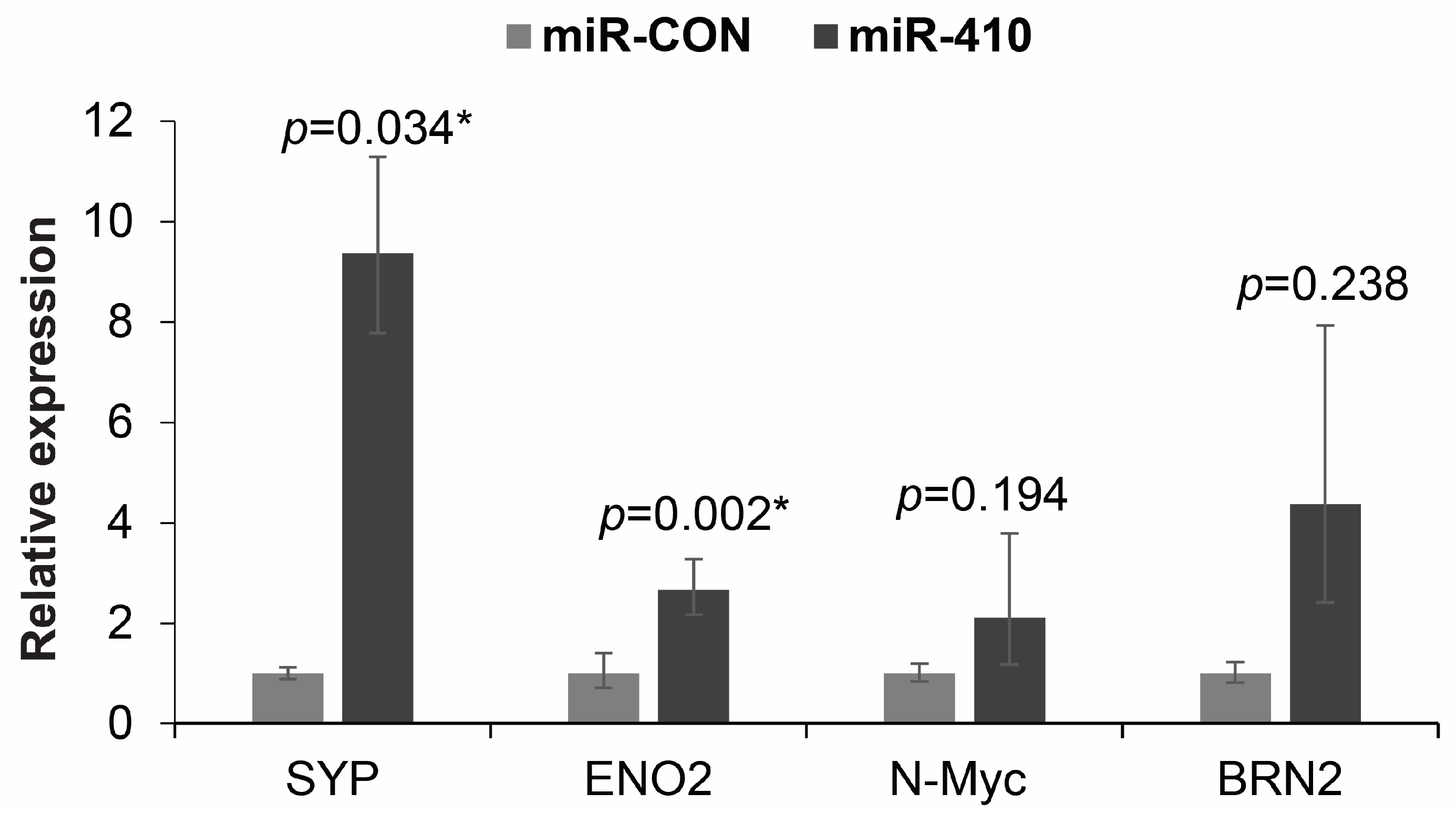
3.9. miR-410 Regulates the Expression of Neuronal Markers in Prostate Cancer
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, K.E.; Scher, H.I. Starving the addiction: New opportunities for durable suppression of AR signaling in prostate cancer. Clin. Cancer Res. 2009, 15, 4792–4798. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.M.; Abate-Shen, C. Molecular genetics of prostate cancer: New prospects for old challenges. Genes Dev. 2010, 24, 1967–2000. [Google Scholar] [CrossRef] [PubMed]
- Loberg, R.D.; Logothetis, C.J.; Keller, E.T.; Pienta, K.J. Pathogenesis and treatment of prostate cancer bone metastases: Targeting the lethal phenotype. J. Clin. Oncol. 2005, 23, 8232–8241. [Google Scholar] [CrossRef] [PubMed]
- Crona, D.J.; Whang, Y.E. Androgen Receptor-Dependent and -Independent Mechanisms Involved in Prostate Cancer Therapy Resistance. Cancers 2017, 9, 67. [Google Scholar] [CrossRef]
- Culig, Z. Molecular Mechanisms of Enzalutamide Resistance in Prostate Cancer. Curr. Mol. Biol. Rep. 2017, 3, 230–235. [Google Scholar] [CrossRef]
- Watson, P.A.; Arora, V.K.; Sawyers, C.L. Emerging mechanisms of resistance to androgen receptor inhibitors in prostate cancer. Nat. Rev. Cancer 2015, 15, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, R.; Zhang, T.; Small, E.J.; Armstrong, A.J. Neuroendocrine prostate cancer: Subtypes, biology, and clinical outcomes. J. Natl. Compr. Cancer Netw. 2014, 12, 719–726. [Google Scholar] [CrossRef]
- Aggarwal, R.R.; Small, E.J. Small-cell/neuroendocrine prostate cancer: A growing threat? Oncology 2014, 28, 838–840. [Google Scholar]
- Bhagirath, D.; Yang, T.L.; Dahiya, R.; Saini, S. MicroRNAs as Regulators of Prostate Cancer Metastasis. Adv. Exp. Med. Biol. 2018, 1095, 83–100. [Google Scholar] [CrossRef]
- Sreekumar, A.; Saini, S. Role of MicroRNAs in Neuroendocrine Prostate Cancer. Noncoding RNA 2022, 8, 25. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Target recognition and regulatory functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.W.; Mendell, J.T. MicroRNAs in cell proliferation, cell death, and tumorigenesis. Br. J. Cancer 2006, 94, 776–780. [Google Scholar] [CrossRef] [PubMed]
- Calin, G.A.; Croce, C.M. MicroRNA signatures in human cancers. Nat. Rev. Cancer 2006, 6, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Akoto, T.; Bhagirath, D.; Saini, S. MicroRNAs in treatment-induced neuroendocrine differentiation in prostate cancer. Cancer Drug. Resist. 2020, 3, 804–818. [Google Scholar] [CrossRef] [PubMed]
- Di Leva, G.; Garofalo, M.; Croce, C.M. MicroRNAs in cancer. Annu. Rev. Pathol. 2014, 9, 287–314. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; He, H.; Zhang, F.; Huang, Z.; Liu, Z.; Jiang, H.; Wu, Q. Spatiotemporal expression pattern of Mirg, an imprinted non-coding gene, during mouse embryogenesis. J. Mol. Histol. 2012, 43, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, D.; Lv, J.; Wang, S.; Zhang, Q. miR-410-3p promotes prostate cancer progression via regulating PTEN/AKT/mTOR signaling pathway. Biochem. Biophys. Res. Commun. 2018, 503, 2459–2465. [Google Scholar] [CrossRef]
- Ke, X.; Yuan, Y.; Guo, C.; Yang, Y.; Pu, Q.; Hu, X.; Tang, K.; Luo, X.; Jiang, Q.; Su, X.; et al. MiR-410 induces stemness by inhibiting Gsk3β but upregulating β-catenin in non-small cells lung cancer. Oncotarget 2017, 8, 11356–11371. [Google Scholar] [CrossRef]
- Chandrashekar, D.S.; Bashel, B.; Balasubramanya, S.A.; Creighton, C.J.; Ponce-Rodriguez, I.; Chakravarthi, B.V.; Varambally, S. UALCAN: A Portal for Facilitating Tumor Subgroup Gene Expression and Survival Analyses. Neoplasia 2017, 19, 649–658. [Google Scholar] [CrossRef]
- Chandrashekar, D.S.; Karthikeyan, S.K.; Korla, P.K.; Patel, H.; Shovon, A.R.; Athar, M.; Netto, G.J.; Qin, Z.S.; Kumar, S.; Manne, U.; et al. UALCAN: An update to the integrated cancer data analysis platform. Neoplasia 2022, 25, 18–27. [Google Scholar] [CrossRef] [PubMed]
- McGeary, S.E.; Lin, K.S.; Shi, C.Y.; Pham, T.M.; Bisaria, N.; Kelley, G.M.; Bartel, D.P. The biochemical basis of microRNA targeting efficacy. Science 2019, 366, eaav1741. [Google Scholar] [CrossRef] [PubMed]
- Grzywa, T.M.; Klicka, K.; Rak, B.; Mehlich, D.; Garbicz, F.; Zieliński, G.; Maksymowicz, M.; Sajjad, E.; Włodarski, P.K. Lineage-dependent role of miR-410-3p as oncomiR in gonadotroph and corticotroph pituitary adenomas or tumor suppressor miR in somatotroph adenomas via MAPK, PTEN/AKT, and STAT3 signaling pathways. Endocrine 2019, 65, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Thiery, J.P. Epithelial-mesenchymal transitions in tumour progression. Nat. Rev. Cancer 2002, 2, 442–454. [Google Scholar] [CrossRef] [PubMed]
- Lamouille, S.; Subramanyam, D.; Blelloch, R.; Derynck, R. Regulation of epithelial-mesenchymal and mesenchymal-epithelial transitions by microRNAs. Curr. Opin. Cell Biol. 2013, 25, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Nagabhushan, M.; Pretlow, T.P.; Amini, S.B.; Pretlow, T.G. Expression of E-cadherin in primary and metastatic prostate cancer. Am. J. Pathol. 1996, 148, 1375–1380. [Google Scholar] [PubMed]
- Nauseef, J.T.; Henry, M.D. Epithelial-to-mesenchymal transition in prostate cancer: Paradigm or puzzle? Nat. Rev. Urol. 2011, 8, 428–439. [Google Scholar] [CrossRef]
- Sethi, S.; Macoska, J.; Chen, W.; Sarkar, F.H. Molecular signature of epithelial-mesenchymal transition (EMT) in human prostate cancer bone metastasis. Am. J. Transl. Res. 2010, 3, 90–99. [Google Scholar]
- Wallerand, H.; Robert, G.; Pasticier, G.; Ravaud, A.; Ballanger, P.; Reiter, R.E.; Ferrière, J.M. The epithelial-mesenchymal transition-inducing factor TWIST is an attractive target in advanced and/or metastatic bladder and prostate cancers. Urol. Oncol. 2010, 28, 473–479. [Google Scholar] [CrossRef]
- Gravdal, K.; Halvorsen, O.J.; Haukaas, S.A.; Akslen, L.A. A switch from E-cadherin to N-cadherin expression indicates epithelial to mesenchymal transition and is of strong and independent importance for the progress of prostate cancer. Clin. Cancer Res. 2007, 13, 7003–7011. [Google Scholar] [CrossRef]
- Sekhon, K.; Bucay, N.; Majid, S.; Dahiya, R.; Saini, S. MicroRNAs and epithelial-mesenchymal transition in prostate cancer. Oncotarget 2016, 7, 67597. [Google Scholar] [CrossRef] [PubMed]
- Bracken, C.P.; Gregory, P.A.; Khew-Goodall, Y.; Goodall, G.J. The role of microRNAs in metastasis and epithelial-mesenchymal transition. Cell. Mol. Life Sci. CMLS 2009, 66, 1682–1699. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Massague, J. Epithelial-mesenchymal transitions: Twist in development and metastasis. Cell 2004, 118, 277–279. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, L. MicroRNA control of epithelial-mesenchymal transition and metastasis. Cancer Metastasis Rev. 2012, 31, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Gregory, P.A.; Bracken, C.P.; Bert, A.G.; Goodall, G.J. MicroRNAs as regulators of epithelial-mesenchymal transition. Cell Cycle 2008, 7, 3112–3118. [Google Scholar] [CrossRef] [PubMed]
- Gregory, P.A.; Bert, A.G.; Paterson, E.L.; Barry, S.C.; Tsykin, A.; Farshid, G.; Vadas, M.A.; Khew-Goodall, Y.; Goodall, G.J. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat. Cell Biol. 2008, 10, 593–601. [Google Scholar] [CrossRef]
- Burk, U.; Schubert, J.; Wellner, U.; Schmalhofer, O.; Vincan, E.; Spaderna, S.; Brabletz, T. A reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cells. EMBO Rep. 2008, 9, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Gandellini, P.; Folini, M.; Longoni, N.; Pennati, M.; Binda, M.; Colecchia, M.; Salvioni, R.; Supino, R.; Moretti, R.; Limonta, P.; et al. miR-205 Exerts tumor-suppressive functions in human prostate through down-regulation of protein kinase Cepsilon. Cancer Res. 2009, 69, 2287–2295. [Google Scholar] [CrossRef]
- Korpal, M.; Kang, Y. The emerging role of miR-200 family of microRNAs in epithelial-mesenchymal transition and cancer metastasis. RNA Biol. 2008, 5, 115–119. [Google Scholar] [CrossRef]
- Korpal, M.; Lee, E.S.; Hu, G.; Kang, Y. The miR-200 family inhibits epithelial-mesenchymal transition and cancer cell migration by direct targeting of E-cadherin transcriptional repressors ZEB1 and ZEB2. J. Biol. Chem. 2008, 283, 14910–14914. [Google Scholar] [CrossRef]
- Park, S.M.; Gaur, A.B.; Lengyel, E.; Peter, M.E. The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev. 2008, 22, 894–907. [Google Scholar] [CrossRef]
- Saini, S.; Majid, S.; Yamamura, S.; Tabatabai, L.; Suh, S.O.; Shahryari, V.; Chen, Y.; Deng, G.; Tanaka, Y.; Dahiya, R. Regulatory Role of mir-203 in Prostate Cancer Progression and Metastasis. Clin. Cancer Res. 2011, 17, 5287–5298. [Google Scholar] [CrossRef] [PubMed]
- Wen, R.U.; Umeano, A.C.; Essegian, D.J.; Sabitaliyevich, U.Y.; Wang, K.; Farooqi, A.A. Role of microRNA-410 in molecular oncology: A double edged sword. J. Cell. Biochem. 2018, 119, 8737–8742. [Google Scholar] [CrossRef] [PubMed]
- Jaggi, M.; Du, C.; Zhang, W.; Balaji, K.C. Protein kinase D1: A protein of emerging translational interest. Front. Biosci. 2007, 12, 3757–3767. [Google Scholar] [CrossRef] [PubMed]
- Biswas, M.H.; Du, C.; Zhang, C.; Straubhaar, J.; Languino, L.R.; Balaji, K.C. Protein kinase D1 inhibits cell proliferation through matrix metalloproteinase-2 and matrix metalloproteinase-9 secretion in prostate cancer. Cancer Res. 2010, 70, 2095–2104. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Du, C.; Zhang, C.; Hassan, S.; Biswas, M.H.; Balaji, K.C. Protein kinase D1 suppresses epithelial-to-mesenchymal transition through phosphorylation of snail. Cancer Res. 2010, 70, 7810–7819. [Google Scholar] [CrossRef] [PubMed]
- Eiseler, T.; Döppler, H.; Yan, I.K.; Goodison, S.; Storz, P. Protein kinase D1 regulates matrix metalloproteinase expression and inhibits breast cancer cell invasion. Breast Cancer Res. BCR 2009, 11, R13. [Google Scholar] [CrossRef]
- Mak, P.; Jaggi, M.; Syed, V.; Chauhan, S.C.; Hassan, S.; Biswas, H.; Balaji, K.C. Protein kinase D1 (PKD1) influences androgen receptor (AR) function in prostate cancer cells. Biochem. Biophys. Res. Commun. 2008, 373, 618–623. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Du, C.; Jaggi, M.; Zhang, C.; Balaji, K.C. Protein kinase D1-mediated phosphorylation and subcellular localization of beta-catenin. Cancer Res. 2009, 69, 1117–1124. [Google Scholar] [CrossRef]
- Yang, N.; Chen, J.; Zhang, H.; Wang, X.; Yao, H.; Peng, Y.; Zhang, W. LncRNA OIP5-AS1 loss-induced microRNA-410 accumulation regulates cell proliferation and apoptosis by targeting KLF10 via activating PTEN/PI3K/AKT pathway in multiple myeloma. Cell. Death Dis. 2017, 8, e2975. [Google Scholar] [CrossRef]
- Gururajan, M.; Josson, S.; Chu, G.C.; Lu, C.L.; Lu, Y.T.; Haga, C.L.; Zhau, H.E.; Liu, C.; Lichterman, J.; Duan, P.; et al. miR-154* and miR-379 in the DLK1-DIO3 microRNA mega-cluster regulate epithelial to mesenchymal transition and bone metastasis of prostate cancer. Clin. Cancer Res. 2014, 20, 6559–6569. [Google Scholar] [CrossRef]
- Beltran, H.; Prandi, D.; Mosquera, J.M.; Benelli, M.; Puca, L.; Cyrta, J.; Marotz, C.; Giannopoulou, E.; Chakravarthi, B.V.; Varambally, S.; et al. Divergent clonal evolution of castration-resistant neuroendocrine prostate cancer. Nat. Med. 2016, 22, 298–305. [Google Scholar] [CrossRef]

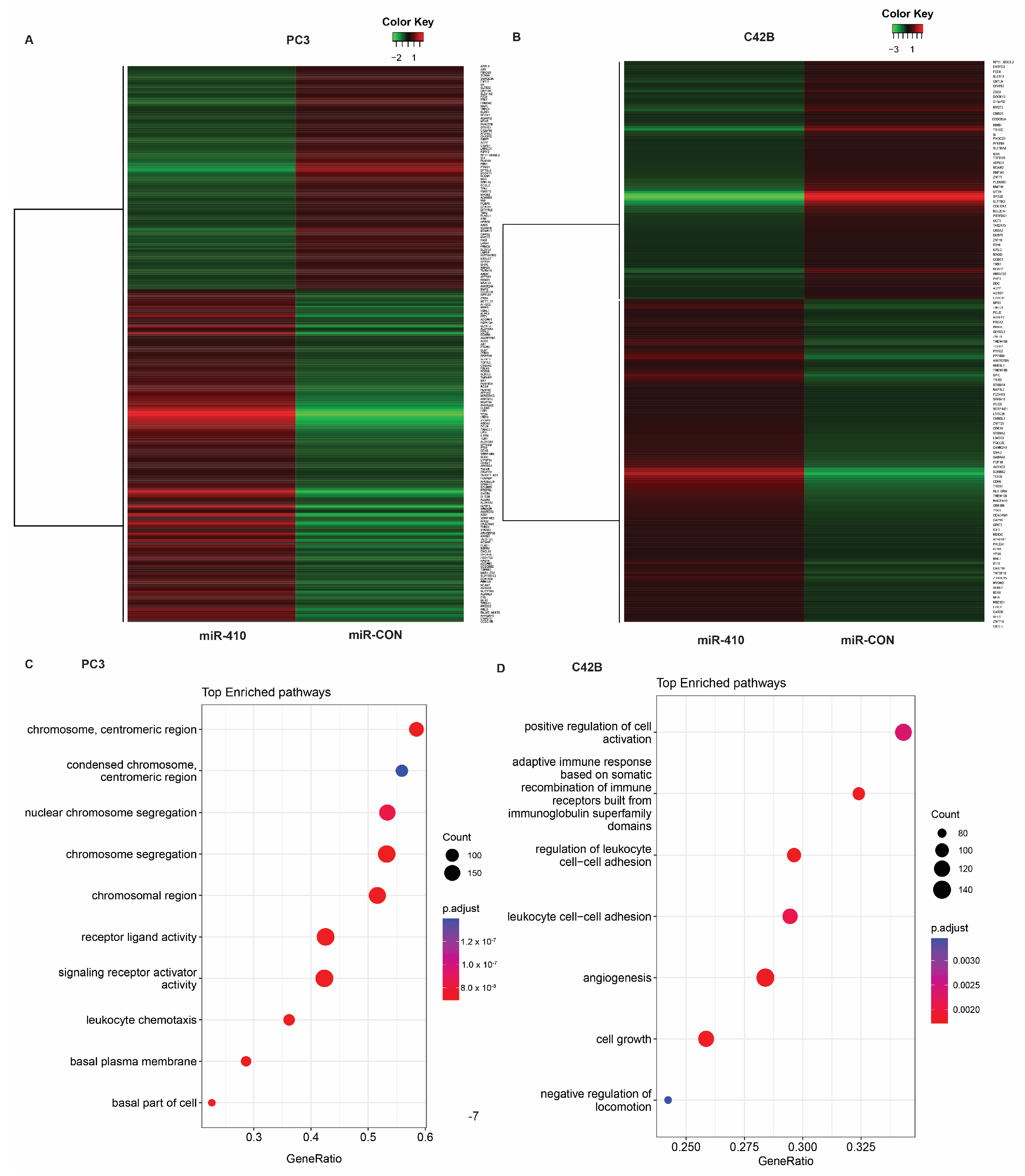

| Cell Line | Gene | Fold Change |
|---|---|---|
| PC3 | PRKD1 |  −7.69474 −7.69474 |
| DPYSL3 |  −7.63739 −7.63739 | |
| C10orf107 |  −6.0686 −6.0686 | |
| FAM20C |  −6.02488 −6.02488 | |
| ZNF385B |  −5.69932 −5.69932 | |
| PDPN |  35.5498 35.5498 | |
| TC2N |  30.7099 30.7099 | |
| SLC6A14 |  23.1731 23.1731 | |
| ESRP1 |  22.6367 22.6367 | |
| CEACAM5 |  20.9757 20.9757 | |
| C42B | SLITRK3 |  −88.5435 −88.5435 |
| CBLN2 |  −51.0595 −51.0595 | |
| BCHE |  −34.1878 −34.1878 | |
| SPG20 |  −16.0452 −16.0452 | |
| COL12A1 |  −11.7891 −11.7891 | |
| IGFBP3 |  24.7997 24.7997 | |
| TEX15 |  12.3031 12.3031 | |
| SYT4 |  11.8595 11.8595 | |
| CDH6 |  11.082 11.082 | |
| WLS |  7.63086 7.63086 |
 Up-regulation;
Up-regulation;  Down-regulation.
Down-regulation.Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asante, D.M.; Sreekumar, A.; Nathani, S.; Lee, T.J.; Sharma, A.; Patel, N.; Simmons, M.N.; Saini, S. miR-410 Is a Key Regulator of Epithelial-to-Mesenchymal Transition with Biphasic Role in Prostate Cancer. Cancers 2024, 16, 48. https://doi.org/10.3390/cancers16010048
Asante DM, Sreekumar A, Nathani S, Lee TJ, Sharma A, Patel N, Simmons MN, Saini S. miR-410 Is a Key Regulator of Epithelial-to-Mesenchymal Transition with Biphasic Role in Prostate Cancer. Cancers. 2024; 16(1):48. https://doi.org/10.3390/cancers16010048
Chicago/Turabian StyleAsante, Diana M., Amritha Sreekumar, Sandip Nathani, Tae Jin Lee, Ashok Sharma, Nikhil Patel, Matthew N. Simmons, and Sharanjot Saini. 2024. "miR-410 Is a Key Regulator of Epithelial-to-Mesenchymal Transition with Biphasic Role in Prostate Cancer" Cancers 16, no. 1: 48. https://doi.org/10.3390/cancers16010048
APA StyleAsante, D. M., Sreekumar, A., Nathani, S., Lee, T. J., Sharma, A., Patel, N., Simmons, M. N., & Saini, S. (2024). miR-410 Is a Key Regulator of Epithelial-to-Mesenchymal Transition with Biphasic Role in Prostate Cancer. Cancers, 16(1), 48. https://doi.org/10.3390/cancers16010048







