Performance of the Fecal Immunochemical Test in Detecting Advanced Colorectal Neoplasms and Colorectal Cancers in People Aged 40–49 Years: A Systematic Review and Meta-Analysis
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Selection Criteria and Data Extraction
2.3. Outcome Assessment
2.4. Statistical Analysis
2.5. Sensitivity Analysis and Risk of Bias Assessment
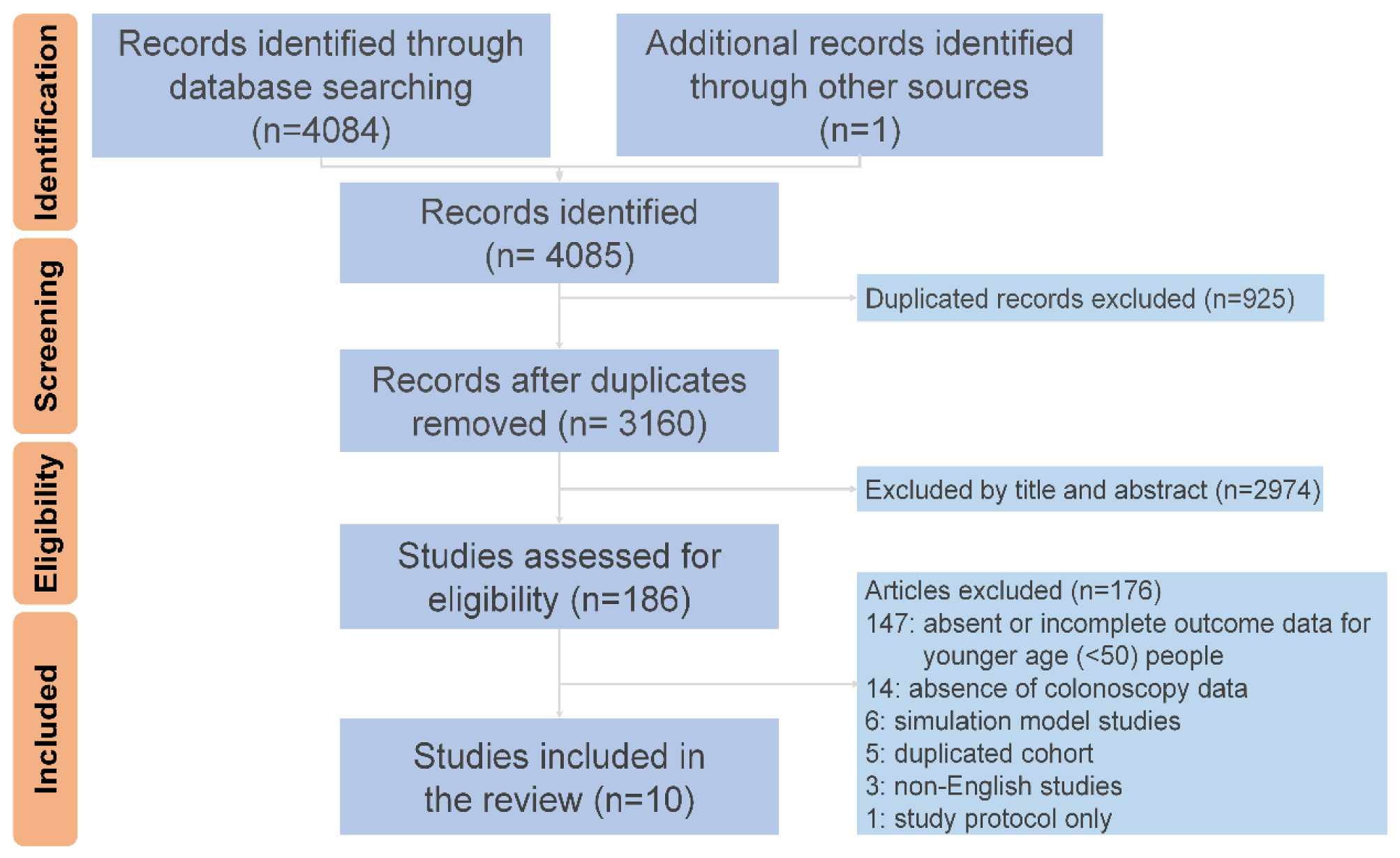
3. Results
3.1. Baseline Characteristics of the Included Studies and Patients
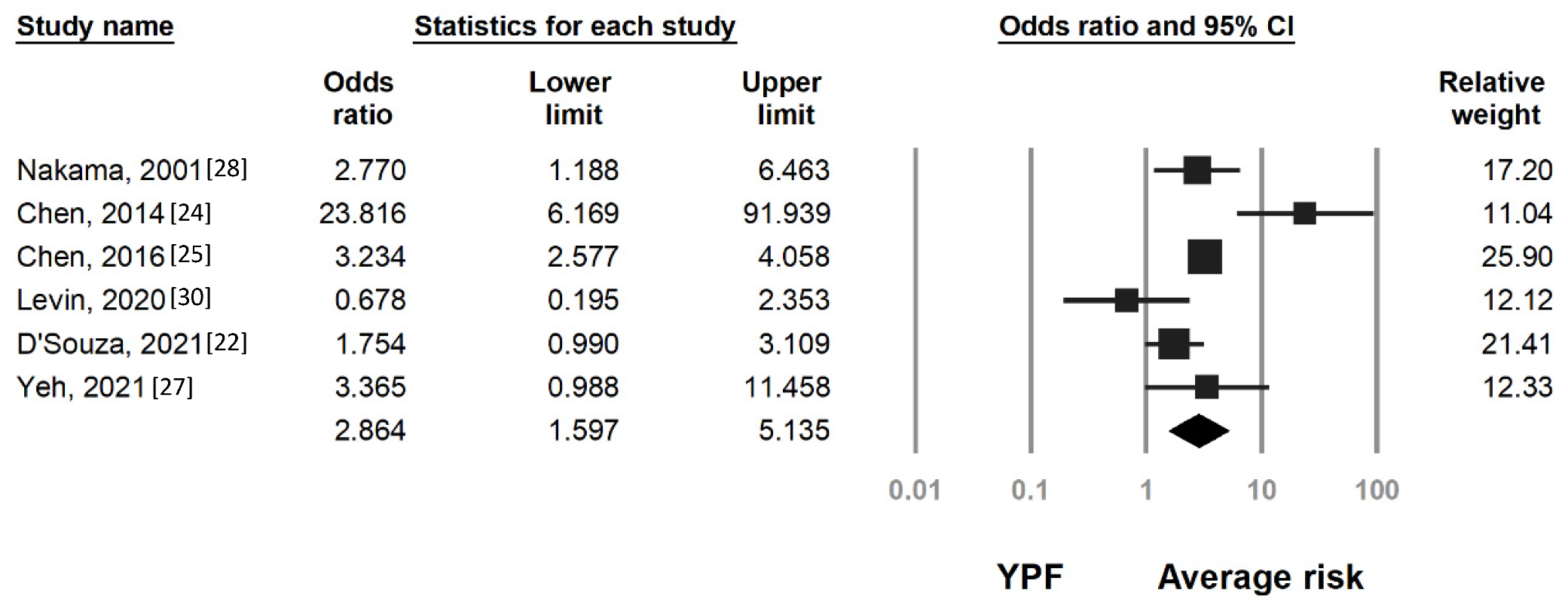
3.2. Risk of Colorectal Neoplasia by Age and FIT Results
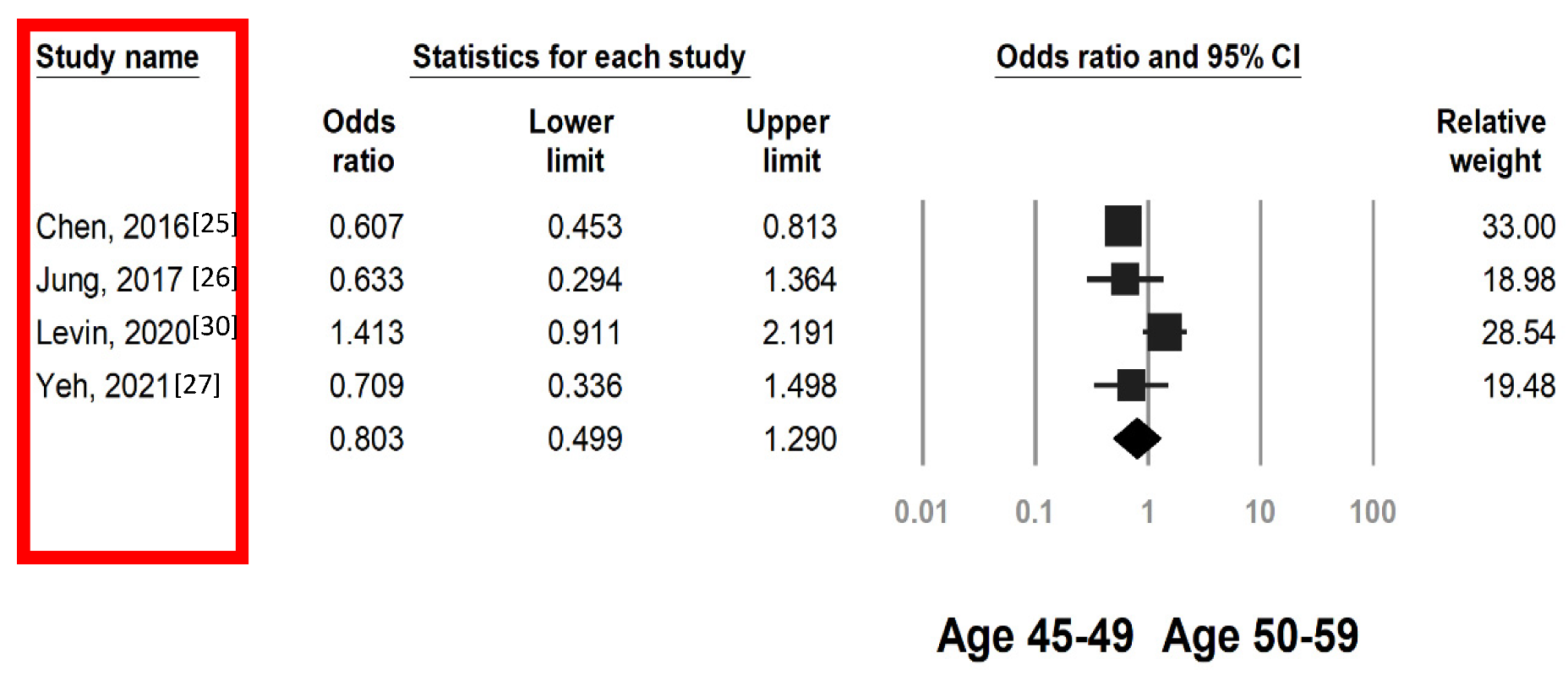
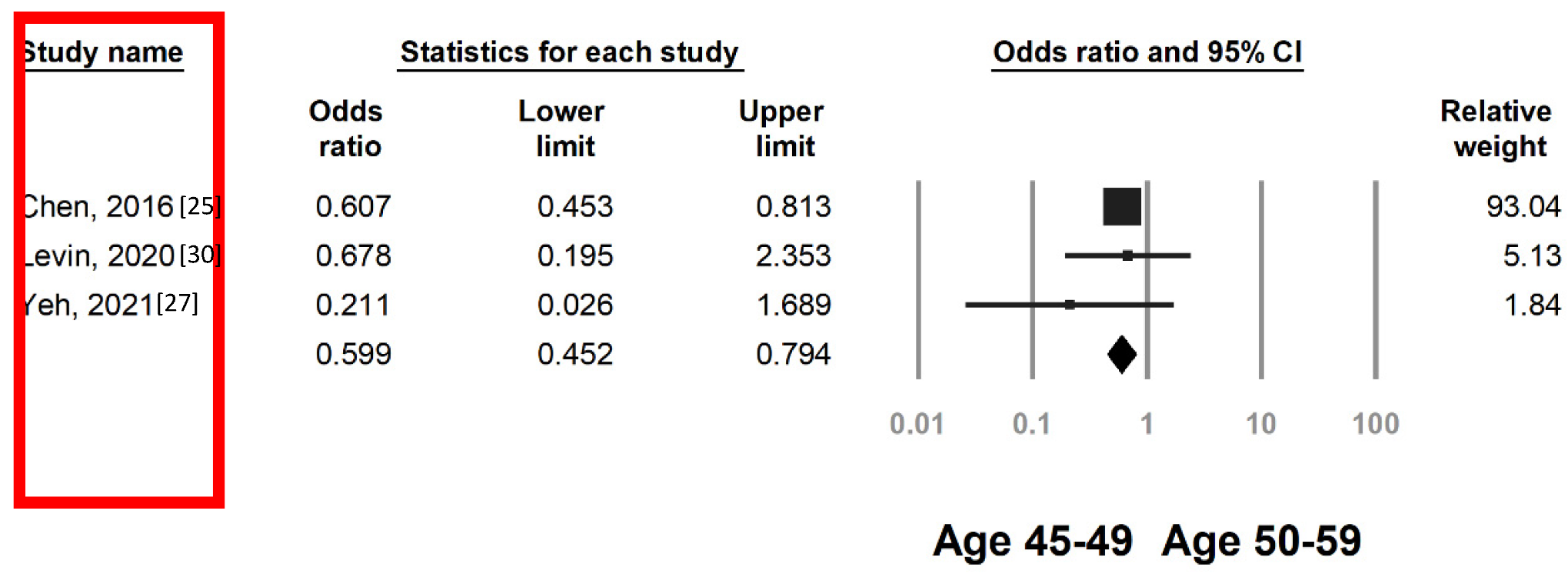
3.3. Colorectal Neoplasia Risk by FIT in Individuals Aged 45–49 and 50–59 Years
3.4. Performance of FIT by Age, Lesion Type, and Cutoff Value
4. Discussion
5. Summary and Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017, 66, 683. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, R.; Guo, F.; Heisser, T.; Hackl, M.; Ihle, P.; De Schutter, H.; Van Damme, N.; Valerianova, Z.; Atanasov, T.; Májek, O.; et al. Colorectal cancer incidence, mortality, and stage distribution in European countries in the colorectal cancer screening era: An international population-based study. Lancet Oncol. 2021, 22, 1002–1013. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.C.S.; Huang, J.; Lok, V.; Wang, J.; Fung, F.; Ding, H.; Zheng, Z.-J. Differences in Incidence and Mortality Trends of Colorectal Cancer Worldwide Based on Sex, Age, and Anatomic Location. Clin. Gastroenterol. Hepatol. 2021, 19, 955–966.e961. [Google Scholar] [CrossRef] [PubMed]
- Gini, A.; Jansen, E.E.L.; Zielonke, N.; Meester, R.G.S.; Senore, C.; Anttila, A.; Segnan, N.; Mlakar, D.N.; de Koning, H.J.; Lansdorp-Vogelaar, I.; et al. Impact of colorectal cancer screening on cancer-specific mortality in Europe: A systematic review. Eur. J. Cancer 2020, 127, 224–235. [Google Scholar] [CrossRef]
- Levin, T.R.; Corley, D.A.; Jensen, C.D.; Schottinger, J.E.; Quinn, V.P.; Zauber, A.G.; Lee, J.K.; Zhao, W.K.; Udaltsova, N.; Ghai, N.R.; et al. Effects of Organized Colorectal Cancer Screening on Cancer Incidence and Mortality in a Large Community-Based Population. Gastroenterology 2018, 155, 1383–1391.e5. [Google Scholar] [CrossRef]
- Siegel, R.L.; Fedewa, S.A.; Anderson, W.F.; Miller, K.D.; Ma, J.; Rosenberg, P.S.; Jemal, A. Colorectal Cancer Incidence Patterns in the United States, 1974–2013. JNCI J. Natl. Cancer Inst. 2017, 109, djw322. [Google Scholar] [CrossRef]
- Bhandari, A.; Woodhouse, M.; Gupta, S. Colorectal cancer is a leading cause of cancer incidence and mortality among adults younger than 50 years in the USA: A SEER-based analysis with comparison to other young-onset cancers. J. Investig. Med. 2017, 65, 311–315. [Google Scholar] [CrossRef]
- Siegel, R.L.; Medhanie, G.A.; Fedewa, S.A.; Jemal, A. State variation in early-onset colorectal cancer in the United States, 1995–2015. JNCI J. Natl. Cancer Inst. 2019, 111, 1104–1106. [Google Scholar] [CrossRef]
- Sung, J.J.Y.; Chiu, H.-M.; Jung, K.-W.; Jun, J.K.; Sekiguchi, M.; Matsuda, T.; Kyaw, M.H. Increasing Trend in Young-Onset Colorectal Cancer in Asia: More Cancers in Men and More Rectal Cancers. Off. J. Am. Coll. Gastroenterol. 2019, 114, 322–329. [Google Scholar] [CrossRef]
- Vuik, F.E.; Nieuwenburg, S.A.; Bardou, M.; Lansdorp-Vogelaar, I.; Dinis-Ribeiro, M.; Bento, M.J.; Zadnik, V.; Pellisé, M.; Esteban, L.; Kaminski, M.F.; et al. Increasing incidence of colorectal cancer in young adults in Europe over the last 25 years. Gut 2019, 68, 1820–1826. [Google Scholar] [CrossRef]
- Archambault, A.N.; Su, Y.R.; Jeon, J.; Thomas, M.; Lin, Y.; Conti, D.V.; Win, A.K.; Sakoda, L.C.; Lansdorp-Vogelaar, I.; Peterse, E.F.P.; et al. Cumulative Burden of Colorectal Cancer-Associated Genetic Variants Is More Strongly Associated with Early-Onset vs Late-Onset Cancer. Gastroenterology 2020, 158, 1274–1286.e1212. [Google Scholar] [CrossRef]
- Pearlman, R.; Frankel, W.L.; Swanson, B.; Zhao, W.; Yilmaz, A.; Miller, K.; Bacher, J.; Bigley, C.; Nelsen, L.; Goodfellow, P.J.; et al. Prevalence and Spectrum of Germline Cancer Susceptibility Gene Mutations among Patients with Early-Onset Colorectal Cancer. JAMA Oncol. 2017, 3, 464–471. [Google Scholar] [CrossRef]
- Stoffel, E.M.; Koeppe, E.; Everett, J.; Ulintz, P.; Kiel, M.; Osborne, J.; Williams, L.; Hanson, K.; Gruber, S.B.; Rozek, L.S. Germline Genetic Features of Young Individuals with Colorectal Cancer. Gastroenterology 2018, 154, 897–905.e1. [Google Scholar] [CrossRef]
- Connell, L.C.; Mota, J.M.; Braghiroli, M.I.; Hoff, P.M. The Rising Incidence of Younger Patients with Colorectal Cancer: Questions About Screening, Biology, and Treatment. Curr. Treat. Options Oncol. 2017, 18, 23. [Google Scholar] [CrossRef]
- US Preventive Services Task Force. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2021, 325, 1965–1977. [Google Scholar] [CrossRef]
- Patel, S.G.; May, F.P.; Anderson, J.C.; Burke, C.A.; Dominitz, J.A.; Gross, S.A.; Jacobson, B.C.; Shaukat, A.; Robertson, D.J. Updates on Age to Start and Stop Colorectal Cancer Screening: Recommendations from the U.S. Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2022, 162, 285–299. [Google Scholar] [CrossRef]
- Shaukat, A.; Kahi, C.J.; Burke, C.A.; Rabeneck, L.; Sauer, B.G.; Rex, D.K. ACG Clinical Guidelines: Colorectal Cancer Screening 2021. Off. J. Am. Coll. Gastroenterol. 2021, 116, 458–479. [Google Scholar] [CrossRef]
- Wolf, A.M.D.; Fontham, E.T.H.; Church, T.R.; Flowers, C.R.; Guerra, C.E.; LaMonte, S.J.; Etzioni, R.; McKenna, M.T.; Oeffinger, K.C.; Shih, Y.-C.T.; et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J. Clin. 2018, 68, 250–281. [Google Scholar] [CrossRef]
- Chen, H.; Shi, J.; Lu, M.; Li, Y.; Du, L.; Liao, X.; Wei, D.; Dong, D.; Gao, Y.; Zhu, C.; et al. Comparison of Colonoscopy, Fecal Immunochemical Test, and Risk-Adapted Approach in a Colorectal Cancer Screening Trial (TARGET-C). Clin. Gastroenterol. Hepatol. 2023, 21, 808–818. [Google Scholar] [CrossRef]
- Zhong, G.-C.; Sun, W.-P.; Wan, L.; Hu, J.-J.; Hao, F.-B. Efficacy and cost-effectiveness of fecal immunochemical test versus colonoscopy in colorectal cancer screening: A systematic review and meta-analysis. Gastrointest. Endosc. 2020, 91, 684–697.e615. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ (Clin. Res. Ed.) 2021, 372, n71. [Google Scholar] [CrossRef]
- D’Souza, N.; Monahan, K.; Benton, S.C.; Wilde, L.; Abulafi, M. Finding the needle in the haystack: The diagnostic accuracy of the faecal immunochemical test for colorectal cancer in younger symptomatic patients. Color. Dis. Off. J. Assoc. Coloproctology Great Br. Irel. 2021, 23, 2539–2549. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, T.; Kato, J.; Yamaji, Y.; Wada, R.; Mitsushima, T.; Sakaguchi, K.; Shiratori, Y. Sensitivity of immunochemical fecal occult blood test to small colorectal adenomas. Am. J. Gastroenterol. 2007, 102, 2259–2264. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Chen, T.H.; Su, M.Y.; Ning, H.C.; Kuo, C.J.; Lin, W.P.; Ho, Y.P.; Lin, C.J.; Hsu, C.M.; Chiu, C.T.; et al. Accuracy of immunochemical fecal occult blood test for detecting colorectal neoplasms in individuals undergoing health check-ups. Adv. Dig. Med. 2014, 1, 74–79. [Google Scholar] [CrossRef]
- Chen, C.H.; Tsai, M.K.; Wen, C.P. Extending Colorectal Cancer Screening to Persons Aged 40 to 49 Years with Immunochemical Fecal Occult Blood Test: A Prospective Cohort Study of 513,283 Individuals. J. Clin. Gastroenterol. 2016, 50, 761–768. [Google Scholar] [CrossRef]
- Jung, Y.S.; Park, C.H.; Kim, N.H.; Park, J.H.; Park, D.I.; Sohn, C.I. Colorectal cancer screening with the fecal immunochemical test in persons aged 30 to 49 years: Focusing on the age for commencing screening. Gastrointest. Endosc. 2017, 86, 892–899. [Google Scholar] [CrossRef]
- Yeh, J.H.; Lin, C.W.; Wang, W.L.; Lee, C.T.; Chen, J.C.; Hsu, C.C.; Wang, J.Y. Positive Fecal Immunochemical Test Strongly Predicts Adenomas in Younger Adults with Fatty Liver and Metabolic Syndrome. Clin. Transl. Gastroenterol. 2021, 12, e00305. [Google Scholar] [CrossRef]
- Nakama, H.; Zhang, B.; Zhang, X.; Fukazawa, K. Age-related cancer detection rate and costs for one cancer detected in one screening by immunochemical fecal occult blood test. Dis. Colon Rectum 2001, 44, 1696–1699. [Google Scholar] [CrossRef]
- Symonds, E.L.; Osborne, J.M.; Cole, S.R.; Bampton, P.A.; Fraser, R.J.; Young, G.P. Factors affecting faecal immunochemical test positive rates: Demographic, pathological, behavioural and environmental variables. J. Med. Screen. 2015, 22, 187–193. [Google Scholar] [CrossRef]
- Levin, T.R.; Jensen, C.D.; Chawla, N.M.; Sakoda, L.C.; Lee, J.K.; Zhao, W.K.; Landau, M.A.; Herm, A.; Eby, E.; Quesenberry, C.P.; et al. Early Screening of African Americans (45–50 Years Old) in a Fecal Immunochemical Test–Based Colorectal Cancer Screening Program. Gastroenterology 2020, 159, 1695–1704.e1691. [Google Scholar] [CrossRef]
- Pin-Vieito, N.; García Nimo, L.; Bujanda, L.; Román Alonso, B.; Gutierrez-Stampa, M.; Aguilar-Gama, V.; Portillo, I.; Cubiella, J. Optimal diagnostic accuracy of quantitative faecal immunochemical test positivity thresholds for colorectal cancer detection in primary health care: A community-based cohort study. United Eur. Gastroenterol. J. 2021, 9, 256–267. [Google Scholar] [CrossRef]
- Butterly, L.F.; Siegel, R.L.; Fedewa, S.; Robinson, C.M.; Jemal, A.; Anderson, J.C. Colonoscopy outcomes in average-risk screening equivalent young adults: Data from the New Hampshire Colonoscopy Registry. Off. J. Am. Coll. Gastroenterol. 2021, 116, 171–179. [Google Scholar] [CrossRef]
- Knudsen, A.; Rutter, C.; Peterse, E. Colorectal Cancer Screening: A Decision Analysis for the US Preventive Services Task Force; Technical Report, No. 202s; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2021. [Google Scholar]
- Doubeni, C.A.; Corley, D.A.; Quinn, V.P.; Jensen, C.D.; Zauber, A.G.; Goodman, M.; Johnson, J.R.; Mehta, S.J.; Becerra, T.A.; Zhao, W.K. Effectiveness of screening colonoscopy in reducing the risk of death from right and left colon cancer: A large community-based study. Gut 2018, 67, 291–298. [Google Scholar] [CrossRef]
- Guo, F.; Chen, C.; Holleczek, B.; Schöttker, B.; Hoffmeister, M.; Brenner, H. Strong reduction of colorectal cancer incidence and mortality after screening colonoscopy: Prospective cohort study from Germany. Off. J. Am. Coll. Gastroenterol. 2021, 116, 967–975. [Google Scholar] [CrossRef]
- Grobbee, E.J.; Wisse, P.H.A.; Schreuders, E.H.; van Roon, A.; van Dam, L.; Zauber, A.G.; Lansdorp-Vogelaar, I.; Bramer, W.; Berhane, S.; Deeks, J.J.; et al. Guaiac-based faecal occult blood tests versus faecal immunochemical tests for colorectal cancer screening in average-risk individuals. Cochrane Database Syst. Rev. 2022. [Google Scholar] [CrossRef]
- Meklin, J.; Syrjänen, K.; Eskelinen, M. Fecal Occult Blood Tests in Colorectal Cancer Screening: Systematic Review and Meta-analysis of Traditional and New-generation Fecal Immunochemical Tests. Anticancer Res. 2020, 40, 3591–3604. [Google Scholar] [CrossRef]
- Murphy, J.; Halloran, S.; Gray, A. Cost-effectiveness of the faecal immunochemical test at a range of positivity thresholds compared with the guaiac faecal occult blood test in the NHS Bowel Cancer Screening Programme in England. BMJ Open 2017, 7, e017186. [Google Scholar] [CrossRef]
- Lansdorp-Vogelaar, I.; Kuntz, K.M.; Knudsen, A.B.; van Ballegooijen, M.; Zauber, A.G.; Jemal, A. Contribution of screening and survival differences to racial disparities in colorectal cancer rates. Cancer Epidemiol. Biomark. Prev. 2012, 21, 728–736. [Google Scholar] [CrossRef]
- Brawley, O.W. Colorectal cancer control: Providing adequate care to those who need it. J. Natl. Cancer Inst. 2014, 106, dju075. [Google Scholar] [CrossRef]
- Hofseth, L.J.; Hebert, J.R.; Chanda, A.; Chen, H.; Love, B.L.; Pena, M.M.; Murphy, E.A.; Sajish, M.; Sheth, A.; Buckhaults, P.J.; et al. Early-onset colorectal cancer: Initial clues and current views. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 352–364. [Google Scholar] [CrossRef]
- Haug, U.; Kuntz, K.M.; Knudsen, A.B.; Hundt, S.; Brenner, H. Sensitivity of immunochemical faecal occult blood testing for detecting left- vs right-sided colorectal neoplasia. Br. J. Cancer 2011, 104, 1779–1785. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.N.; Peeters, M.; Hoeck, S.; Van Hal, G.; Janssens, S.; De Schutter, H. Optimizing the colorectal cancer screening programme using faecal immunochemical test (FIT) in Flanders, Belgium from the “interval cancer” perspective. Br. J. Cancer 2022, 126, 1091–1099. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.-C.; Shun, C.-T.; Hsu, W.-F.; Tu, C.-H.; Tsai, P.-Y.; Lin, B.-R.; Liang, J.-T.; Wu, M.-S.; Chiu, H.-M. Fecal immunochemical test detects sessile serrated adenomas and polyps with a low level of sensitivity. Clin. Gastroenterol. Hepatol. 2017, 15, 872–879.e871. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, Y.; Diaz-Meco, M.T.; Moscat, J. Serrated colorectal cancer: The road less travelled? Trends Cancer 2019, 5, 742–754. [Google Scholar] [CrossRef]

| Study | Region | Patients by Age, N | Age Started to Screen | Gender (Male, %) | Product Name of FIT | Cutoff Value of FIT (µg Hb/g Feces) | |
|---|---|---|---|---|---|---|---|
| 40–49 | ≥50 | ||||||
| Nakama, 2001 [28] | Japan | 2382 | 5018 | 40 | NA | OC-Hemodia | NA |
| Morikawa, 2007 [23] | Japan | 12,696 | 9109 | 40 | 71.9 | Magstream 1000 Hem SP | NA |
| Chen YY, 2014 [24] | Taiwan | 2214 | 3882 | 40 | 56.0 | OC-Light | 10 |
| Symonds, 2015 [29] | Australia | 1849 † | 19,839 | 19 | 45.2 | OC-Sensor | 20 |
| Chen CH, 2016 [25] | Taiwan | 92,062 | 141,982 | 20 | 47.6 | OC-Sensor | 20 |
| Jung, 2017 [26] | Australia | 8819 | 2233 | 30 | 74.7 | OC-Sensor Diana | 20 |
| Levin, 2020 [30] | US | 3390 | 13,442 | 45 | 52.2 | OC-Sensor Diana | 20 |
| D’Souza, 2021 [22] | England | 1103 † | 8719 | 17 | 45.0 | HM-Jack analyser | 10 |
| Pin-Vieito, 2021 [31] | Spanish | 8866 † | 29,809 | 18 | 46.0 | OC-SensorTM | 20 |
| Yeh, 2021 [27] | Taiwan | 1857 | 3150 | 20 | 57.6 | OC-Sensor | 20 |
| Study § | FIT Positive Patients by Age, N | Total Colono- scopy Exams by Age, N | Total CRN by Age, N | CRN of FIT Positive Patients by Age, N (%) | ACRN of FIT Positive Patients, by Age, N (%) | CRC of FIT Positive Patients, by Age, N (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 40–49 | ≥50 | 40–49 | ≥50 | 40–49 | ≥50 | 40–49 | ≥50 | 40–49 | ≥50 | 40–49 | ≥50 | |
| Nakama, 2001 [28] | 138 | 315 | 2382 | 5018 | 8 | 81 | 6 (4.3) | 56 (17.8) | 6 (4.3) | 56 (17.8) | 6 (4.3) | 56 (17.8) |
| Morikawa, 2007 [23] | 1231 (total cases) | 12,696 | 9109 | See footnotes below a | ||||||||
| Chen YY, 2014 [24] | 64 | 165 | 2214 | 3882 | 380 | 1096 | 25 (39.1) | 78 (47.3) | 18 (28.1) | 38 (23.0) | 3 (4.7) | 6 (3.6) |
| Symonds, 2015 [29] | 73 † | 1104 | 67 † | 1090 | NA | NA | 15 † (20.5) | 512 (46.4) | NA | NA | NA | NA |
| Chen CH, ‡§ 2016 [25] | 3728 | 3835 | NA | NA | 272 | 554 | 89 ‡ (2.3) | 213 ‡ (5.5) | 89 ‡ (2.3) | 213 ‡ (5.5) | 89 ‡ (2.3) | 213 ‡ (5.5) |
| Jung, 2017 [26] | 258 | 94 | 8819 | 2233 | 1634 | 643 | NA | NA | 26 (10.0) | 20 (21.2) | NA | NA |
| Levin, 2020 [30] | 136 | 575 | 116 | 451 | NA | NA | 67 (49.3) | 298 (51.8) | 39 (28.7) | 119 (20.7) | 3 (2.2) | 17 (3.0) |
| D’Souza, 2021 [22] | 212 † | 1674 | 1103 | 8719 | 189 † | 2882 | NA | NA | 29 † (13.7) | 461 (27.5) | 13 † (6.1) | 286 (17.1) |
| Pin-Vieito, § 2021 [31] | 754 † | 5900 | NA | NA | See footnotes below b | |||||||
| Yeh, 2021 [27] | 109 | 207 | 1857 | 3150 | 389 | 897 | 37 (33.9) | 101 (48.7) | 18 (16.5) | 64 (30.9) | 3 (2.7) | 17 (8.2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeh, J.-H.; Tseng, C.-H.; Wang, W.-L.; Chen, C.-I.; Liu, Y.-P.; Lee, Y.-C.; Wang, J.-Y.; Lin, Y.-C. Performance of the Fecal Immunochemical Test in Detecting Advanced Colorectal Neoplasms and Colorectal Cancers in People Aged 40–49 Years: A Systematic Review and Meta-Analysis. Cancers 2023, 15, 3006. https://doi.org/10.3390/cancers15113006
Yeh J-H, Tseng C-H, Wang W-L, Chen C-I, Liu Y-P, Lee Y-C, Wang J-Y, Lin Y-C. Performance of the Fecal Immunochemical Test in Detecting Advanced Colorectal Neoplasms and Colorectal Cancers in People Aged 40–49 Years: A Systematic Review and Meta-Analysis. Cancers. 2023; 15(11):3006. https://doi.org/10.3390/cancers15113006
Chicago/Turabian StyleYeh, Jen-Hao, Cheng-Hao Tseng, Wen-Lun Wang, Chih-I Chen, Yu-Peng Liu, Yi-Chia Lee, Jaw-Yuan Wang, and Yu-Ching Lin. 2023. "Performance of the Fecal Immunochemical Test in Detecting Advanced Colorectal Neoplasms and Colorectal Cancers in People Aged 40–49 Years: A Systematic Review and Meta-Analysis" Cancers 15, no. 11: 3006. https://doi.org/10.3390/cancers15113006
APA StyleYeh, J.-H., Tseng, C.-H., Wang, W.-L., Chen, C.-I., Liu, Y.-P., Lee, Y.-C., Wang, J.-Y., & Lin, Y.-C. (2023). Performance of the Fecal Immunochemical Test in Detecting Advanced Colorectal Neoplasms and Colorectal Cancers in People Aged 40–49 Years: A Systematic Review and Meta-Analysis. Cancers, 15(11), 3006. https://doi.org/10.3390/cancers15113006









