Preoperative 18F-FDG PET/CT in Patients with Presumed Localized Colon Cancer: A Prospective Study with Long-Term Follow-Up
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. 18F-FDG PET/CT
2.3. Gold Standard
2.4. Statistical Analysis
3. Results
3.1. Patient Population
3.2. Primary Tumor Assessment
3.3. Nodal Involvement and Distant Metastases Assessment
3.4. Survival Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Allemani, C.; Weir, H.K.; Carreira, H.; Harewood, R.; Spika, D.; Wang, X.S.; Bannon, F.; Ahn, J.V.; Johnson, C.J.; Bonaventure, A.; et al. Global surveillance of cancer survival 1995–2009: Analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet Lond. Engl. 2015, 385, 977–1010. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Argilés, G.; Tabernero, J.; Labianca, R.; Hochhauser, D.; Salazar, R.; Iveson, T.; Laurent-Puig, P.; Quirke, P.; Yoshino, T.; Taieb, J.; et al. Localised colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 1291–1305. [Google Scholar] [CrossRef] [PubMed]
- Gómez-España, M.A.; Gallego, J.; González-Flores, E.; Maurel, J.; Páez, D.; Sastre, J.; Aparicio, J.; Benavides, M.; Feliu, J.; Vera, R. SEOM clinical guidelines for diagnosis and treatment of metastatic colorectal cancer. Clin. Transl. Oncol. 2019, 21, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Gómez-España, M.A.; Gallego, J.; González-Flores, E.; Maurel, J.; Páez, D.; Sastre, J.; Aparicio, J.; Benavides, M.; Feliu, J.; Vera, R. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 2016, 27, 1386–1422. [Google Scholar]
- Glynne-Jones, R.; Wyrwicz, L.; Tiret, E.; Brown, G.; Rödel, C.; Cervantes, A.; Arnold, D. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29 (Suppl. S4), iv263. [Google Scholar] [CrossRef]
- Boyle, P.; Leon, M.E. Epidemiology of colorectal cancer. Br. Med. Bull. 2002, 64, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Favoriti, P.; Carbone, G.; Greco, M.; Pirozzi, F.; Pirozzi, R.E.M.; Corcione, F. Worldwide burden of colorectal cancer: A review. Updat. Surg. Mars 2016, 68, 7–11. [Google Scholar] [CrossRef]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef]
- Llamas-Elvira, J.M.; Rodríguez-Fernández, A.; Gutiérrez-Sáinz, J.; Gomez-Rio, M.; Bellon-Guardia, M.; Ramos-Font, C.; Rebollo-Aguirre, A.C.; Cabello-García, D.; Ferrón-Orihuela, A. Fluorine-18 fluorodeoxyglucose PET in the preoperative staging of colorectal cancer. Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 859–867. [Google Scholar] [CrossRef]
- Shin, S.S.; Jeong, Y.Y.; Min, J.J.; Kim, H.R.; Chung, T.W.; Kang, H.K. Preoperative staging of colorectal cancer: CT vs. integrated FDG PET/CT. Abdom. Imaging 2008, 33, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Sato, T.; Kitamura, H.; Ito, M.; Tsunoda, Y.; Hirayama, A.; Kurosawa, H.; Tanaka, T.; Fukushi, M.; Moriyama, N. Diagnosis supporting algorithm for lymph node metastases from colorectal carcinoma on 18F-FDG PET/CT. Ann. Nucl. Med. 2008, 22, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Park, I.J.; Kim, H.C.; Yu, C.S.; Ryu, M.H.; Chang, H.M.; Kim, J.H.; Ryu, J.S.; Yeo, J.S.; Kim, J.C. Efficacy of PET/CT in the accurate evaluation of primary colorectal carcinoma. Eur. J. Surg. Oncol. 2006, 32, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, H.; Ikuma, H.; Seki, A.; Yokoe, K.; Yuen, S.; Aramaki, T.; Yamagushi, S. Positron emission tomography scanning is not superior to whole body multidetector helical computed tomography in the preoperative staging of colorectal cancer. Gut 2006, 55, 1007–1011. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Nabi, H.; Doerr, R.J.; Lamonica, D.M.; Cronin, V.R.; Galantowicz, P.J.; Carbone, G.M.; Spaulding, M.B. Staging of primary colorectal carcinomas with fluorine-18 fluorodeoxyglucose whole-body PET: Correlation with histopathologic and CT findings. Radiology 1998, 206, 755–760. [Google Scholar] [CrossRef] [PubMed]
- Kantorová, I.; Lipská, L.; Bêlohlávek, O.; Visokai, V.; Trubaĉ, M.; Schneiderová, M. Routine (18)F-FDG PET preoperative staging of colorectal cancer: Comparison with conventional staging and its impact on treatment decision making. J. Nucl. Med. 2003, 44, 1784–1788. [Google Scholar] [PubMed]
- Selzner, M.; Hany, T.F.; Wildbrett, P.; McCormack, L.; Kadry, Z.; Clavien, P.A. Does the Novel PET/CT Imaging Modality Impact on the Treatment of Patients with Metastatic Colorectal Cancer of the Liver? Ann. Surg. 2004, 240, 1027–1036. [Google Scholar] [CrossRef]
- Doi, H.; Kitajima, K.; Fukushima, K.; Kawanaka, Y.; Mouri, M.; Yamamoto, S.; Ishikura, R.; Terada, T.; Noguchi, K.; Hirota, S. SUVmax on FDG-PET is a predictor of prognosis in patients with maxillary sinus cancer. Jpn. J. Radiol. 2016, 34, 349–355. [Google Scholar] [CrossRef]
- Nakamura, K.; Hongo, A.; Kodama, J.; Hiramatsu, Y. The measurement of SUVmax of the primary tumor is predictive of prognosis for patients with endometrial cancer. Gynecol. Oncol. 2011, 123, 82–87. [Google Scholar] [CrossRef]
- Xia, X.; Wang, Y.; Yuan, J.; Sun, W.; Jiang, J.; Liu, C.; Zhang, Q.; Ma, X. Baseline SUVmax of 18F-FDG PET-CT indicates prognosis of extranodal natural killer/T-cell lymphoma. Medicine 2020, 99, e22143. [Google Scholar] [CrossRef]
- Kato, H.; Nakajima, M.; Sohda, M.; Tanaka, N.; Inose, T.; Miyazaki, T.; Fukuchi, M.; Oriuchi, N.; Endo, K.; Kuwano, H. The clinical application of (18)F-fluorodeoxyglucose positron emission tomography to predict survival in patients with operable esophageal cancer. Cancer 2009, 115, 3196–3203. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.W.; Lee, E.J.; Cho, Y.H.; Yoon, S.Y.; So, Y.; Kim, S.Y.; Lee, M.H.; Kim, J.H.; Lee, S.Y.; Sung, I.K.; et al. High FDG uptake in PET/CT predicts worse prognosis in patients with metastatic gastric adenocarcinoma. J Cancer Res. Clin. Oncol. 2010, 136, 1929–1935. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Cai, G.; Peng, J.; Li, D.; Li, X.; Xu, Y.; Cai, S. The preoperative SUVmax for 18F-FDG uptake predicts survival in patients with colorectal cancer. BMC Cancer 2015, 15, 991. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Kim, S.W.; Kim, J.S.; Choi, K.Y.; Kang, W.K.; Oh, S.T.; Yoo, I.e.R.; Kim, S.H. Prognostic value of 18-fluorodeoxyglucose positron emission tomography-computed tomography in resectable colorectal cancer. World J. Gastroenterol. 2012, 18, 5072–5077. [Google Scholar] [CrossRef] [PubMed]
- Soyluoglu, S.; Gunay, B.O. Contribution of Metabolic Parameters and Pericolic Fat Stranding on Preoperative 18F-FDG PET/CT in Predicting Post-operative Histopathology and Outcome in Colorectal Cancer. Nucl. Med. Mol. Imaging 2023, 57, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Thoeni, R.F. Colorectal cancer. Radiologic staging. Radiol. Clin. N. Am. Mars 1997, 35, 457–485. [Google Scholar] [CrossRef]
- Maeda, C.; Endo, S.; Mori, Y.; Mukai, S.; Hidaka, E.; Ishida, F.; Kudo, S.E. The ability of positron emission tomography/computed tomography to detect synchronous colonic cancers in patients with obstructive colorectal cancer. Mol. Clin. Oncol. 2019, 10, 425–429. [Google Scholar] [CrossRef]
- Dahmarde, H.; Parooie, F.; Salarzaei, M. Is 18F-FDG PET/CT an Accurate Way to Detect Lymph Node Metastasis in Colorectal Cancer: A Systematic Review and Meta-Analysis. Contrast Media Mol. Imaging 2020, 2020, 5439378. [Google Scholar] [CrossRef]
- Lu, Y.Y.; Chen, J.H.; Ding, H.J.; Chien, C.R.; Lin, W.Y.; Kao, C.H. A systematic review and meta-analysis of pretherapeutic lymph node staging of colorectal cancer by 18F-FDG PET or PET/CT. Nucl. Med. Commun. 2012, 33, 1127–1133. [Google Scholar] [CrossRef]
- Arena, V.; Skanjeti, A.; Casoni, R.; Douroukas, A.; Pelosi, E. Dual-phase FDG-PET: Delayed acquisition improves hepatic detectability of pathological uptake. Radiol. Med. 2008, 113, 875–886. [Google Scholar] [CrossRef]
- Sargent, D.; Sobrero, A.; Grothey, A.; O’Connell, M.J.; Buyse, M.; Andre, T.; Zheng, Y.; Green, E.; Labianca, R.; O’Callaghan, C.; et al. Evidence for Cure by Adjuvant Therapy in Colon Cancer: Observations Based on Individual Patient Data From 20,898 Patients on 18 Randomized Trials. J. Clin. Oncol. 2009, 27, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lee, H.S.; Kim, S.; Park, E.J.; Baik, S.H.; Jeon, T.J.; Lee, K.Y.; Ryu, Y.H.; Kang, J. Prognostic significance of bone marrow and spleen 18F-FDG uptake in patients with colorectal cancer. Sci Rep. 2021, 11, 12137. [Google Scholar] [CrossRef] [PubMed]
- Mirshahvalad, S.A.; Hinzpeter, R.; Kohan, A.; Anconina, R.; Kulanthaivelu, R.; Ortega, C.; Metser, U.; Veit-Haibach, P. Diagnostic performance of [18F]-FDG PET/MR in evaluating colorectal cancer: A systematic review and meta-analysis. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 4205–4217. [Google Scholar] [CrossRef] [PubMed]
- Kömek, H.; Can, C.; Kaplan, İ.; Gündoğan, C.; Kepenek, F.; Karaoglan, H.; Demirkıran, A.; Ebinç, S.; Güzel, Y.; Gündeş, E. Comparison of [68Ga]Ga-DOTA-FAPI-04 PET/CT and [18F]FDG PET/CT in colorectal cancer. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 3898–3909. [Google Scholar] [CrossRef]
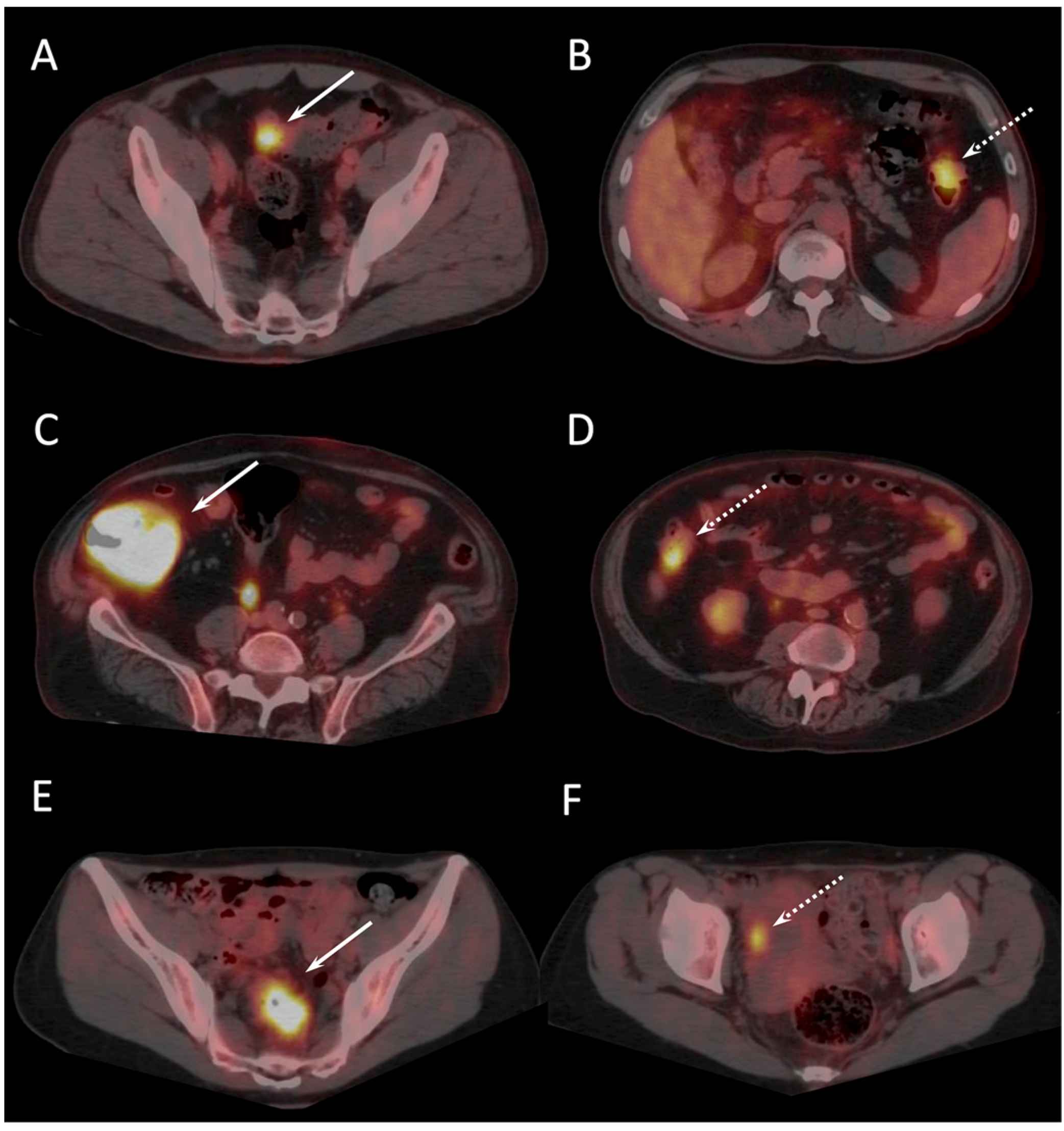
| Number of patients | 48 |
| Sex | 25 men (52%) 23 women (48%) |
| Age | 67 ± 16 |
| Diabetes | 7/48 (14.6%) |
| Colonoscopy | Complete colonoscopy: 42/48 (88%) Incomplete colonoscopy: 5/48 (10%) Not feasible: 1/48 (2%) |
| CECT | 48/48 (100%) |
| 18F-PET/CT | Whole-body standard imaging: 48/48 (100%) Additional delayed imaging: 36/48 (75%) |
| Pathological findings (103 lesions) | Low-grade dysplasia: 38 (37%) High-grade dysplasia: 3 (2.9%) pTis: 4 (3.9%) pT1: 10 (9.7%) pT2: 5 (4.9%) pT3: 40 (38.8%) pT4: 3 (2.9%) |
| Localization of pre-malignant and malignant lesions | Caecum: 14 (21.5%) Right colon: 17 (26.2%) Transverse colon: 5 (7.7%) Left colon: 8 (12.3%) Sigmoid: 21 (32.3%) |
| T grade (62 lesions) | PTis: 4 (6.5%) PT1: 10 (16.1%) PT2: 5 (8%) PT3: 40 (64.5%) PT4: 3 (4.8%) |
| Main size (cm) (65 lesions) | 4.77 ± 2.02 (range: 1–11) |
| Tumor histology | Adenocarcinoma: 48/48 (100%) Mucinous subtype: 5/48 (10.4%) |
| Adenocarcinoma differentiation | Well differentiated: 40 (83.3%) Moderately differentiated: 2 (4.2%) Poorly differentiated: 6 (12.5%) |
| Microsatellite instability (patients) | 8/48 (16.7%) |
| Angioinvasion (patients) | 6/48 (12.5%) |
| N+ (patients) | 18/48 (37.5%) |
| Pathology after Surgical Resection | Conventional Workup (Colonoscopy and CECT) | 18F-FDG PET/CT | ||
|---|---|---|---|---|
| Adenoma with low-grade dysplasia | 38 | 1 | 5 | |
| Adenoma with high-grade dysplasia | 3 | 0 | 3 | |
| In situ carcinoma | 4 | 2 | 4 | |
| Invasive carcinoma (total) | 58 | 49 | 54 | |
| T1 | 10 | 6 | 6 | |
| T2 | 5 | 3 | 5 | |
| T3 | 40 | 37 | 40 | |
| T4 | 3 | 3 | 3 | |
| Tumoral and precancerous lesions * | 65 | 51 | 61 | |
| Blockage at Endoscopy | Cause of Blockage | Tumor Topography (T Stage) | Endoscopic Exploration of Colon Segment with CC (+/−) | Standard Workup (+/−) | 18F-FDG PET/CT (+/−) | PET/CT Incremental Value (y/n) |
|---|---|---|---|---|---|---|
| LC | Looping | S (T3) | + | + | + | no |
| S | Looping | C (T3) | − | + | + | no |
| S | Tumor | S (T3) | + | + | + | no |
| LC (T2) | − | − | + | yes | ||
| C (T2) | − | − | + | yes | ||
| LC | Tumor | S (T3) | + | + | + | no |
| LC (T3) | + | + | + | no | ||
| S | Tumor | S (T4) | + | + | + | no |
| Endoscopy not performed | Patient refusal | LC (T1) | / | − | + | yes |
| Tumor Site | Tumor Size (mm) | Standard Workup | PET/CT | Incremental Value of PET/CT |
|---|---|---|---|---|
| T (T1) | 40 | TP | TP | Detection of two additional adenomas with high-grade dysplasia |
| RC (HG) | 25 | - | TP | |
| RC (HG) | 30 | - | TP | |
| S (T1) | 50 | TP | TP | Detection of one additional adenoma with high grade dysplasia |
| T (HG) | 12 | - | TP | |
| S (is) | 10 | FN | TP | Detection of one synchronous in situ adenocarcinomas not reported at colonoscopy |
| C (T3) | 60 | TP | TP | |
| S (T3) | 30 | FN | TP | Detection of one synchronous invasive adenocarcinoma and one additional in situ carcinoma |
| CG (is) | 15 | FN | TP | |
| S (T3) | 55 | TP | TP | Detection of two additional synchronous invasive adenocarcinomas beyond a sigmoid tumoral stenosis |
| LC (T2) | 25 | FN | TP | |
| C (T2) | 30 | FN | TP | |
| S (T3) | 70 | FN | TP | Detection of one synchronous invasive adenocarcinoma not reported at colonoscopy |
| CG (is) | 60 | TP | TP | |
| S (T3) | 45 | TP | TP | Detection of one synchronous invasive adenocarcinoma not reported at colonoscopy |
| S (T3) | 25 | FN | TP | |
| S (is) | 15 | FN | FN | |
| CG (is) | 15 | FN | FN | |
| S (LG) | 35 | TP | TP | None (perioperative detection of a caecal in situ adenocarcinoma) |
| C (is) | 25 | FN | FN | |
| C (T3) | 55 | TP | TP | |
| S (T1) | 10 | FN | FN | None (perioperative detection of a sigmoid invasive adenocarcinoma) |
| C (T2) | 50 | TP | TP | |
| T (T3) | 45 | TP | n/a | None (diffuse intestinal uptake related to metformin) |
| C (T3) | 30 | TP | n/a | None (diffuse intestinal uptake related to metformin) |
| LC (T3) | 70 | TP | TP | None (false-positive results) |
| - | - | - | FP |
| SUVmax | SUVpeak | TLG | MTV | |
|---|---|---|---|---|
| LG dysplasia | 6.02 ± 0.66 | 4.48 ± 0.71 | 25.93 ± 12.48 | 7.42 ± 3.89 |
| HG dysplasia | 6.41 ± 0.94 | 4.13 ± 0.39 | 13.25 ± 9.57 | 3.85 ± 3.53 |
| In situ carcinoma | 5.64 ± 1.5 | 4.2 ± 1.4 | 47.2 ± 53.1 | 15.47 ± 19.49 |
| T1 carcinoma | 10.99 ± 1.84 | 8.46 ± 2.56 | 66.97 ± 65.37 | 9.19 ± 8.38 |
| T2 carcinoma | 14.52 ± 10.27 | 10.55 ± 7.34 | 62.1 ± 52.93 | 6.77 ± 3.75 |
| T3 carcinoma | 19.41 ± 10.03 | 15.34 ± 7.32 | 269.08 ± 245.22 | 22.86 ± 17.58 |
| T4 carcinoma | 13.19 ± 0.98 | 10.82 ± 0.5 | 189.3 ± 68 | 24.36 ± 7.82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aymard, S.; Rust, E.; Kaseb, A.; Liu, D.; Hubele, F.; Romain, B.; Averous, G.; Brigand, C.; Imperiale, A. Preoperative 18F-FDG PET/CT in Patients with Presumed Localized Colon Cancer: A Prospective Study with Long-Term Follow-Up. Cancers 2024, 16, 233. https://doi.org/10.3390/cancers16010233
Aymard S, Rust E, Kaseb A, Liu D, Hubele F, Romain B, Averous G, Brigand C, Imperiale A. Preoperative 18F-FDG PET/CT in Patients with Presumed Localized Colon Cancer: A Prospective Study with Long-Term Follow-Up. Cancers. 2024; 16(1):233. https://doi.org/10.3390/cancers16010233
Chicago/Turabian StyleAymard, Samuel, Edmond Rust, Ashjan Kaseb, David Liu, Fabrice Hubele, Benoit Romain, Gerlinde Averous, Cecile Brigand, and Alessio Imperiale. 2024. "Preoperative 18F-FDG PET/CT in Patients with Presumed Localized Colon Cancer: A Prospective Study with Long-Term Follow-Up" Cancers 16, no. 1: 233. https://doi.org/10.3390/cancers16010233
APA StyleAymard, S., Rust, E., Kaseb, A., Liu, D., Hubele, F., Romain, B., Averous, G., Brigand, C., & Imperiale, A. (2024). Preoperative 18F-FDG PET/CT in Patients with Presumed Localized Colon Cancer: A Prospective Study with Long-Term Follow-Up. Cancers, 16(1), 233. https://doi.org/10.3390/cancers16010233





