Gender Differences in Soft Tissue and Bone Sarcoma: A Narrative Review
Abstract
:Simple Summary
Abstract
1. Introduction
1.1. Environmental Factors and Sarcomas
1.2. Genetic Susceptibility and Sarcoma
- Familial gastrointestinal stromal tumor syndrome (GIST)
- Li–Fraumeni syndrome (LFS)
- Neurofibromatosis (NF1)
- Retinoblastoma (Rb)
- Bloom syndrome (BS)
- Rothmund–Thompson syndrome
- Werner syndrome
1.3. Sex Differences in Sarcomas
1.4. Sex Differences in Sarcoma in Childhood
1.5. Sex Biological Differences in Sarcoma in Childhood
2. Conclusions
| Study Design, Data Source, Years Include | Relevant Study Population | Key Results | |
|---|---|---|---|
| Part A: Evidence for adult | |||
| Rong J et al., 2020 [35] | Retrospective registry-based cohort (CHINA-USA) 2010–2016 | 1050 | Data from gastric GIST patients were collected from the SEER database. Propensity score matching (PSM) was performed to reduce confounding factors, and the clinicopathological features and prognosis of GIST patients were comprehensively evaluated. Gender could be a prognostic factor for gastric GIST survival, and male patients had a higher risk of death. |
| Mo Chen et al., 2018 [36] | Retrospective registry-based cohort (CHINA-USA) 1973–2013 | 6582 | Data from gastric GIST patients were collected from the SEER database. The study investigated the impact of marital status on the overall survival (OS) and cancer-specific survival (CSS) of operable GIST cases. The marriage could be a protective prognostic factor for survival, and widowed patients had a higher risk of death. |
| Neal D Freedman et al., 2007 [38] | Retrospective registry-based cohort (USA) | 154 | The study investigated the association of menstrual and reproductive factors and gastric cancer risk. No associations were observed between gastric cancer risk and age of menarche, number of children, breast feeding, or oral contraceptive use. In contrast, associations were observed with age of menopause, years of fertility, years since menopause, and intrauterine device use. |
| M Lindblad et al., 2006 [39] | Retrospective (SWEDEN) 1994–2001 | 612 | Esophageal and gastric adenocarcinoma share an unexplained male predominance, A nested case–control study of hormone replacement therapy (HRT) was conducted among 299 women with esophageal cancer, 313 with gastric cancer, and 3191 randomly selected control women. Among 1,619,563 person-years of follow-up, more than 50% reduced risk of gastric adenocarcinoa was found among users of HRT compared to non-users. This inverse association appeared to be stronger for gastric noncardia and weaker for gastric cardia tumors. There was no association between HRT and esophageal adenocarcinoma. |
| Giun-Yi Hung et al., 2019 [41] | Retrospective registry-based cohort (TAIWAN) 2007–2013 | 11,393 | STS data were acquired from the population-based 2007–2013 Taiwan Cancer Registry of the Health and Welfare Data Science Center, Taiwan. In total, 11,393 patients with an age-standardized incidence rate of 5.62 per 100,000 person-years were identified. Overall, a male predominance and the rate increased with age, peaking at >75 years. |
| Mei-Chin Hsieh et al., 2013 [42] | Retrospective registry-based cohort (USA) 1995–2008 | 10,289 | STS data were obtained from the North American Association of Central Cancer Registries (NAACCR). The incidence of all STSs combined was higher in males than females. |
| Rouhani P et al., 2008 [44] | Retrospective registry-based cohort (USA) 1992–2004 | 12,114 | Data from cutaneous soft tissue sarcoma (CSTS) patients were collected from the SEER database confirmed that the incidence of all CSTSs combined was higher in males than females. |
| Part B: Evidence for childhood and adolescent | |||
| Cole S et al., 2022 [47] | Retrospective registry-based cohort (USA) 1975–2017 | 5016 | Data from osteosarcoma patients were collected from the SEER database. The findings confirm in cases 0 to 9 years old, incidence of primary osteosarcoma was similar between the sexes and increased significantly throughout the study period. Overall, survival rates for all cases have remained relatively unchanged over recent decades, with worse survival observed in males. |
| Ognjanovic S et al., 2009 [55] | Retrospective registry-based cohort (USA) 1975–2005 | 987 | Data from childhood rhabdomyosarcoma (RMS) patients were collected from the SEER database. The findings revealed the incidence of an embryonal rhabdomyosarcoma (ERMS) was higher in male than females and, more specifically, a smaller peak of embryonal rhabdomyosarcoma (ERMS) incidence rates was observed during adolescence in males which may be related to only those sex-specific hormonal differences. |
| Ward E et al., 2014 [56] | Retrospective registry-based cohort (USA) 1975–2010 | 15,780 | Data from children and adolescent patients diagnosed with cancer were collected from the SEER database and The North American Association of CentralCancer Registries (NAACCR). The findings confirm that gender disparity has been found in most pediatric cancers, acute lymphoblastic leukemia, non-Hodgkin’s lymphoma, medulloblastoma, hepatic tumors, osteosarcoma, and germ cell tumors, showing that the direct effect of male sex is significant for several tumor types. |
| Williams LA et al., 2019 [69] | Retrospective registry-based cohort (USA) 2000–2015 | 71,906 | Cancer cases aged 0–19 years were identified using the SEER Program. Male sex was positively associated with most cancers. The higher incidence rates observed in males remained consistent over the childhood and adolescent periods, suggesting that childhood and adolescent hormonal fluctuations may not be the primary driving factor for the sex disparities in childhood cancer. The observed incidence disparities may be due to sex differences in exposures, genetics, or immune responses. |
| Williams LA et al., 2019 [58] | Retrospective registry-based cohort (USA) 2000–2014 | 57,004 | Cancer cases aged 0–19 years were identified using the the SEER program. Males had worse overall survival and a higher risk of death for acute lymphoblastic leukemia, ependymoma, neuroblastoma, osteosarcoma, thyroid carcinoma, and malignant melanoma. |
Author Contributions
Funding
Conflicts of Interest
References
- Burningham, Z.; Hashibe, M.; Spector, L.; Schiffman, J.D. The Epidemiology of Sarcoma. Clin. Sarcoma Res. 2012, 2, 14. [Google Scholar] [CrossRef] [PubMed]
- Jo, V.Y.; Fletcher, C.D.M. WHO classification of soft tissue tumours: An update based on the 2013 (4th) edition. Pathology 2014, 46, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Mastrangelo, G.; Fadda, E.; Cegolon, L.; Montesco, M.C.; Ray-Coquard, I.; Buja, A.; Fedeli, U.; Frasson, A.; Spolaore, P.; Rossi, C.R. A European project on incidence, treatment, and outcome of sarcoma. BMC Public Health 2010, 10, 188. [Google Scholar] [CrossRef] [PubMed]
- Ducimetière, F.; Lurkin, A.; Ranchère-Vince, D.; Decouvelaere, A.V.; Isaac, S.; Claret-Tournier, C.; Suignard, Y.; Salameire, D.; Cellier, D.; Alberti, L.; et al. Incidence rate, epidemiology of sarcoma and molecular biology. Preliminary results from EMS study in the Rhône-Alpes region. Bull Cancer 2010, 97, 629–641. [Google Scholar] [PubMed]
- Laurino, S.; Omer, L.C.; Albano, F.; Marino, G.; Bianculli, A.; Solazzo, A.P.; Sgambato, A.; Falco, G.; Russi, S.; Bochicchio, A.M. Radiation-induced sarcomas: A single referral cancer center experience and literature review. Front. Oncol. 2022, 12, 986123. [Google Scholar] [CrossRef] [PubMed]
- Xi, M.; Liu, M.Z.; Wang, H.X.; Cai, L.; Zhang, L.; Xie, C.F.; Li, Q.Q. Radiation-induced sarcoma in patients with nasopharyngeal carcinoma: A single-institution study. Cancer 2010, 116, 5479–5486. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.; Tmanova, L.; Mydlarz, W.K.; Page, B.; Richmon, J.D.; Quon, H.; Schmitt, N.C. Radiation-Associated Sarcoma of the Neck: Case Series and Systematic Review. Ann. Otol. Rhinol. Laryngol. 2018, 127, 735–740. [Google Scholar] [CrossRef]
- Gladdy, R.A.; Qin, L.X.; Moraco, N.; Edgar, M.A.; Antonescu, C.R.; Alektiar, K.M.; Brennan, M.F.; Singer, S. Do radiation-associated soft tissue sarcomas have the same prognosis as sporadic soft tissue sarcomas? J. Clin. Oncol. 2010, 28, 2064–2069. [Google Scholar] [CrossRef]
- Lazarides, A.L.; Burke, Z.D.C.; Gundavda, M.K.; Novak, R.; Ghert, M.; Wilson, D.A.; Rose, P.S.; Wong, P.; Griffin, A.M.; Ferguson, P.C.; et al. How Do the Outcomes of Radiation-Associated Pelvic and Sacral Bone Sarcomas Compare to Primary Osteosarcomas following Surgical Resection? Cancers 2022, 14, 2179. [Google Scholar] [CrossRef]
- Snow, A.; Ring, A.; Struycken, L.; Mack, W.; Koç, M.; Lang, J.E. Incidence of radiation induced sarcoma attributable to radiotherapy in adults: A retrospective cohort study in the SEER cancer registries across 17 primary tumor sites. Cancer Epidemiol. 2021, 70, 101857. [Google Scholar] [CrossRef]
- Hardell, L. Malignant mesenchymal tumours and exposure to phenoxy acids. A clinical observation. Lakartidningen 1977, 74, 7974021. [Google Scholar]
- Hardell, L.; Eriksson, M.; Degerman, A. Meta-analysis of four Swedish case-control studies on exposure to pesticides as risk-factor for soft-tissue sarcoma including the relation to tumour localization and histopathological type. Int. J. Oncol. 1995, 6, 847–851. [Google Scholar] [PubMed]
- Edwards, D.; Voronina, A.; Attwood, K.; Grand’Maison, A. Association between occupational exposures and sarcoma incidence and mortality: Systematic review and meta-analysis. Syst. Rev. 2021, 10, 231. [Google Scholar] [CrossRef] [PubMed]
- Ward, E.; Boffetta, P.; Andersen, A.; Colin, D.; Comba, P.; Deddens, J.A.; De Santis, M.; Engholm, G.; Hagmar, L.; Langard, S.; et al. Update of the follow-up of mortality and cancer incidence among European workers employed in the vinyl chloride industry. Epidemiology 2001, 12, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Hossain, A.; McDuffie, H.H.; Bickis, M.G.; Pahwa, P. Case-control study on occupational risk factors for soft-tissue sarcoma. J. Occup. Environ. Med. 2007, 49, 1386–1393. [Google Scholar] [CrossRef] [PubMed]
- Bhalla, A.D.; Landers, S.M.; Singh, A.K.; Landry, J.P.; Yeagley, M.G.; Myerson, G.S.B.; Delgado-Baez, C.B.; Dunnand, S.; Nguyen, T.; Ma, X.; et al. Experimental models of undifferentiated pleomorphic sarcoma and malignant peripheral nerve sheath tumor. Lab. Investig. 2022, 102, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Lahat, G.; Lazar, A.; Lev, D. Sarcoma Epidemiology and Etiology: Potential Environmental and Genetic Factors. Surg. Clin. N. Am. 2008, 88, 451–481. [Google Scholar] [CrossRef] [PubMed]
- Patil, D.T.; Rubin, B.P. Genetics of Gastrointestinal Stromal Tumors a Heterogeneous Family of Tumors? Surg. Pathol. Clin. 2015, 8, 515–524. [Google Scholar] [CrossRef]
- Li, F.P.; Fraumeni, J.F.; Mulvihill, J.J.; Blattner, W.A.; Dreyfus, M.G.; Tucker, M.A.; Miller, R.W. A Cancer Family Syndrome in Twenty-four Kindreds. Cancer Res. 1988, 48, 5358–5362. [Google Scholar]
- Tinat, J.; Bougeard, G.; Baert-Desurmont, S.; Vasseur, S.; Martin, C.; Bouvignies, E.; Caron, O.; Bressac-de Paillerets, B.; Berthet, P.; Dugast, C.; et al. 2009 Version of the Chompret Criteria for Li Fraumeni Syndrome. J. Clin. Oncol. 2009, 27, e108–e109. [Google Scholar] [CrossRef]
- Guha, T.; Malkin, D. Inherited TP53 mutations and the Li-fraumeni syndrome. Cold Spring Harb. Perspect. Med. 2017, 7, a026187. [Google Scholar] [CrossRef] [PubMed]
- Villani, A.; Shore, A.; Wasserman, J.D.; Stephens, D.; Kim, R.H.; Druker, H.; Gallinger, B.; Naumer, A.; Kohlmann, W.; Novokmet, A.; et al. Biochemical and imaging surveillance in germline TP53 mutation carriers with Li-Fraumeni syndrome: 11 year follow-up of a prospective observational study. Lancet Oncol. 2016, 17, 1295–1305. [Google Scholar] [CrossRef] [PubMed]
- Tamura, R. Current understanding of neurofibromatosis type 1, 2, and schwannomatosis. Int. J. Mol. Sci. 2021, 22, 5850. [Google Scholar] [CrossRef] [PubMed]
- Wong, F.L. Cancer Incidence After Retinoblastoma. JAMA 1997, 278, 1262. [Google Scholar] [CrossRef]
- Ottaviani, G.; Jaffe, N. The etiology of osteosarcoma. Cancer Treat. Res. 2009, 152, 15–32. [Google Scholar] [PubMed]
- Hansen, M.F.; Koufos, A.; Gallie, B.L.; Phillips, R.A.; Fodstad, O.; Brøgger, A.; Gedde-Dahl, T.; Cavenee, W.K. Osteosarcoma and retinoblastoma: A shared chromosomal mechanism revealing recessive predisposition. Proc. Natl. Acad. Sci. USA 1985, 82, 6216–6220. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, O.; Easton, D.; Anderson, K.; Gilham, C.; Jay, M.; Peto, J. Lifetime risks of common cancers among retinoblastoma survivors. J. Natl. Cancer Inst. 2004, 96, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Cunniff, C.; Bassetti, J.A.; Ellis, N.A. Bloom’s syndrome: Clinical spectrum, molecular pathogenesis, and cancer predisposition. Mol. Syndromol. 2017, 8, 4–23. [Google Scholar] [CrossRef]
- Salih, A.; Inoue, S.; Onwuzurike, N. Rothmund-Thomson syndrome (RTS) with osteosarcoma due to RECQL4 mutation. BMJ Case Rep. 2018, 2018, bcr2017222384. [Google Scholar] [CrossRef]
- Oshima, J.; Sidorova, J.M.; Monnat, R.J. Werner syndrome: Clinical features, pathogenesis and potential therapeutic interventions. Ageing Res. Rev. 2017, 33, 105–114. [Google Scholar] [CrossRef]
- Whitmire, P.; Rickertsen, C.R.; Hawkins-Daarud, A.; Carrasco, E.; Lorence, J.; De Leon, G.; Curtin, L.; Bayless, S.; Clark-Swanson, K.; Peeri, N.C.; et al. Sex-specific impact of patterns of imageable tumor growth on survival of primary glioblastoma patients. BMC Cancer 2020, 20, 447. [Google Scholar] [CrossRef]
- Miller, K.D.; Nogueira, L.; Devasia, T.; Mariotto, A.B.; Yabroff, K.R.; Jemal, A.; Kramer, J.; Siegel, R.L. Cancer treatment and survivorship statistics, 2022. CA Cancer J. Clin. 2022, 72, 409–436. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Research UK. Soft tissue sarcoma statistics. CA Cancer J. Clin. 2022, 72, 7–33. Available online: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/soft-tissue-sarcoma (accessed on 20 January 2022). [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Rong, J.; Chen, S.; Song, C.; Wang, H.; Zhao, Q.; Zhao, R.; He, Y.; Yan, L.; Song, Y.; Wang, F.; et al. The prognostic value of gender in gastric gastrointestinal stromal tumors: A propensity score matching analysis. Biol. Sex. Differ. 2020, 11, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wang, X.; Wei, R.; Wang, Z. The influence of marital status on the survival of patients with operable gastrointestinal stromal tumor: A SEER-based study. Int. J. Health Plan. Manag. 2019, 34, e447–e463. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.; Xie, R.; Tuo, B. Effects of Estrogen on the Gastrointestinal Tract. Dig. Dis. Sci. 2018, 63, 583–596. [Google Scholar] [CrossRef] [PubMed]
- Freedman, N.D.; Chow, W.H.; Gao, Y.T.; Shu, X.O.; Ji, B.T.; Yang, G.; Lubin, J.H.; Li, H.L.; Rothman, N.; Zheng, W.; et al. Menstrual and reproductive factors and gastric cancer risk in a large prospective study of women. Gut 2007, 56, 1671–1677. [Google Scholar] [CrossRef]
- Lindblad, M.; García Rodríguez, L.A.; Chandanos, E.; Lagergren, J. Hormone replacement therapy and risks of oesophageal and gastric adenocarcinomas. Br. J. Cancer 2006, 94, 136–141. [Google Scholar] [CrossRef]
- Jang, Y.C.; Leung, C.Y.; Huang, H.L. Association of hormone replacement therapy with risk of gastric cancer: A systematic review and meta-analysis. Sci. Rep. 2022, 12, 1–7. [Google Scholar]
- Hung, G.Y.; Horng, J.L.; Chen, P.C.H.; Lin, L.Y.; Chen, J.Y.; Chuang, P.H.; Chao, T.-C.; Yen, C.-C. Incidence of soft tissue sarcoma in Taiwan: A nationwide population-based study (2007–2013). Cancer Epidemiol. 2019, 60, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.C.; Wu, X.C.; Andrews, P.A.; Chen, V.W. Racial and Ethnic Disparities in the Incidence and Trends of Soft Tissue Sarcoma among Adolescents and Young Adults in the United States, 1995–2008. J. Adolesc. Young Adult Oncol. 2013, 2, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Van Herk-Sukel, M.P.P.; Shantakumar, S.; Overbeek, L.I.H.; Van Boven, H.; Penning-Van Beest, F.J.A.; Herings, R.M.C. Occurrence of comorbidities before and after soft tissue sarcoma diagnosis. Sarcoma 2012, 2012, 402109. [Google Scholar] [CrossRef] [PubMed]
- Rouhani, P.; Fletcher, C.D.M.; Devesa, S.S.; Toro, J.R. Cutaneous soft tissue sarcoma incidence patterns in the U.S. Cancer 2008, 113, 616–627. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.J.; Pappoppula, L.; Guddati, A.K. Analysis of trends in race and gender disparities in incidence-based mortality in patients diagnosed with soft tissue sarcomas from 2000 to 2016. Int. J. Gen. Med. 2021, 14, 3787–3791. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society. Key Statistics for Soft Tissue Sarcomas. Available online: https://www.cancer.org/cancer/soft-tissue-sarcoma/about/key-statistics.html (accessed on 20 January 2022).
- Cole, S.; Gianferante, D.M.; Zhu, B.; Mirabello, L. Osteosarcoma: A Surveillance, Epidemiology, and End Results program-based analysis from 1975 to 2017. Cancer 2022, 128, 2107–2118. [Google Scholar] [CrossRef] [PubMed]
- Brown, H.K.; Schiavone, K.; Gouin, F.; Heymann, M.F.; Heymann, D. Biology of Bone Sarcomas and New Therapeutic Developments. Calcif. Tissue Int. 2018, 102, 174–195. [Google Scholar] [CrossRef] [PubMed]
- Dermawan, J.K.; Chi, P.; Tap, W.D.; Rosenbaum, E.; D’Angelo, S.; Alektiar, K.M.; Antonescu, C.R. Distinct genomic landscapes in radiation-associated angiosarcoma compared with other radiation-associated sarcoma histologies. J. Pathol. 2023, 260, 465–477. [Google Scholar] [CrossRef]
- Inoue, Y.Z.; Frassica, F.J.; Sim, F.H.; Unni, K.K.; Petersen, I.A.; McLeod, R.A. Clinicopathologic features and treatment of postirradiation sarcoma of bone and soft tissue. J. Surg. Oncol. 2000, 75, 42–50. [Google Scholar] [CrossRef]
- Ognjanovic, S.; Olivier, M.; Bergemann, T.L.; Hainaut, P. Sarcomas in TP53 germline mutation carriers: A review of the IARC TP53 database. Cancer 2012, 118, 1387–1396. [Google Scholar] [CrossRef]
- Farid, M.; Ngeow, J. Sarcomas Associated with Genetic Cancer Predisposition Syndromes: A Review. Oncologist 2016, 21, 1002–1013. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.M.; Savage, S.A.; Bond, G.L. Hereditary and environmental epidemiology of sarcomas. Clin. Sarcoma. Res. 2012, 2, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Jawad, M.U.; Cheung, M.C.; Min, E.S.; Schneiderbauer, M.M.; Koniaris, L.G.; Scully, S.P. Ewing sarcoma demonstrates racial disparities in incidence-related and sex-related differences in outcome: An analysis of 1631 cases from the SEER database, 1973–2005. Cancer 2009, 115, 3526–3536. [Google Scholar] [CrossRef] [PubMed]
- Ognjanovic, S.; Linabery, A.M.; Charbonneau, B.; Ross, J.A. Trends in childhood rhabdomyosarcoma incidence and survival in the United States, 1975–2005. Cancer 2009, 115, 4218–4226. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society, Cancer in children & adolescents. Spec. Sect. Cancer Child. Adolesc. 2014, 1, 25–42.
- Williams, L.A.; Richardson, M.; Kehm, R.D.; McLaughlin, C.C.; Mueller, B.A.; Chow, E.J.; Spector, L.G. The association between sex and most childhood cancers is not mediated by birthweight. Cancer Epidemiol. 2018, 57, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.A.; Spector, L.G. Survival Differences between Males and Females Diagnosed with Childhood Cancer. JNCI Cancer Spectr. 2019, 3, pkz032. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.W.; Keegan, T.; Hamilton, A.; Lynch, C.; Wu, X.C.; Schwartz, S.M.; Kato, I.; Cress, R.; Harlan, L.; AYA HOPE Study Collaborative Group. Understanding care and outcomes in adolescents and young adult with Cancer: A review of the AYA HOPE study. Pediatr. Blood Cancer 2019, 66, e27486. [Google Scholar] [CrossRef]
- Agaram, N.P. Evolving classification of rhabdomyosarcoma. Histopathology 2022, 80, 98–108. [Google Scholar] [CrossRef]
- Locatelli, V.; Bianchi, V.E. Effect of GH/IGF-1 on Bone Metabolism and Osteoporsosis. Int. J. Endocrinol. 2014, 2014, 235060. [Google Scholar] [CrossRef]
- Singh, D.; Sanyal, S.; Chattopadhyay, N. The role of estrogen in bone growth and formation: Changes at puberty. Cell Health Cytoskelet. 2011, 3, 2–12. [Google Scholar] [CrossRef]
- Laitinen, M.K.; Albergo, J.I.; Stevenson, J.D.; Farfalli, G.L.; Aponte-Tinao, L.A.; Grimer, R.J.; Jeys, L.M.; Parry, M.C. Female gender in the hormonally active age group plays a major role in high-grade chondrosarcoma survival. Acta Oncol. 2020, 59, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Frank, G.R. Role of estrogen and androgen in pubertal skeletal physiology. Med. Pediatr. Oncol. 2003, 41, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Kukurba, K.R.; Parsana, P.; Balliu, B.; Smith, K.S.; Zappala, Z.; Knowles, D.A.; Favé, M.-J.; Davis, J.R.; Li, X.; Zhu, X.; et al. Impact of the X chromosome and sex on regulatory variation. Genome Res. 2016, 26, 768–777. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, I.; Lleo, A.; Gershwin, M.E.; Invernizzi, P. The X chromosome and immune associated genes. J. Autoimmun. 2012, 38, J187–J192. [Google Scholar] [CrossRef] [PubMed]
- Libert, C.; Dejager, L.; Pinheiro, I. The X chromosome in immune functions: When a chromosome makes the difference. Nat. Rev. Immunol. 2010, 10, 594–604. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.H.; Li, Y.Y.; Qiao, H.; Wang, H.C.; Yang, X.A.; Zhang, H.G.; Pang, X.-W.; Zhang, Y.; Chen, W.-F. TSPY is a cancer testis antigen expressed in human hepatocellular carcinoma. Br. J. Cancer 2005, 93, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.A.; Richardson, M.; Marcotte, E.L.; Poynter, J.N.; Spector, L.G. Sex ratio among childhood cancers by single year of age. Pediatr. Blood Cancer 2019, 66, e27620. [Google Scholar] [CrossRef]
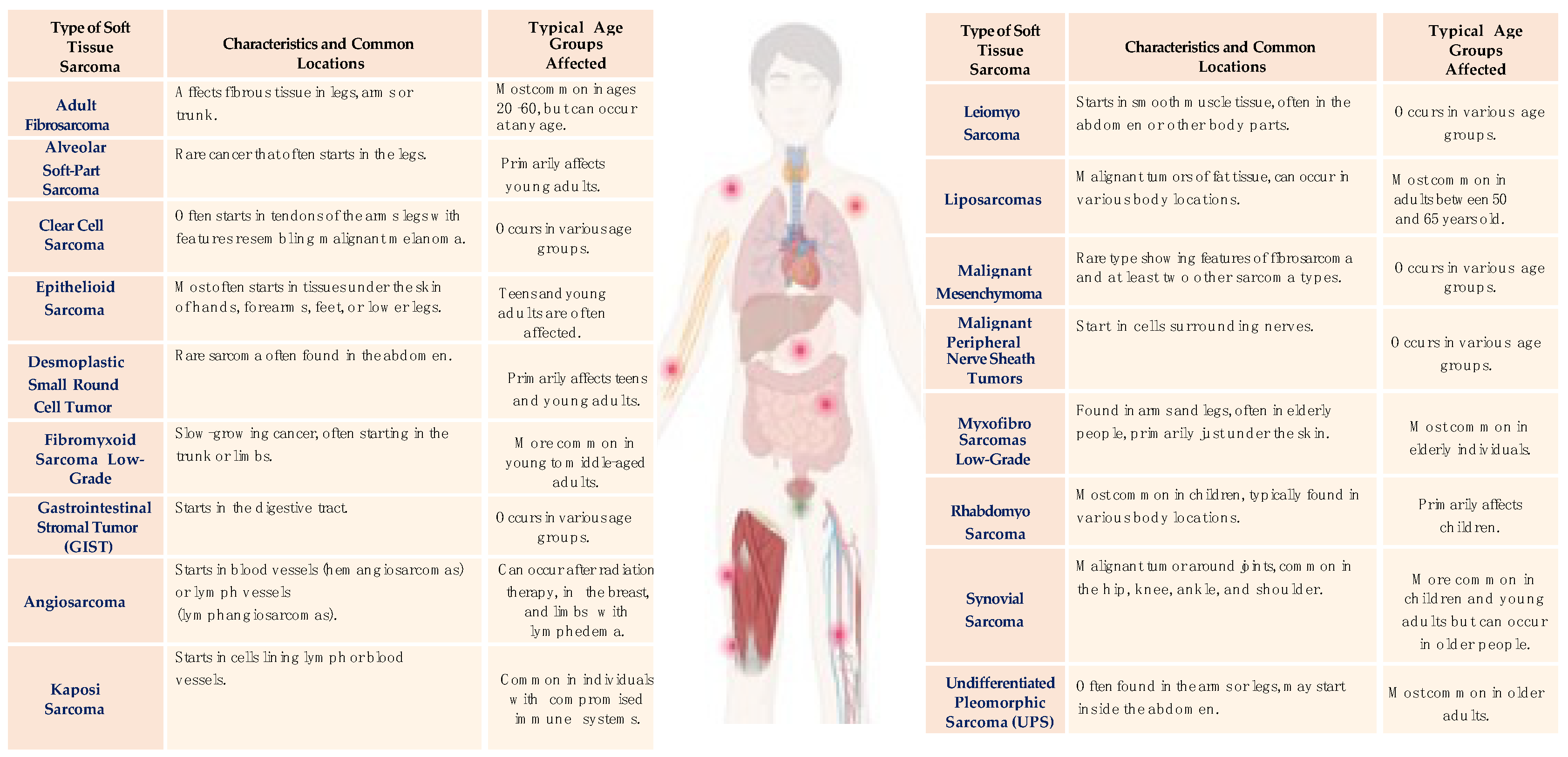
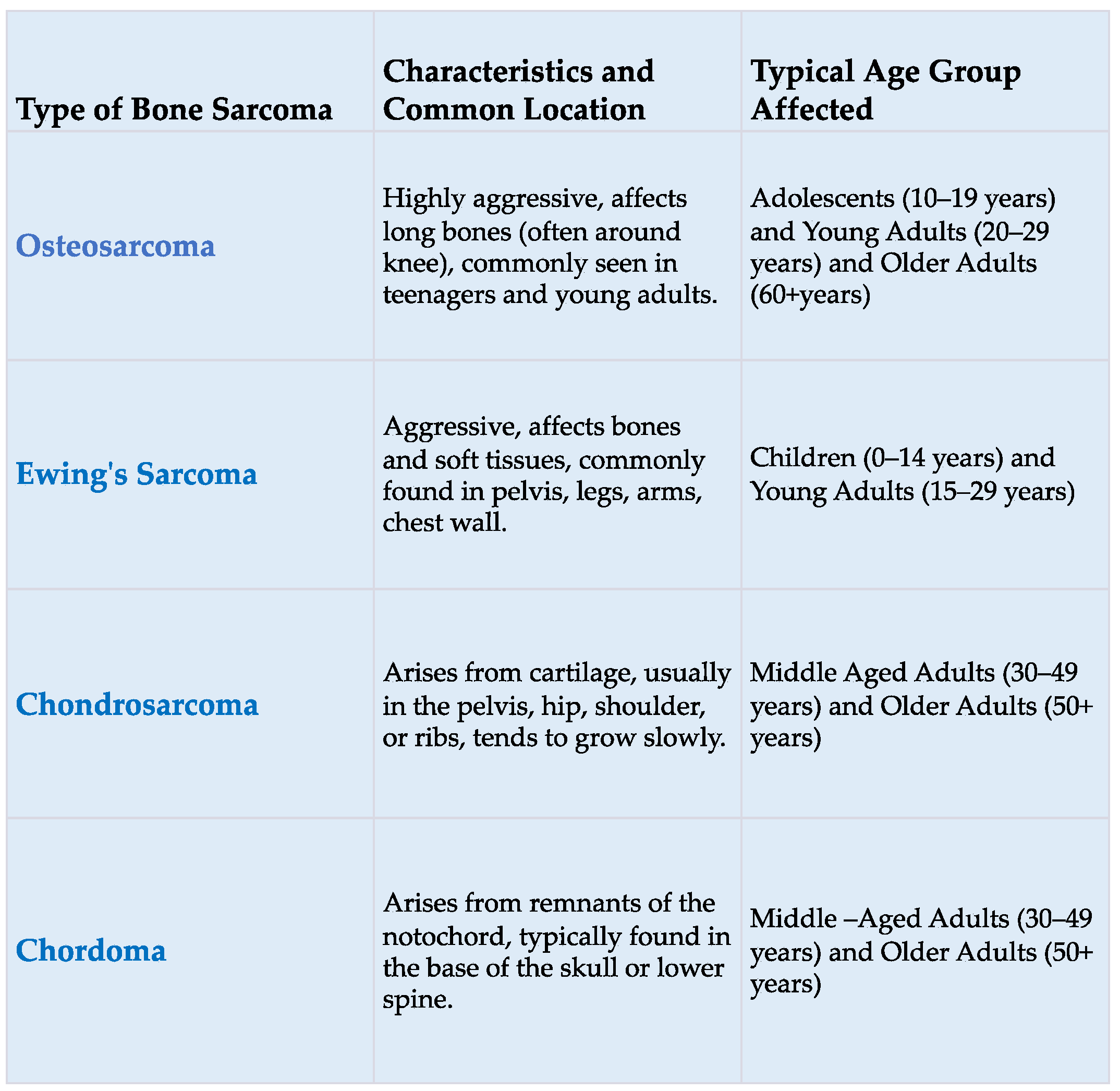
| Criterion | Description |
|---|---|
| I. | Proband with a tumor belonging to the LFS tumor spectrum (e.g., soft tissue sarcoma, osteosarcoma, brain tumor, premenopausal breast cancer, adrenocortical carcinoma, leukemia, and lung bronchoalveolar cancer) before the age of 46 years and at least one first- or second-degree relative with an LFS tumor (except breast cancer if the proband has breast cancer) before the age of 56 years or with multiple tumors. |
| II. | Proband with multiple tumors (except multiple breast tumors), two of which belong to the LFS tumor spectrum, and the first of which occurred before the age of 46 years. |
| III. | Patient with adrenocortical carcinoma or choroid plexus tumor, irrespective of family history. |
| Abbreviations | LFS, Li–Fraumeni syndrome |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cosci, I.; Del Fiore, P.; Mocellin, S.; Ferlin, A. Gender Differences in Soft Tissue and Bone Sarcoma: A Narrative Review. Cancers 2024, 16, 201. https://doi.org/10.3390/cancers16010201
Cosci I, Del Fiore P, Mocellin S, Ferlin A. Gender Differences in Soft Tissue and Bone Sarcoma: A Narrative Review. Cancers. 2024; 16(1):201. https://doi.org/10.3390/cancers16010201
Chicago/Turabian StyleCosci, Ilaria, Paolo Del Fiore, Simone Mocellin, and Alberto Ferlin. 2024. "Gender Differences in Soft Tissue and Bone Sarcoma: A Narrative Review" Cancers 16, no. 1: 201. https://doi.org/10.3390/cancers16010201
APA StyleCosci, I., Del Fiore, P., Mocellin, S., & Ferlin, A. (2024). Gender Differences in Soft Tissue and Bone Sarcoma: A Narrative Review. Cancers, 16(1), 201. https://doi.org/10.3390/cancers16010201







