Short-Term Outcomes after D2 Gastrectomy with Complete Mesogastric Excision in Patients with Locally Advanced Gastric Cancer: A Systematic Review and Meta-Analysis of High-Quality Studies
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion Criteria
- -
- Population (P): patients suffering from non-metastatic gastric cancer;
- -
- Intervention (I): D2 gastrectomy with complete mesogastric excision;
- -
- Comparison (C): conventional D2 gastrectomy;
- -
- Outcomes (O): intraoperative and short-term postoperative outcomes (2.6 Primary and secondary outcomes);
- -
- Study design (S): all study designs.
2.2. Exclusion Criteria
- (1)
- Studies including patients suffering from gastric neoplasms different from adenocarcinoma;
- (2)
- Studies including patients suffering from esophagogastric junction without separate outcome data;
- (3)
- Studies reporting overlapping series;
- (4)
- Case reports, editorials, abstracts, unpublished studies, book chapters, and commentaries;
- (5)
- Previously published reviews.
2.3. Systematic Review Process and Data Extraction
2.4. Assessment of the Risk of Bias
2.5. Primary and Secondary Outcomes
2.6. Statistical Analysis
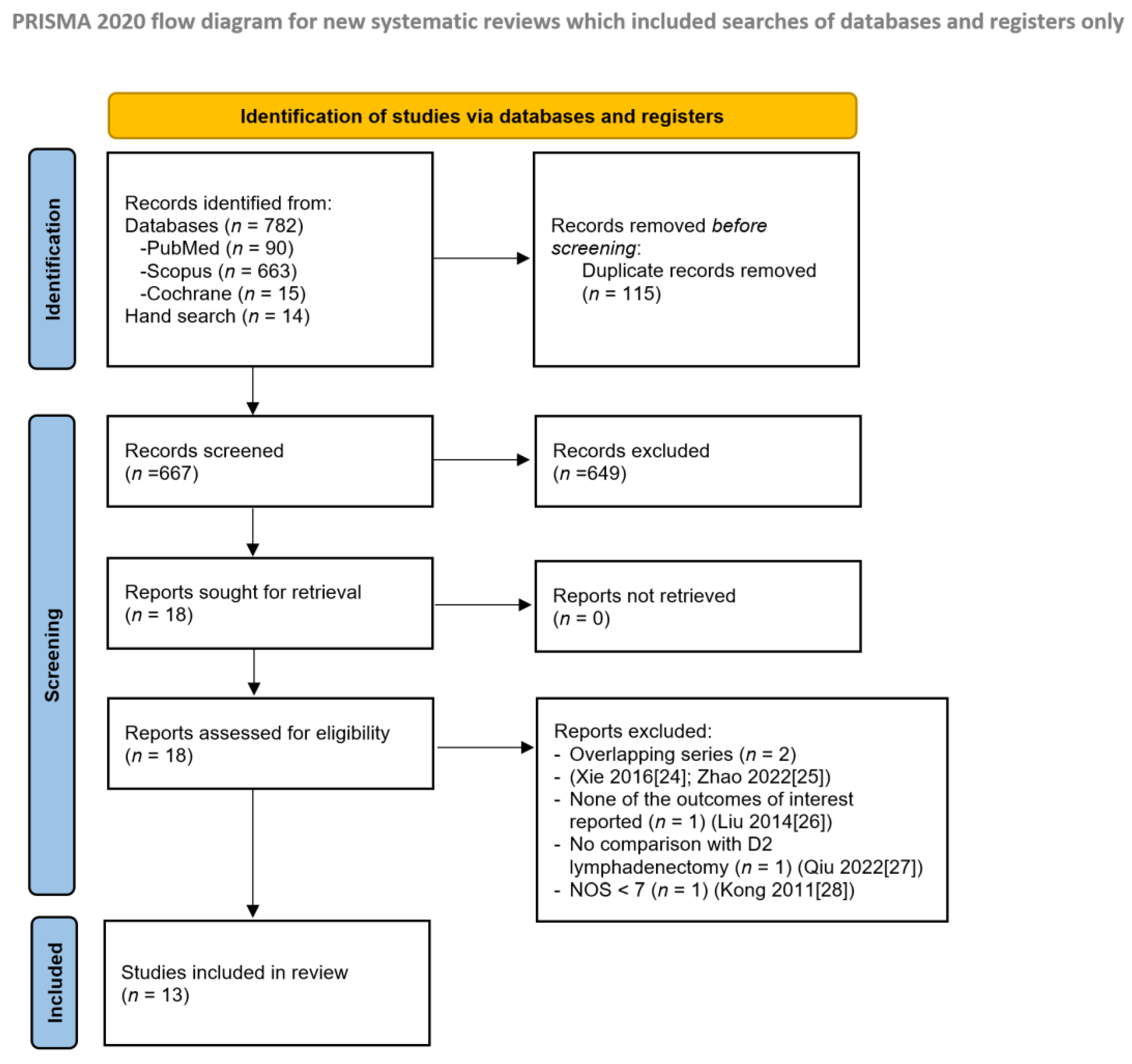
3. Results
3.1. Descriptive Noncomparative Analysis of Included Studies
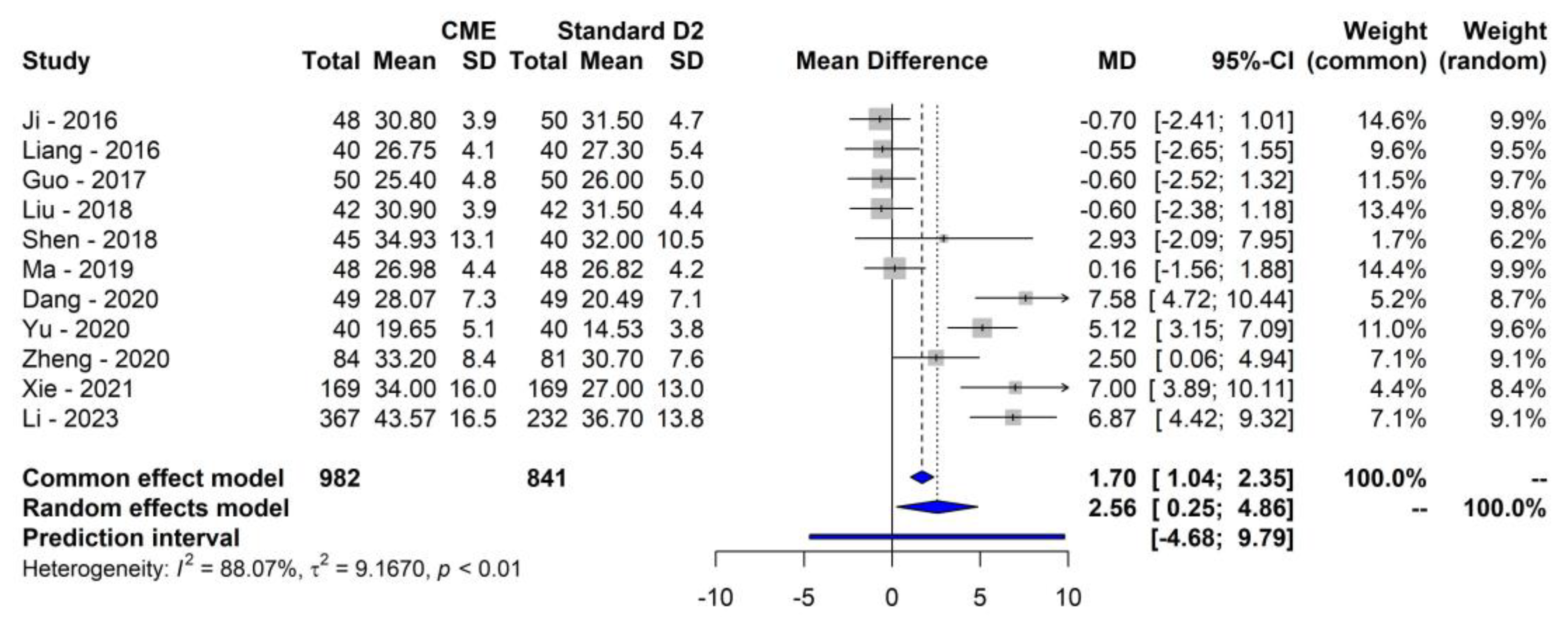
3.2. Primary Outcome
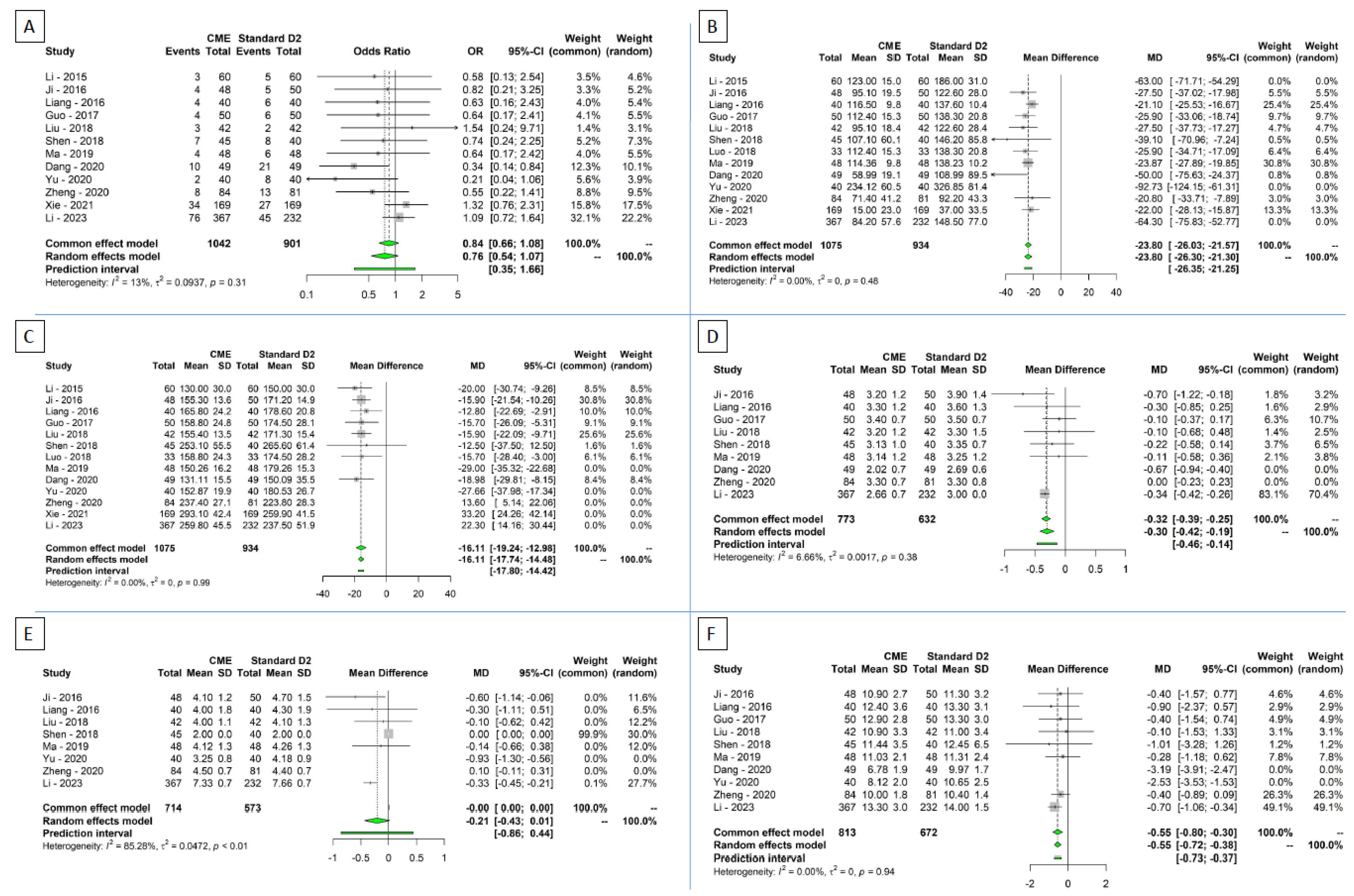
3.3. Secondary Outcomes
3.4. Sensitivity Analysis
3.5. Subgroup Analysis and Metaregression of Primary Endpoint
3.6. Quality of the Studies and Risk of Bias Assessment
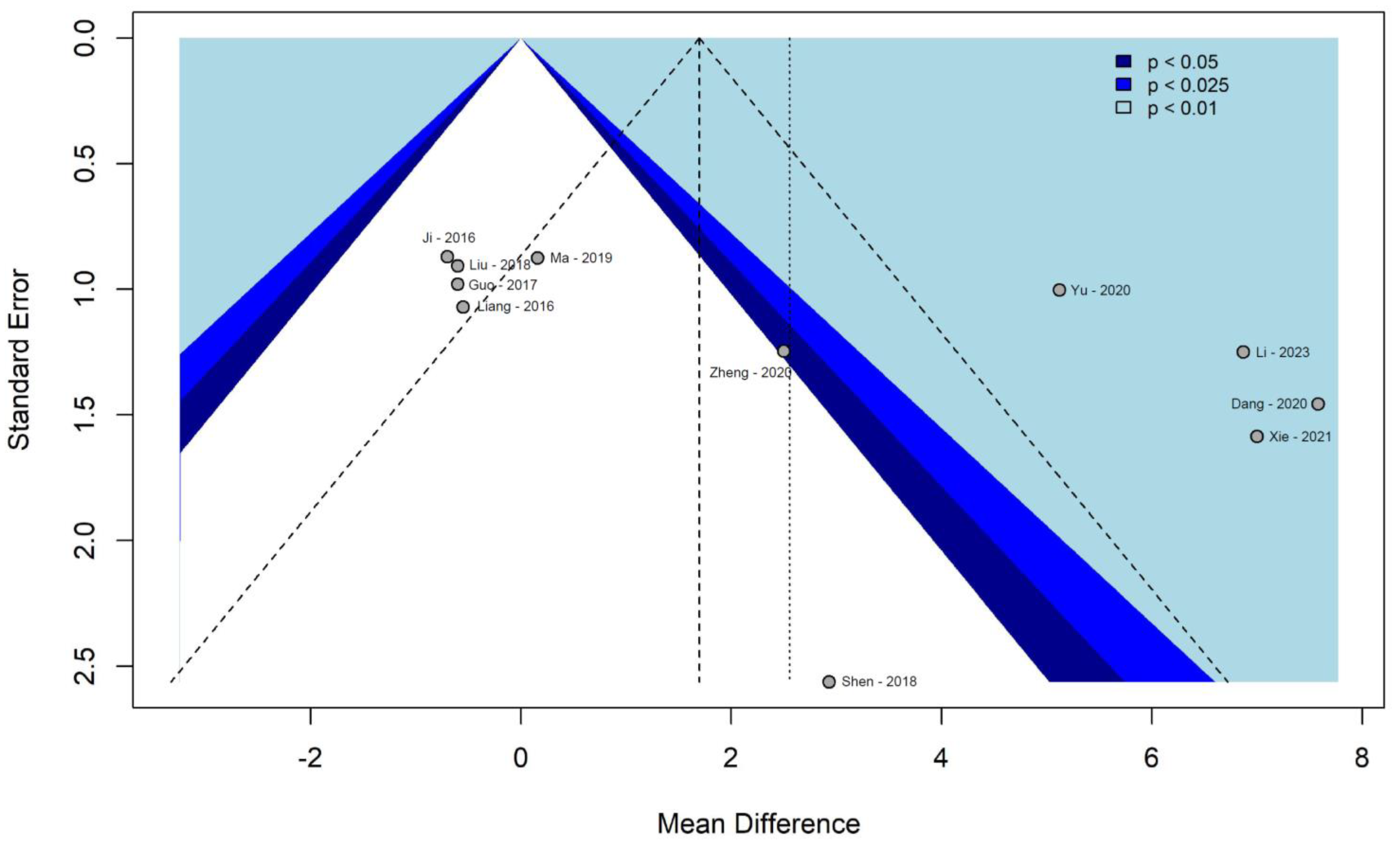
3.7. Assessment of Publication Bias
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Ajani, J.A.; D’Amico, T.A.; Bentrem, D.J.; Chao, J.; Cooke, D.; Corvera, C.; Das, P.; Enzinger, P.C.; Enzler, T.; Fanta, P.; et al. Gastric Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 167–192. [Google Scholar] [CrossRef] [PubMed]
- Jiao, X.; Wang, Y.; Wang, F.; Wang, X. Recurrence Pattern and Its Predictors for Advanced Gastric Cancer after Total Gastrectomy. Medicine 2020, 99, 23795. [Google Scholar] [CrossRef] [PubMed]
- Davis, N.M.; Kurpios, N.A.; Sun, X.; Gros, J.; Martin, J.F.; Tabin, C.J. The Chirality of Gut Rotation Derives from Left-Right Asymmetric Changes in the Architecture of the Dorsal Mesentery. Dev. Cell 2008, 15, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Osaiweran, H.; Liu, L.; Wang, X.; Yu, C.; Tong, Y.; Hu, J.; Gong, J. Mesogastrium: A Fifth Route of Metastasis in Gastric Cancer? Med. Hypotheses 2013, 80, 498–500. [Google Scholar] [CrossRef] [PubMed]
- Borghi, F.; Gattolin, A.; Bogliatto, F.; Garavoglia, M.; Levi, A.C. Relationships between Gastric Development and Anatomic Bases of Radical Surgery for Cancer. World J. Surg. 2002, 26, 1139–1144. [Google Scholar] [CrossRef] [PubMed]
- Stecco, C.; Sfriso, M.M.; Porzionato, A.; Rambaldo, A.; Albertin, G.; Macchi, V.; De Caro, R. Microscopic Anatomy of the Visceral Fasciae. J. Anat. 2017, 231, 121–128. [Google Scholar] [CrossRef]
- Heald, R.J. The “Holy Plane” of Rectal Surgery. J. R. Soc. Med. 1988, 81, 1003–1009. [Google Scholar] [CrossRef]
- Santoro, R.; Ettorre, G.M.; Santoro, E. Subtotal Gastrectomy for Gastric Cancer. World J. Gastroenterol. 2014, 20, 13667. [Google Scholar] [CrossRef]
- Giacopuzzi, S.; Bencivenga, M.; Cipollari, C.; Weindelmayer, J.; De Manzoni, G. Lymphadenectomy: How to Do It? Transl. Gastroenterol. Hepatol. 2017, 1–4. [Google Scholar] [CrossRef]
- Ju, M.R.; Wang, S.C.; Zeh, H.J.; Porembka, M.R. Minimally Invasive Gastrectomy for Cancer and Anastomotic Options. J. Surg. Oncol. 2020, 122, 49–60. [Google Scholar] [CrossRef] [PubMed]
- D’Annibale, A.; Pende, V.; Pernazza, G.; Monsellato, I.; Mazzocchi, P.; Lucandri, G.; Morpurgo, E.; Contardo, T.; Sovernigo, G. Full Robotic Gastrectomy with Extended (D2) Lymphadenectomy for Gastric Cancer: Surgical Technique and Preliminary Results. J. Surg. Res. 2011, 166, 881. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, H.; Kurahashi, Y.; Ishida, Y. Gastric Equivalent of the ‘Holy Plane’ to Standardize the Surgical Concept of Stomach Cancer to Mesogastric Excision: Updating Jamieson and Dobson’s Historic Schema. Gastric Cancer 2021, 24, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Gao, C.; Lu, A.; Liu, L.; Yu, C.; Hu, J.; Gong, J. Proximal Segmentation of the Dorsal Mesogastrium Reveals New Anatomical Implications for Laparoscopic Surgery. Sci. Rep. 2015, 5, 16287. [Google Scholar] [CrossRef] [PubMed]
- Kumamoto, T.; Kurahashi, Y.; Niwa, H.; Nakanishi, Y.; Ozawa, R.; Okumura, K.; Ishida, Y.; Shinohara, H. Laparoscopic Suprapancreatic Lymph Node Dissection Using a Systematic Mesogastric Excision Concept for Gastric Cancer. Ann. Surg. Oncol. 2020, 27, 529–531. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Liu, L.; Osaiweran, H.; Yu, C.; Sheng, F.; Gao, C.; Hu, J.; Gong, J. Detection and Characterization of Metastatic Cancer Cells in the Mesogastrium of Gastric Cancer Patients. PLoS ONE 2015, 10, 142970. [Google Scholar] [CrossRef]
- Kumamoto, T.; Kurahashi, Y.; Haruta, S.; Niwa, H.; Nakanishi, Y.; Ozawa, R.; Okumura, K.; Ishida, Y.; Shinohara, H. Laparoscopic Modified Lymphadenectomy in Gastric Cancer Surgery Using Systematic Mesogastric Excision: A Novel Technique Based on a Concept. Langenbecks Arch. Surg. 2019, 404, 369–374. [Google Scholar] [CrossRef]
- Shinohara, H.; Kurahashi, Y.; Haruta, S.; Ishida, Y.; Sasako, M. Universalization of the Operative Strategy by Systematic Mesogastric Excision for Stomach Cancer with That for Total Mesorectal Excision and Complete Mesocolic Excision Colorectal Counterparts. Ann. Gastroenterol. Surg. 2018, 2, 28–36. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Altman, D.; Antes, G.; Atkins, D.; Barbour, V.; Barrowman, N.; Berlin, J.A.; et al. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 151, 264–269. [Google Scholar]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A Critical Appraisal Tool for Systematic Reviews That Include Randomised or Non-Randomised Studies of Healthcare Interventions, or Both. BMJ 2017, 358, 1–9. [Google Scholar] [CrossRef]
- Japanese Gastric Cancer Association. Japanese Gastric Cancer Treatment Guidelines 2018. Gastric. Cancer 2021, 24, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality If Nonrandomized Studies in Meta-Analyses. 2012. Available online: http://www.Ohri.Ca/Programs/Clin._Epidemiol./Oxf.Asp (accessed on 9 November 2023). [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A Web and Mobile App for Systematic Reviews. Syst. Rev. 2016, 5, 210. Available online: https://www.rayyan.ai/ (accessed on 9 November 2023). [CrossRef] [PubMed]
- Xie, D.; Yu, C.; Liu, L.; Osaiweran, H.; Gao, C.; Hu, J.; Gong, J. Short-Term Outcomes of Laparoscopic D2 Lymphadenectomy with Complete Mesogastrium Excision for Advanced Gastric Cancer. Surg. Endosc. 2016, 30, 4847. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Deng, J.; Cao, B.; Shen, J.; Liu, L.; Xiao, A.; Yin, P.; Xie, D.; Gong, J. Short-Term Outcomes of D2 Lymphadenectomy plus Complete Mesogastric Excision for Gastric Cancer: A Propensity Score Matching Analysis. Surg. Endosc. 2022, 36, 5921–5929. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.L. The Application Value of the Complete Mesogastrium Excision in the Radical Surgery of Gastric Cancer. Mod. Diagn. Treat. 2014, 10, 809–810. [Google Scholar]
- Qiu, X.T.; Zheng, C.Y.; Liang, Y.L.; Zheng, L.Z.; Zu, B.; Chen, H.H.; Dong, Z.Y.; Zhu, L.M.; Lin, W. Totally Laparoscopic Total Gastrectomy Using the “Enjoyable Space” Approach Coupled with Self-Pulling and Latter Transection Reconstruction versus Laparoscopic-Assisted Total Gastrectomy for Upper Gastric Cancer: Short-Term Outcomes. Wideochirurgia I Inne Tech. Maloinwazyjne 2022, 17, 113568. [Google Scholar] [CrossRef]
- Kong, Y.; Hua, Y.-W.; Zhang, Z.-D. D2 Gastric Resection along the Mesangial Compartment Excision of Clinical Research. Chin. J. Pract. Surg. 2011, 9–10. [Google Scholar]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J. (Eds.) Chapter 8: Assessing Risk of Bias in a Randomized Trial. Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (Updated September 2020); Wiley-Blackwell: Hoboken, NJ, USA, 2020. [Google Scholar]
- Sterne, J.A.C.; Hernán, M.A.; McAleenan, A.; Reeves, B.C.; Higgins, J.P.T. Assessing Risk of Bias in a Non-Randomized Study. Cochrane Handb. Syst. Rev. Interv. 2019, 2, 621–641. [Google Scholar] [CrossRef]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the Sample Mean and Standard Deviation from the Sample Size, Median, Range and/or Interquartile Range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring Inconsistency in Meta-Analyses. Br. Med. J. 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Team, R.C. R: A Language and Environment for Statistical Computing; Team, R.C.: Vienna, Austria, 2019. [Google Scholar]
- Schwarzer, G.; Carpenter, J.R.; Rücker, G. An Introduction to Meta-Analysis in R. In Meta-Analysis with R. Use R! Springer: Cham, Switzerland, 2015; pp. 3–17. [Google Scholar]
- Viechtbauer, W. Conducting Meta-Analyses in R with the Metafor. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef]
- McGrath, S.; Sohn, H.; Steele, R.; Benedetti, A. Meta-Analysis of the Difference of Medians. Biom. J. 2020, 62, 69–98. [Google Scholar] [CrossRef] [PubMed]
- Harrer, M.; Cuijpers, P.; Furukawa, T.A.; Ebert, D.D. Doing Meta-Analysis in R: A Hands-on Guide. Chapman and Hall/CRC: Boca Raton, FL, USA, 2021. [Google Scholar]
- Li, B.; Wang, G.Z.; Zhou, L. The Application of Complete Mesogastrium Excision in Laparoscopic Distal Gastrectomy for Gastric Cancer. Chin. J. Mod. Drug Appl. 2015, 9, 41–43. [Google Scholar] [CrossRef]
- Ji, F.; Fang, X.; Jiang, J.; Wu, Y.; Feng, Y.; Guo, H. Application of En-Bloc Mesogastric Excision in the Treatment of Advanced Gastric Cancer. Chin. J. Gastrointest. Surg. 2016, 19, 1097–1100. [Google Scholar] [CrossRef]
- Liang, Y.; Xu, Y.X.; Song, H.; Xu, T.; Fan Rui, Z.; Cao, M.; Xu, W. A Comparative Study of Complete Mesogastrium Excision and Standard D2 Radical Resection in the Treatment of Advanced Gastric Cancer. Mod. Dig. Interv. 2016, 21, 875–877. [Google Scholar] [CrossRef]
- Guo, Y.F.; Zhang, H.W.; Li, Y.L.; Qiao, W.; Luo, K.L.; Han, B. Comparative Study of Total Mesentery Excision and Traditional Radical Gastrectomy for Proximal Gastric Cancer. J. Laparosc. Surg. 2017, 22, 730–733. [Google Scholar] [CrossRef]
- Liu, A. Clinical Value of Total Mesenterectomy in the Treatment of Advanced Gastric Cancer. Chin. Foreign Med. Res. 2018, 16, 14–15. [Google Scholar] [CrossRef]
- Shen, J.; Dong, X.; Liu, Z.; Wang, G.; Yang, J.; Zhou, F.; Lu, M.; Ma, X.; Li, Y.; Tang, C.; et al. Modularized Laparoscopic Regional En Bloc Mesogastrium Excision (REME) Based on Membrane Anatomy for Distal Gastric Cancer. Surg. Endosc. 2018, 32, 4698–4705. [Google Scholar] [CrossRef]
- Luo, X.-Z. Clinical Comparative Study of Two Operative Regimens for the Treatment of Advanced Middle and Upper Gastric Carcinoma. Smart Healthc. 2018, 4, 135–136. [Google Scholar] [CrossRef]
- Ma, W. Comparison of the Clinical Effect of Complete Mesogastrium Excision and Standard D2 Radical Resection for Total Gastrectomy in the Treatment of Advanced Gastric Cancer. Med. J. Chin. People’s Health 2019, 31, 65–67. [Google Scholar] [CrossRef]
- Dang, P.; Zhang, L.; Zhang, W.; Zhu, Y.; Chen, W.; Wu, G.; Sun, P. Laparoscopic D2 Radical Gastrectomy Combined with Complete Mesocolic Excision for Advanced Gastric Cancer. Chin. J. Pract. Diagn. Treat. 2020, 34, 20–23. [Google Scholar] [CrossRef]
- Yu, D. Clinical Efficacy of Enbloc Mesogastric Excision and Standard D2 Radical Prostatectomy in Patients with Advanced Gastric Cancer. Contemp. Med. 2020, 26, 3–6. [Google Scholar] [CrossRef]
- Zheng, C.-Y.; Dong, Z.-Y.; Zheng, L.-Z.; Qiu, X.-T.; Zu, B.; Xu, R.; Lin, W. Laparoscopic D2 plus Complete Mesogastrium Excision Using the “Enjoyable Space” Approach versus Conventional D2 Total Gastrectomy for Local Advanced Gastric Cancer: Short-Term Outcomes. Videosurgery Other Miniinvasive Tech. 2020, 15, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Shen, J.; Liu, L.; Cao, B.; Wang, Y.; Qin, J.; Wu, J.; Yan, Q.; Hu, Y.; Yang, C.; et al. Complete Mesogastric Excision for Locally Advanced Gastric Cancer: Short-Term Outcomes of a Randomized Clinical Trial. Cell Rep. Med. 2021, 2, 100217. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wu, H.; Lin, H.; Li, J.; Guo, Z.; Pan, G.; Guo, Y.; Zheng, P.; Cai, Z.; Ren, J.; et al. The Short- and Long-Term Effect of Membrane Anatomy-Guided Laparoscopic D2 Lymphadenectomy plus Regional Complete Mesogastrium Excision for Locally Advanced Gastric Cancer. Surg. Endosc. 2023, 37, 4990–5003. [Google Scholar] [CrossRef] [PubMed]
- Dickson, J.L.; Cunningham, D. Systemic Treatment of Gastric Cancer. Eur. J. Gastroenterol. Hepatol. 2004, 16, 255–263. [Google Scholar] [CrossRef]
- Hohenberger, W.; Weber, K.; Matzel, K.; Papadopoulos, T.; Merkel, S. Standardized Surgery for Colonic Cancer: Complete Mesocolic Excision and Central Ligation—Technical Notes and Outcome. Color. Dis. 2009, 11, 1735. [Google Scholar] [CrossRef]
- Bunt, A.M.; Hermans, J.; van de Velde, C.J.; Sasako, M.; Hoefsloot, F.A.; Fleuren, G.; Bruijn, J.A. Lymph Node Retrieval in a Randomized Trial on Western-Type versus Japanese-Type Surgery in Gastric Cancer. J. Clin. Oncol. 1996, 14, 2289. [Google Scholar] [CrossRef]
- Meng, X.; Wang, L.; Liu, G.; Zhang, J.; Wang, Y.; Yang, D.; Zheng, G.; Zhang, T.; Zheng, Z.; Zhao, Y. D2 Lymphadenectomy with Complete Mesogastrium Excision vs. Conventional D2 Gastrectomy for Advanced Gastric Cancer. Chin. Med. J. 2022, 135, 1223–1230. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions, and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content. |

| Study | Years of Enrollment | Study Design | Risk of Bias | RoB Tool Used | Total Pts | CME | Standard D2 | Age D2 | Age CME | Male Pts | Female Pts | Surgical Approach: Open (O) vs. Minimally Invasive (MI) | CME Type of Gastrectomy | D2 Type of Gastrectomy | TNM Stage CME | TNM Stage D2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Li—2015 [38] | 2006–2011 | Retrospective—random selection | Moderate | RoB 2 | 120 | 60 | 60 | 52.4 | 51.7 | 67 | 53 | 100% MI | Distal Gastrectomy 100% | Distal Gastrectomy 100% | ||
| Ji—2016 [39] | 2013–2015 | Retrospective | Serious | ROBINS-I | 98 | 48 | 50 | 55 | 43 | (not specified) | Total Gastrectomy 100% | Total Gastrectomy 100% | ||||
| Liang—2016 [40] | 2010–2013 | Retrospective—random selection | Low | RoB 2 | 80 | 40 | 40 | 55.5 | 55.2 | 46 | 34 | (not specified) | Total Gastrectomy 17,5%; Distal Gastrectomy 82,5% | Total Gastrectomy 22,5%; Distal Gastrectomy 77,5% | II 65%; III 35% | II 70%; III 30% |
| Guo—2017 [41] | 2011–2014 | Retrospective | Moderate | RoB 2 | 100 | 50 | 50 | 57.3 | 57.5 | 56 | 44 | 100% MI | Total Gastrectomy 100% | Total Gastrectomy 100% | II 58%; III 42% | II 64%; III 36% |
| Liu—2018 [42] | 2016–2017 | Retrospective—random selection | Moderate | ROBINS-I | 84 | 42 | 42 | 54.6 | 54.3 | 42 | 42 | (not specified) | Total Gastrectomy 100% | Total Gastrectomy 100% | ||
| Shen—2018 [43] | 2014–2017 | Retrospective | Serious | ROBINS-I | 85 | 45 | 40 | 63.2 | 62 | 54 | 31 | 100% MI | Distal gastrectomy 100% | Distal gastrectomy 100% | I 42.22% II 31.11% III 26.67% | I 47.5% II 12.5% III 40% |
| Luo—2018 [44] | 2013–2015 | Retrospective—random selection | Serious | ROBINS-I | 66 | 33 | 33 | 57.4 | 57.3 | 41 | 25 | 100% MI for D2 type CME not specified | Total Gastrectomy 100% | Total Gastrectomy 100% | ||
| Ma—2019 [45] | 2014–2016 | Retrospective—random selection | Low | RoB 2 | 96 | 48 | 48 | 61.6 | 61.5 | 54 | 42 | (not specified) | Total Gastrectomy 100% | Total Gastrectomy 100% | I 43.7%; II 29.2%; II 27.1% | I 41.7%; II 33.3%; II 25% |
| Dang—2020 [46] | 2018–2019 | Retrospective—random selection | Low | RoB 2 | 98 | 49 | 49 | 51.4 | 49,8 | 70 | 28 | 100% MI | (not specified) | (not specified) | II 48.9%; III 51.1% | II: 44.9%; III: 55.1% |
| Yu—2020 [47] | 2012–2017 | Retrospective—random selection | Low | RoB 2 | 80 | 40 | 40 | 49.12 | 49.67 | 45 | 35 | 100% O | Total Gastrectomy 100% | Total Gastrectomy 100% | ||
| Zheng—2020 [48] | 2015–2017 | Retrospective | Serious | ROBINS-I | 165 | 84 | 81 | 63.0 | 63.1 | 137 | 28 | 100% MI | Total Gastrectomy 100% | Total Gastrectomy 100% | IB 10.71% IIA 30.95% IIB 15.47% IIIA 21.43% IIIB 15.47% IIIC 5.95% | IB 9.88% IIA 28.4% IIB 19.75 % IIIA 18.5% 18.5% 4.94% |
| Xie—2021 [49] | 2014–2018 | RCT | Low | RoB 2 | 338 | 169 | 169 | 54.5 | 54.8 | 213 | 125 | 100% MI | Distal gastrectomy 100% | Distal gastrectomy 100% | IB 20.7% IIA 29.6% IIB 15.4% IIIA 14.2% IIIB 18.9% IIIC 1.2% | IB 18.3% IIA 23.1% IIB 13.6% IIIA 20.1% IIIB 20.7% IIIC 4.2% |
| Li—2023 [50] | 2014–2019 | Retrospective | Moderate | ROBINS-I | 599 | 367 | 232 | 65 | 63.7 | 434 | 165 | 100% MI | 80.93% total gastrectomy; 18.8% distal gastrectomy; 0,.7% proximal gastrectomy | 78.88% total gastrectomy; 19.4% distal gastrectomy; 1.72% proximal gastrectomy | I 10.89% II 36.78% III 52.32% | I 13.36% II 34.1% III 52.59% |
| Subgroup Analysis | ||||||||
| Variable | Number of Studies | MD | 95% CI | I2 (%) | p (Q Test—between Groups Differences) | |||
| Lower | Upper | |||||||
| Study design | 0.04 | |||||||
| Retrospective | 5 | 2.07 | −1.91 | 6.04 | 86.76 | |||
| Retrospective—random selection | 5 | 2.23 | −2.38 | 6.84 | 90.42 | |||
| RCT | 1 | 7.00 | 3.89 | 10.11 | - | |||
| Surgical approach | 0.65 | |||||||
| Open | 1 | 5.12 | 3.15 | 7.08 | - | |||
| Minimally invasive | 6 | 4.35 | 0.83 | 7.88 | 86.87 | |||
| Type of gastrectomy (total gastrectomy) | 0.039 | |||||||
| Distal | 2 | 5.87 | 3.23 | 8.51 | 45.16 | |||
| Total | 6 | 0.92 | −1.54 | 3.39 | 82.33 | |||
| Risk of bias | 0.458 | |||||||
| Low | 5 | 3.73 | −1.01 | 8.47 | 90.47 | |||
| Moderate | 3 | 1.82 | −8.82 | 12.45 | 92.81 | |||
| Serious | 3 | 1.14 | −3.93 | 6.22 | 62.75 | |||
| Meta Regression Analysis | ||||||||
| Variable | Number of Studies | Estimate | 95% CI | p | I2 (%) | p(Q Test) | R2 (%) | |
| Lower | Upper | |||||||
| Year of publication | 11 | 1.31 | 0.69 | 1.93 | 0.001 | 62.89 | 0.0039 | 76.87 |
| Years of enrollment | 11 | 1.08 | −0.53 | 2.71 | 0.16 | 84.54 | <0.0001 | 24.85 |
| Total number of patients | 11 | 0.012 | −0.0007 | 0.025 | 0.062 | 83.63 | <0.0001 | 32.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Granieri, S.; Sileo, A.; Altomare, M.; Frassini, S.; Gjoni, E.; Germini, A.; Bonomi, A.; Akimoto, E.; Wong, C.L.; Cotsoglou, C. Short-Term Outcomes after D2 Gastrectomy with Complete Mesogastric Excision in Patients with Locally Advanced Gastric Cancer: A Systematic Review and Meta-Analysis of High-Quality Studies. Cancers 2024, 16, 199. https://doi.org/10.3390/cancers16010199
Granieri S, Sileo A, Altomare M, Frassini S, Gjoni E, Germini A, Bonomi A, Akimoto E, Wong CL, Cotsoglou C. Short-Term Outcomes after D2 Gastrectomy with Complete Mesogastric Excision in Patients with Locally Advanced Gastric Cancer: A Systematic Review and Meta-Analysis of High-Quality Studies. Cancers. 2024; 16(1):199. https://doi.org/10.3390/cancers16010199
Chicago/Turabian StyleGranieri, Stefano, Annaclara Sileo, Michele Altomare, Simone Frassini, Elson Gjoni, Alessandro Germini, Alessandro Bonomi, Eigo Akimoto, Chun Lam Wong, and Christian Cotsoglou. 2024. "Short-Term Outcomes after D2 Gastrectomy with Complete Mesogastric Excision in Patients with Locally Advanced Gastric Cancer: A Systematic Review and Meta-Analysis of High-Quality Studies" Cancers 16, no. 1: 199. https://doi.org/10.3390/cancers16010199
APA StyleGranieri, S., Sileo, A., Altomare, M., Frassini, S., Gjoni, E., Germini, A., Bonomi, A., Akimoto, E., Wong, C. L., & Cotsoglou, C. (2024). Short-Term Outcomes after D2 Gastrectomy with Complete Mesogastric Excision in Patients with Locally Advanced Gastric Cancer: A Systematic Review and Meta-Analysis of High-Quality Studies. Cancers, 16(1), 199. https://doi.org/10.3390/cancers16010199