The Role of Non-Peripancreatic Lymph Nodes in the Survival of Patients Suffering from Pancreatic Cancer of the Body and Tail: A Systematic Review and Meta-Analysis of High-Quality Studies
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion Criteria
- -
- Population (P): patients suffering from pancreatic ductal adenocarcinoma undergoing distal pancreatosplenectomy with lymphadenectomy;
- -
- Intervention (I): lymphadenectomy including non-PLN stations according to the ISGPS (stations 10–11–18);
- -
- Comparison I: lymphadenectomy involving only PLN stations according to the ISGPS (stations 10–11–18); this criterion was not mandatory;
- -
- Outcomes (O): overall mortality;
- -
- Study design (S): all study designs.
- -
- Studies with insufficient reporting of the PICOS criteria were excluded.
2.3. Exclusion Criteria and Quality Assessment of Included Studies
- (1)
- Studies also including patients suffering from PDAC of the head without reporting separate data;
- (2)
- Studies reporting overlapping series;
- (3)
- Studies with a less than 3-year follow-up period;
- (4)
- Non-English language papers;
- (5)
- Case reports, editorials, abstracts, unpublished studies, book chapters, and commentaries;
- (6)
- Previously published reviews.
2.4. Systematic Review Process and Data Extraction
2.5. Assessment of Risk of Bias
2.6. Endpoints
2.7. Statistical Analysis
3. Results
3.1. Descriptive Noncomparative Analysis of Included Studies and Primary Endpoint
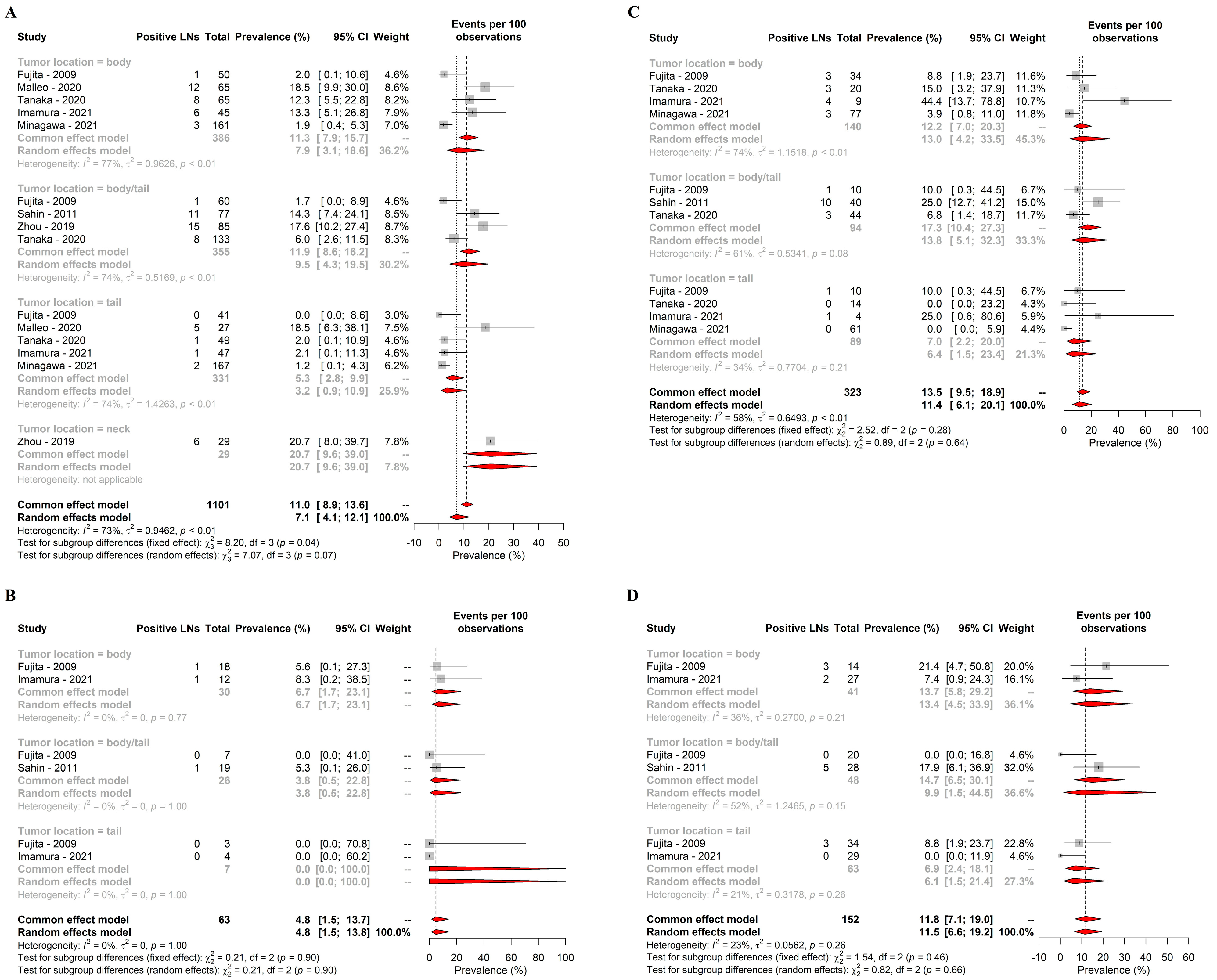
3.2. Primary Endpoint
3.3. Secondary Endpoint
3.4. Quality of the Studies and Risk of Bias Assessment
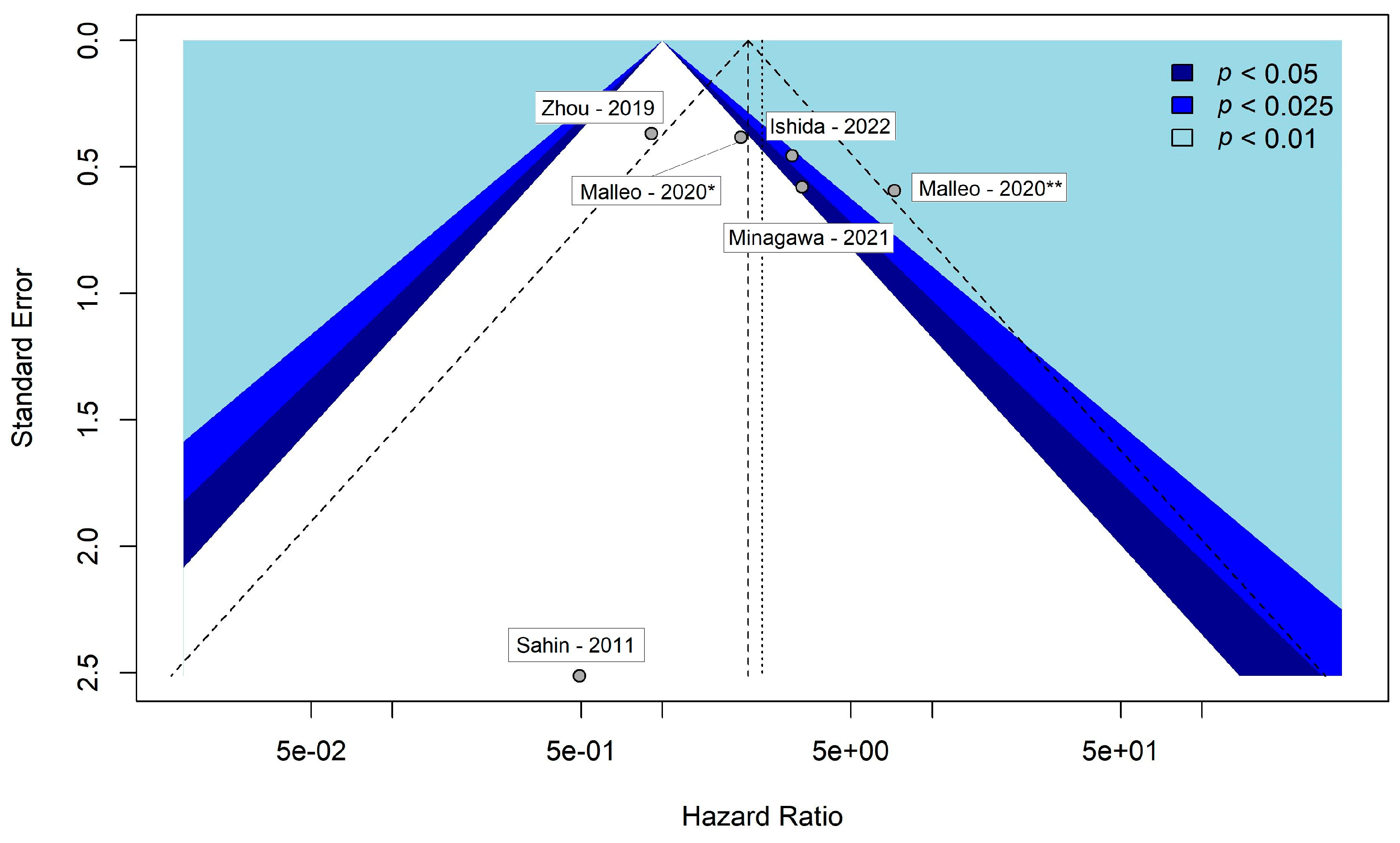
3.5. Assessment of Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Lau, M.K.; Davila, J.A.; Shaib, Y.H. Incidence and Survival of Pancreatic Head and Body and Tail Cancers: A Population-Based Study in the United States. Pancreas 2010, 39, 458–462. [Google Scholar] [CrossRef]
- Elshaer, M.; Gravante, G.; Kosmin, M.; Riaz, A.; Al-Bahrani, A. A Systematic Review of the Prognostic Value of Lymph Node Ratio, Number of Positive Nodes and Total Nodes Examined in Pancreatic Ductal Adenocarcinoma. Ann. R. Coll. Surg. Engl. 2017, 99, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Strobel, O.; Hinz, U.; Gluth, A.; Hank, T.; Hackert, T.; Bergmann, F.; Werner, J.; Büchler, M.W. Pancreatic Adenocarcinoma: Number of Positive Nodes Allows to Distinguish Several N Categories. Ann. Surg. 2015, 261, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Pedrazzoli, S.; DiCarlo, V.; Dionigi, R.; Mosca, F.; Pederzoli, P.; Pasquali, C.; Klöppel, G.; Dhaene, K.; Michelassi, F. Standard versus Extended Lymphadenectomy Associated with Pancreatoduodenectomy in the Surgical Treatment of Adenocarcinoma of the Head of the Pancreas: A Multicenter, Prospective, Randomized Study. Ann. Surg. 1998, 228, 508. [Google Scholar] [CrossRef] [PubMed]
- Yeo, C.J.; Cameron, J.L.; Sohn, T.A.; Coleman, J.A.; Sauter, P.K.; Hruban, R.H.; Pitt, H.A.; Lillemoe, K.D. Pancreaticoduodenectomy with or without Extended Retroperitoneal Lymphadenectomy for Periampullary Adenocarcinoma: Comparison of Morbidity and Mortality and Short-Term Outcome. Ann. Surg. 1999, 229, 613. [Google Scholar] [CrossRef]
- Yeo, C.J.; Cameron, J.L.; Lillemoe, K.D.; Sohn, T.A.; Campbell, K.A.; Sauter, P.K.; Coleman, J.A.; Abrams, R.A.; Hruban, R.H. Pancreaticoduodenectomy with or without Distal Gastrectomy and Extended Retroperitoneal Lymphadenectomy for Periampullary Adenocarcinoma, Part 2: Randomized Controlled Trial Evaluating Survival, Morbidity, and Mortality. Ann. Surg. 2002, 236, 355. [Google Scholar] [CrossRef] [PubMed]
- Farnell, M.B.; Pearson, R.K.; Sarr, M.G.; DiMagno, E.P.; Burgart, L.J.; Dahl, T.R.; Foster, N.; Sargent, D.J. A Prospective Randomized Trial Comparing Standard Pancreatoduodenectomy with Pancreatoduodenectomy with Extended Lymphadenectomy in Resectable Pancreatic Head Adenocarcinoma. Surgery 2005, 138, 618–630. [Google Scholar] [CrossRef] [PubMed]
- Nimura, Y.; Nagino, M.; Takao, S.; Takada, T.; Miyazaki, K.; Kawarada, Y.; Miyagawa, S.; Yamaguchi, A.; Ishiyama, S.; Takeda, Y.; et al. Standard versus Extended Lymphadenectomy in Radical Pancreatoduodenectomy for Ductal Adenocarcinoma of the Head of the Pancreas: Long-Term Results of a Japanese Multicenter Randomized Controlled Trial. J. Hepatobiliary Pancreat. Sci. 2012, 19, 230–241. [Google Scholar] [CrossRef]
- Tol, J.A.M.G.; Gouma, D.J.; Bassi, C.; Dervenis, C.; Montorsi, M.; Adham, M.; Andrén-Sandberg, A.; Asbun, H.J.; Bockhorn, M.; Büchler, M.W.; et al. Definition of a Standard Lymphadenectomy in Surgery for Pancreatic Ductal Adenocarcinoma: A Consensus Statement by the International Study Group on Pancreatic Surgery (ISGPS). Surgery 2014, 156, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Tempero, M.A.; Malafa, M.P.; Al-Hawary, M.; Behrman, S.W.; Benson, A.B.; Cardin, D.B.; Chiorean, E.G.; Chung, V.; Czito, B.; Del Chiaro, M.; et al. Pancreatic Adenocarcinoma, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 439–457. [Google Scholar] [CrossRef] [PubMed]
- Ducreux, M.; Cuhna, A.S.; Caramella, C.; Hollebecque, A.; Burtin, P.; Goéré, D.; Seufferlein, T.; Haustermans, K.; Van Laethem, J.L.; Conroy, T.; et al. Cancer of the Pancreas: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2015, 26 (Suppl. S5), v56–v68. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Han, S.-S.; Kang, C.M.; Kwon, W.; Paik, K.Y.; Song, K.B.; Yang, J.D.; Chung, J.C.; Jeong, C.-Y.; Kim, S.-W. Korean Surgical Practice Guideline for Pancreatic Cancer 2022: A Summary of Evidence-Based Surgical Approaches. Ann. Hepato-Biliary-Pancreat. Surg. 2022, 26, 1–16. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Altman, D.; Antes, G.; Atkins, D.; Barbour, V.; Barrowman, N.; Berlin, J.A.; et al. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A Critical Appraisal Tool for Systematic Reviews That Include Randomised or Non-Randomised Studies of Healthcare Interventions, or Both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality If Nonrandomized Studies in Meta-Analyses. 2012. Available online: http//www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 13 December 2022).
- Sterne, J.A.C.; Hernán, M.A.; McAleenan, A.; Reeves, B.C.; Higgins, J.P.T. Assessing Risk of Bias in a Non-Randomized Study. Cochrane Handb. Syst. Rev. Interv. 2019, 2, 621–641. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Chapter 8: Assessing Risk of Bias in a Randomized Trial. In Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (Updated September 2020); Wiley-Blackwell: Hoboken, NJ, USA, 2020. [Google Scholar]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring Inconsistency in Meta-Analyses. Br. Med. J. 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Tierney, J.F.; Stewart, L.A.; Ghersi, D.; Burdett, S.; Sydes, M.R. Practical Methods for Incorporating Summary Time-to-Event Data into Meta-Analysis. Trials 2007, 8, 16. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Schwarzer, G.; Carpenter, J.R.; Rücker, G. An Introduction to Meta-Analysis in R. In Meta-Analysis with R; Springer: Cham, Switzerland, 2015. [Google Scholar]
- Viechtbauer, W. Conducting Meta-Analyses in R with the Metafor Package. J. Stat. Softw. 2010, 36, 48. [Google Scholar] [CrossRef]
- Harrer, M.; Cuijpers, P.; Furukawa, T.; Ebert, D.D. Doing Meta-Analysis in R: A Hands-On Guide; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar]
- Fujita, T.; Nakagohri, T.; Gotohda, N.; Takahashi, S.; Konishi, M.; Kojima, M.; Kinoshita, T. Evaluation of the Prognostic Factors and Significance of Lymph Node Status in Invasive Ductal Carcinoma of the Body or Tail of the Pancreas. Pancreas 2010, 39, e48–e54. [Google Scholar] [CrossRef]
- Sahin, T.T.; Fujii, T.; Kanda, M.; Nagai, S.; Kodera, Y.; Kanzaki, A.; Yamamura, K.; Sugimoto, H.; Kasuya, H.; Nomoto, S.; et al. Prognostic Implications of Lymph Node Metastases in Carcinoma of the Body and Tail of the Pancreas. Pancreas 2011, 40, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Malleo, G.; Maggino, L.; Nobile, S.; Casciani, F.; Cacciatori, N.; Paiella, S.; Luchini, C.; Rusev, B.; Capelli, P.; Marchegiani, G.; et al. Reappraisal of Nodal Staging and Study of Lymph Node Station Involvement in Distal Pancreatectomy for Body-Tail Pancreatic Ductal Adenocarcinoma. Eur. J. Surg. Oncol. 2020, 46, 1734–1741. [Google Scholar] [CrossRef]
- Tanaka, K.; Nakamura, T.; Asano, T.; Nakanishi, Y.; Noji, T.; Tsuchikawa, T.; Okamura, K.; Shichinohe, T.; Hirano, S. Pancreatic Body and Tail Cancer and Favorable Metastatic Lymph Node Behavior on the Left Edge of the Aorta. Pancreatology 2020, 20, 1451–1457. [Google Scholar] [CrossRef]
- Imamura, T.; Yamamoto, Y.; Sugiura, T.; Okamura, Y.; Ito, T.; Ashida, R.; Ohgi, K.; Uesaka, K. Reconsidering the Optimal Regional Lymph Node Station According to Tumor Location for Pancreatic Cancer. Ann. Surg. Oncol. 2021, 28, 1602–1611. [Google Scholar] [CrossRef] [PubMed]
- Minagawa, T.; Sugiura, T.; Okamura, Y.; Ito, T.; Yamamoto, Y.; Ashida, R.; Ohgi, K.; Sasaki, K.; Uesaka, K. Clinical Implications of Lymphadenectomy for Invasive Ductal Carcinoma of the Body or Tail of the Pancreas. Ann. Gastroenterol. Surg. 2022, 6, 531–542. [Google Scholar] [CrossRef]
- Ishida, H.; Ogura, T.; Takahashi, A.; Miyamoto, R.; Matsudaira, S.; Amikura, K.; Tanabe, M.; Kawashima, Y. Optimal Region of Lymph Node Dissection in Distal Pancreatectomy for Left-Sided Pancreatic Cancer Based on Tumor Location. Ann. Surg. Oncol. 2022, 29, 2414–2424. [Google Scholar] [CrossRef]
- Zhou, Y.; Lin, J.; Wang, W.; Chen, H.; Deng, X.; Peng, C.; Cheng, D.; Shen, B. Should a Standard Lymphadenectomy Include the No. 9 Lymph Nodes for Body and Tail Pancreatic Ductal Adenocarcinoma? Pancreatology 2019, 19, 414–418. [Google Scholar] [CrossRef]
- Hirashita, T.; Iwashita, Y.; Fujinaga, A.; Nakanuma, H.; Masuda, T.; Endo, Y.; Ohta, M.; Inomata, M. Relationship between the Tumor Location and Clinicopathological Features in Left-Sided Pancreatic Ductal Adenocarcinoma. Surg. Today 2021, 51, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Nakao, A.; Harada, A.; Nonami, T.; Kaneko, T.; Nomoto, S.; Koyama, H.; Kanazumi, N.; Nakashima, N.; Takagi, H. Lymph Node Metastasis in Carcinoma of the Body and Tail of the Pancreas. Br. J. Surg. 1997, 84, 1090–1092. [Google Scholar] [CrossRef] [PubMed]
- Kanda, M.; Fujii, T.; Nagai, S.; Kodera, Y.; Kanzaki, A.; Sahin, T.T.; Hayashi, M.; Yamada, S.; Sugimoto, H.; Nomoto, S.; et al. Pattern of Lymph Node Metastasis Spread in Pancreatic Cancer. Pancreas 2011, 40, 951–955. [Google Scholar] [CrossRef] [PubMed]
- McGrath, S.; Zhao, X.F.; Steele, R.; Thombs, B.D.; Benedetti, A.; Levis, B.; Riehm, K.E.; Saadat, N.; Levis, A.W.; Azar, M.; et al. Estimating the Sample Mean and Standard Deviation from Commonly Reported Quantiles in Meta-Analysis. Stat. Methods Med. Res. 2020, 29, 2520–2537. [Google Scholar] [CrossRef] [PubMed]
- Okusaka, T.; Nakamura, M.; Yoshida, M.; Kitano, M.; Uesaka, K.; Ito, Y.; Furuse, J.; Hanada, K.; Okazaki, K. Clinical Practice Guidelines for Pancreatic Cancer 2019 from the Japan Pancreas Society: A Synopsis. Pancreas 2020, 49, 326. [Google Scholar] [CrossRef]
- Lee, H.; Heo, J.S.; Choi, S.H.; Choi, D.W. Extended versus Peripancreatic Lymph Node Dissection for the Treatment of Left-Sided Pancreatic Cancer. Ann. Surg. Treat. Res. 2017, 92, 411–418. [Google Scholar] [CrossRef]
- Sohn, T.A.; Yeo, C.J.; Cameron, J.L.; Koniaris, L.; Kaushal, S.; Abrams, R.A.; Sauter, P.K.; Coleman, J.; Hruban, R.H.; Lillemoe, K.D. Resected Adenocarcinoma of the Pancreas—616 Patients: Results, Outcomes, and Prognostic Indicators. J. Gastrointest. Surg. 2000, 4, 567–579. [Google Scholar] [CrossRef] [PubMed]
- Riediger, H.; Keck, T.; Wellner, U.; Zur Hausen, A.; Adam, U.; Hopt, U.T.; Makowiec, F. The Lymph Node Ratio Is the Strongest Prognostic Factor after Resection of Pancreatic Cancer. J. Gastrointest. Surg. 2009, 13, 1337–1344. [Google Scholar] [CrossRef]
- House, M.G.; Gönen, M.; Jarnagin, W.R.; D’Angelica, M.; Dematteo, R.P.; Fong, Y.; Brennan, M.F.; Allen, P.J. Prognostic Significance of Pathologic Nodal Status in Patients with Resected Pancreatic Cancer. J. Gastrointest. Surg. 2007, 11, 1549–1555. [Google Scholar] [CrossRef]
- Pawlik, T.M.; Gleisner, A.L.; Cameron, J.L.; Winter, J.M.; Assumpcao, L.; Lillemoe, K.D.; Wolfgang, C.; Hruban, R.H.; Schulick, R.D.; Yeo, C.J.; et al. Prognostic Relevance of Lymph Node Ratio Following Pancreaticoduodenectomy for Pancreatic Cancer. Surgery 2007, 141, 610–618. [Google Scholar] [CrossRef]
- Berger, A.C.; Watson, J.C.; Ross, E.A.; Hoffman, J.P. The Metastatic/Examined Lymph Node Ratio Is an Important Prognostic Factor after Pancreaticoduodenectomy for Pancreatic Adenocarcinoma. Am. Surg. 2004, 70, 235–240. [Google Scholar] [CrossRef]
- Malleo, G.; Maggino, L.; Ferrone, C.R.; Marchegiani, G.; Mino-Kenudson, M.; Capelli, P.; Rusev, B.; Lillemoe, K.D.; Bassi, C.; Fernàndez-Del Castillo, C.; et al. Number of Examined Lymph Nodes and Nodal Status Assessment in Distal Pancreatectomy for Body/Tail Ductal Adenocarcinoma. Ann. Surg. 2019, 270, 1138–1146. [Google Scholar] [CrossRef]
- Watanabe, J.; Rifu, K.; Sasanuma, H.; Kotani, K.; Sata, N. The Efficacy of Radical Antegrade Modular Pancreatosplenectomy: A Systematic Review and Meta-Analysis. J. Hepato-Biliary-Pancreat. Sci. 2022, 29, 1156–1165. [Google Scholar] [CrossRef] [PubMed]
- Murakami, Y.; Uemura, K.; Sudo, T.; Hayashidani, Y.; Hashimoto, Y.; Nakashima, A.; Yuasa, Y.; Kondo, N.; Ohge, H.; Sueda, T. Number of Metastatic Lymph Nodes, but Not Lymph Node Ratio, Is an Independent Prognostic Factor after Resection of Pancreatic Carcinoma. J. Am. Coll. Surg. 2010, 211, 196–204. [Google Scholar] [CrossRef] [PubMed]



| Author | Year of Publication | Years of Enrollment | Country | NOS | Age | N° of Pts | LN Stations Involved in Lymphadenectomy |
LN Stations
Definition | Tumor Location | |
|---|---|---|---|---|---|---|---|---|---|---|
| Fujita [26] | 2009 | 1993–2008 | Japan | 7 | 64.2 (40–81) (Mean—range) | 50 | 8-9-10-11-12-13-14-16-17-18 | JPS | 50% body; 34% body/tail; 16% tail | |
| Sahin [27] | 2011 | 1991–2010 | Japan | 7 | 63 (38–79) (Median—range) | 85 | 8-10-11-18 (adjacent LNs) | 9-12-13-14-15-16-17 (distant LNs) | JPS | 82.3% body; 17.7% tail |
| Zhou [33] | 2019 | 2013–2016 | China | 8 | NS | 169 | 9-10-11-18 (standard LND) | 8-14-16 (extended LND) | ISGPS | 17.2% neck; 82.8% body/tail |
| Malleo [28] | 2020 | 2001–2017 | Italy | 8 | 68 (59–74) (Median—IQR) | 100 | 8-10-11-18 (+9 in selected cases) | ISGPS | 69% body; 31% tail | |
| Tanaka [29] | 2020 | 2002–2019 | Japan | 8 | 69 (44–85) (Median—range) | 100 | 8-9-10-11-14-16-18 | JPS | 23% body; 52% body/tail; 25% tail | |
| Imamura [30] | 2021 | 2002–2015 | Japan | 7 | 68 (67–70) (Median—IQR) * | 135 | 8-9-10-11-12-13-14-16-18 | AJCC | 44% body; 66% tail | |
| Minagawa [31] | 2021 | 2007–2018 | Japan | 7 | 71 (68–73) (Median—IQR) * | 120 | 8-9-10-11-18 (+14a for PDAC of the body) | ISGPS | 48.3% body; 51.7% tail | |
| Ishida [32] | 2022 | 2007–2020 | Japan | 7 | 73 (47–89) (Median—range) | 110 | 7-8-9-10-11-14-18 | JPS | NR | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Granieri, S.; Kersik, A.; Bonomi, A.; Frassini, S.; Bernasconi, D.; Paleino, S.; Germini, A.; Gjoni, E.; Cotsoglou, C. The Role of Non-Peripancreatic Lymph Nodes in the Survival of Patients Suffering from Pancreatic Cancer of the Body and Tail: A Systematic Review and Meta-Analysis of High-Quality Studies. Cancers 2023, 15, 2322. https://doi.org/10.3390/cancers15082322
Granieri S, Kersik A, Bonomi A, Frassini S, Bernasconi D, Paleino S, Germini A, Gjoni E, Cotsoglou C. The Role of Non-Peripancreatic Lymph Nodes in the Survival of Patients Suffering from Pancreatic Cancer of the Body and Tail: A Systematic Review and Meta-Analysis of High-Quality Studies. Cancers. 2023; 15(8):2322. https://doi.org/10.3390/cancers15082322
Chicago/Turabian StyleGranieri, Stefano, Alessia Kersik, Alessandro Bonomi, Simone Frassini, Davide Bernasconi, Sissi Paleino, Alessandro Germini, Elson Gjoni, and Christian Cotsoglou. 2023. "The Role of Non-Peripancreatic Lymph Nodes in the Survival of Patients Suffering from Pancreatic Cancer of the Body and Tail: A Systematic Review and Meta-Analysis of High-Quality Studies" Cancers 15, no. 8: 2322. https://doi.org/10.3390/cancers15082322
APA StyleGranieri, S., Kersik, A., Bonomi, A., Frassini, S., Bernasconi, D., Paleino, S., Germini, A., Gjoni, E., & Cotsoglou, C. (2023). The Role of Non-Peripancreatic Lymph Nodes in the Survival of Patients Suffering from Pancreatic Cancer of the Body and Tail: A Systematic Review and Meta-Analysis of High-Quality Studies. Cancers, 15(8), 2322. https://doi.org/10.3390/cancers15082322






