EBP50 Depletion and Nuclear β-Catenin Accumulation Engender Aggressive Behavior of Colorectal Carcinoma through Induction of Tumor Budding
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Cases and Evaluation of Tumor Budding Findings
2.2. Antibodies
2.3. Immunohistochemistry (IHC) Quantification
2.4. Immunofluorescence
2.5. Plasmids and Cell Lines
2.6. GST Pull-Down Assay
2.7. Transfection
2.8. Western Blot Assays and Co-Immunoprecipitation Assays
2.9. Flow Cytometry and Aldefluor Assay
2.10. Wound Healing Assay
2.11. Migration Assay
2.12. Spheroid Assay
2.13. TCGA Data Analysis
2.14. Statistical Analysis
3. Results
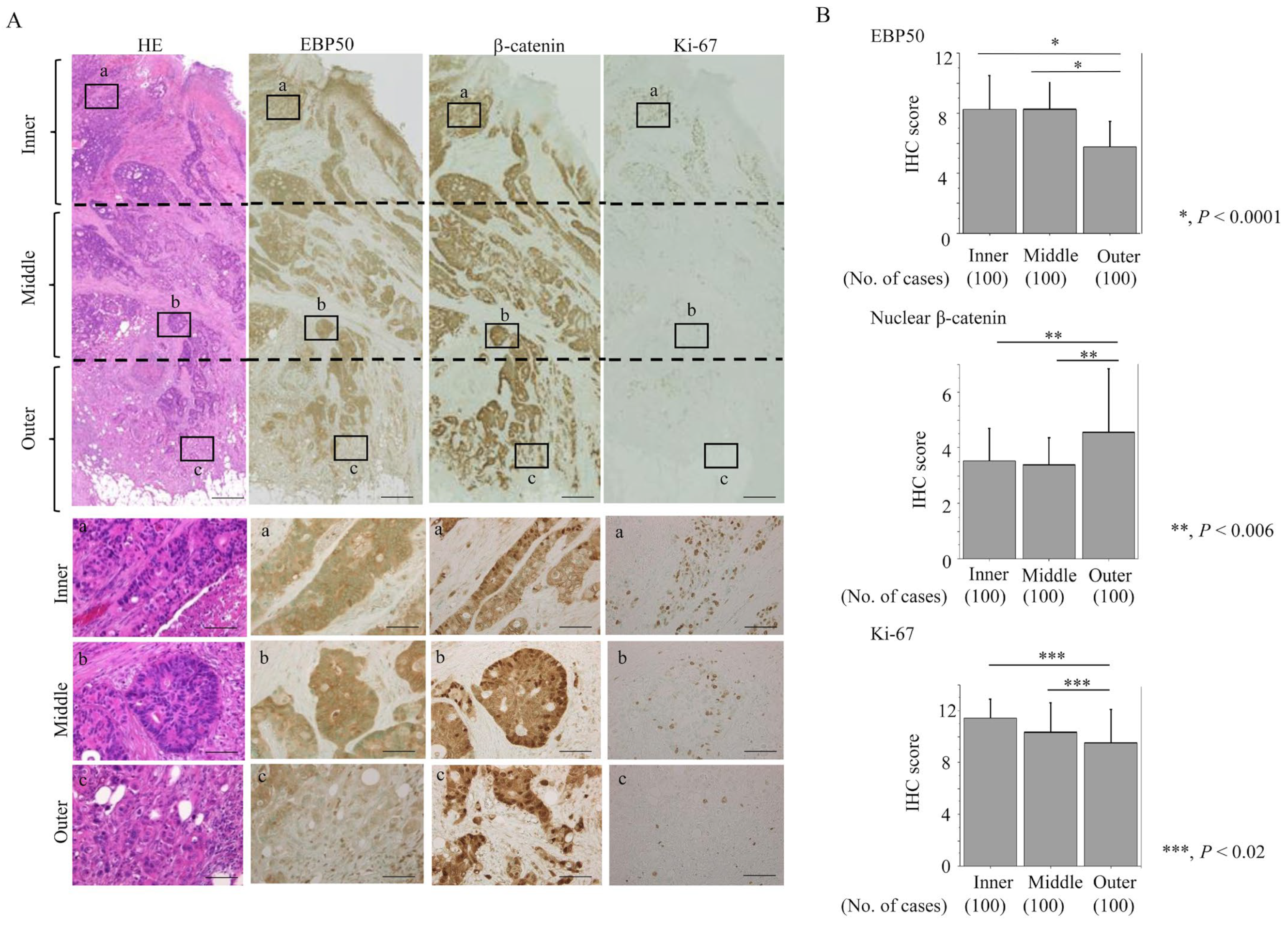
3.1. EBP50 Expression during Adenoma-Carcinoma Progression and TCGA Data Analysis in CRC
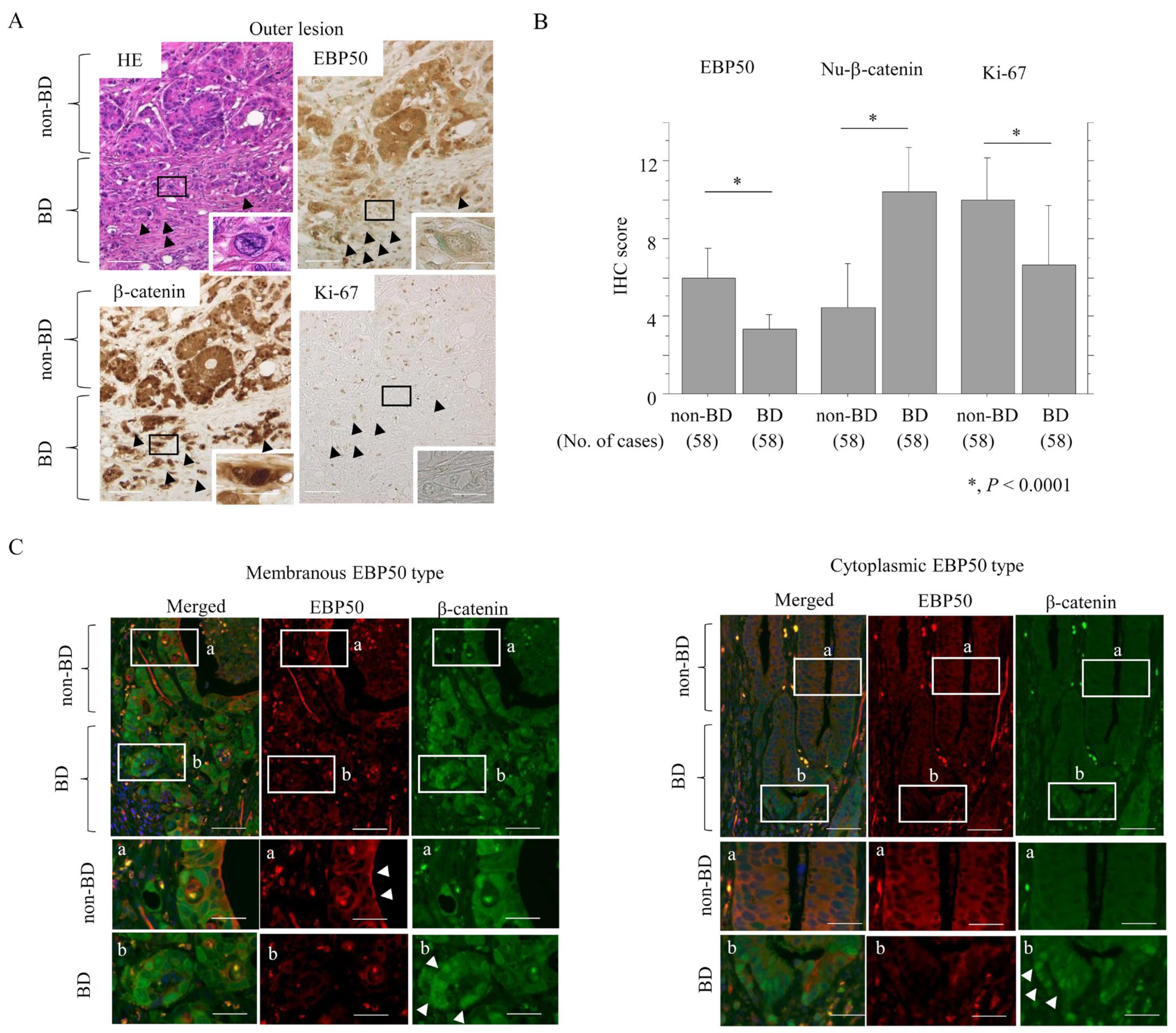
3.2. Reduced EBP50 Expression in Outer Lesions of CRC Is Associated with Aggressive Tumor Behavior
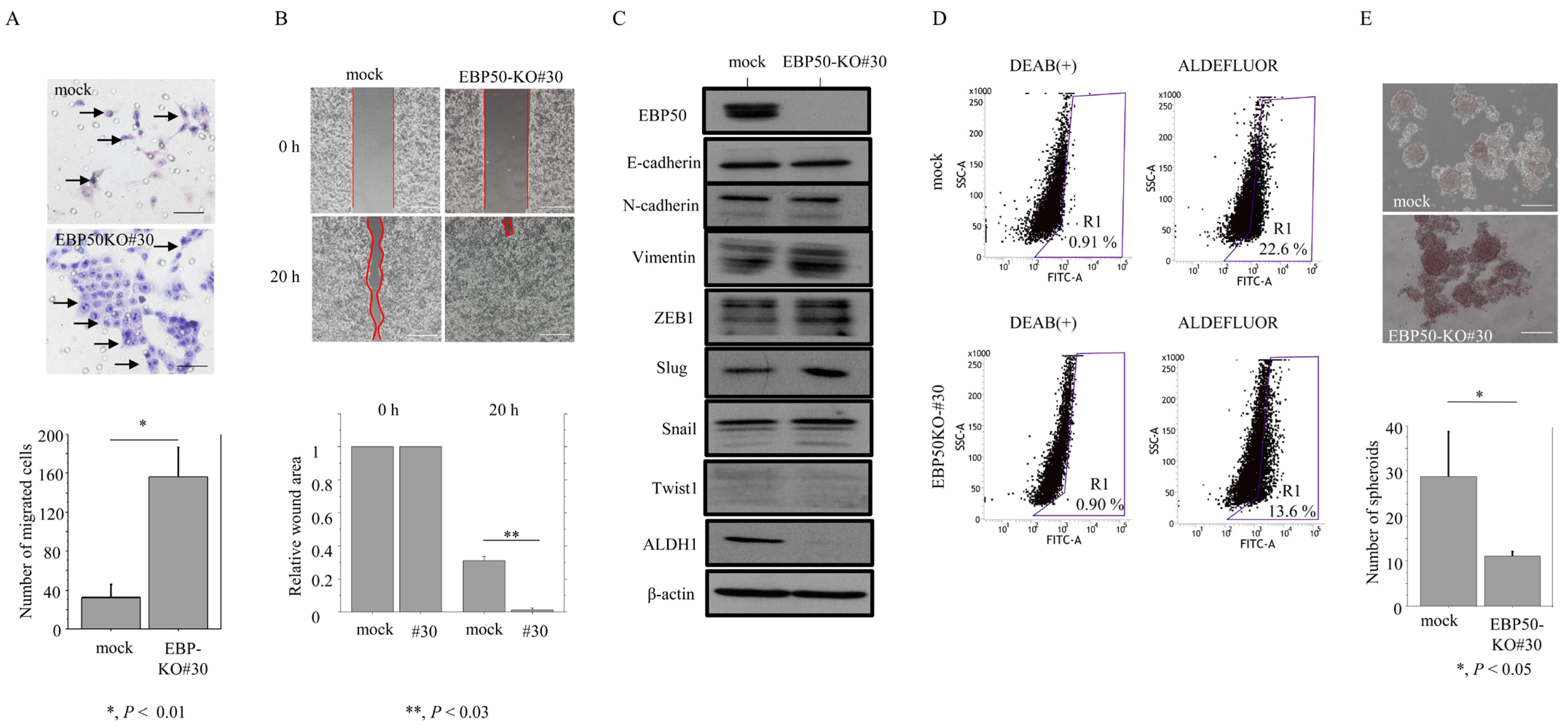
3.3. Knockout of EBP50 Is Associated with Decreased Proliferation and Accelerated Migration Capability
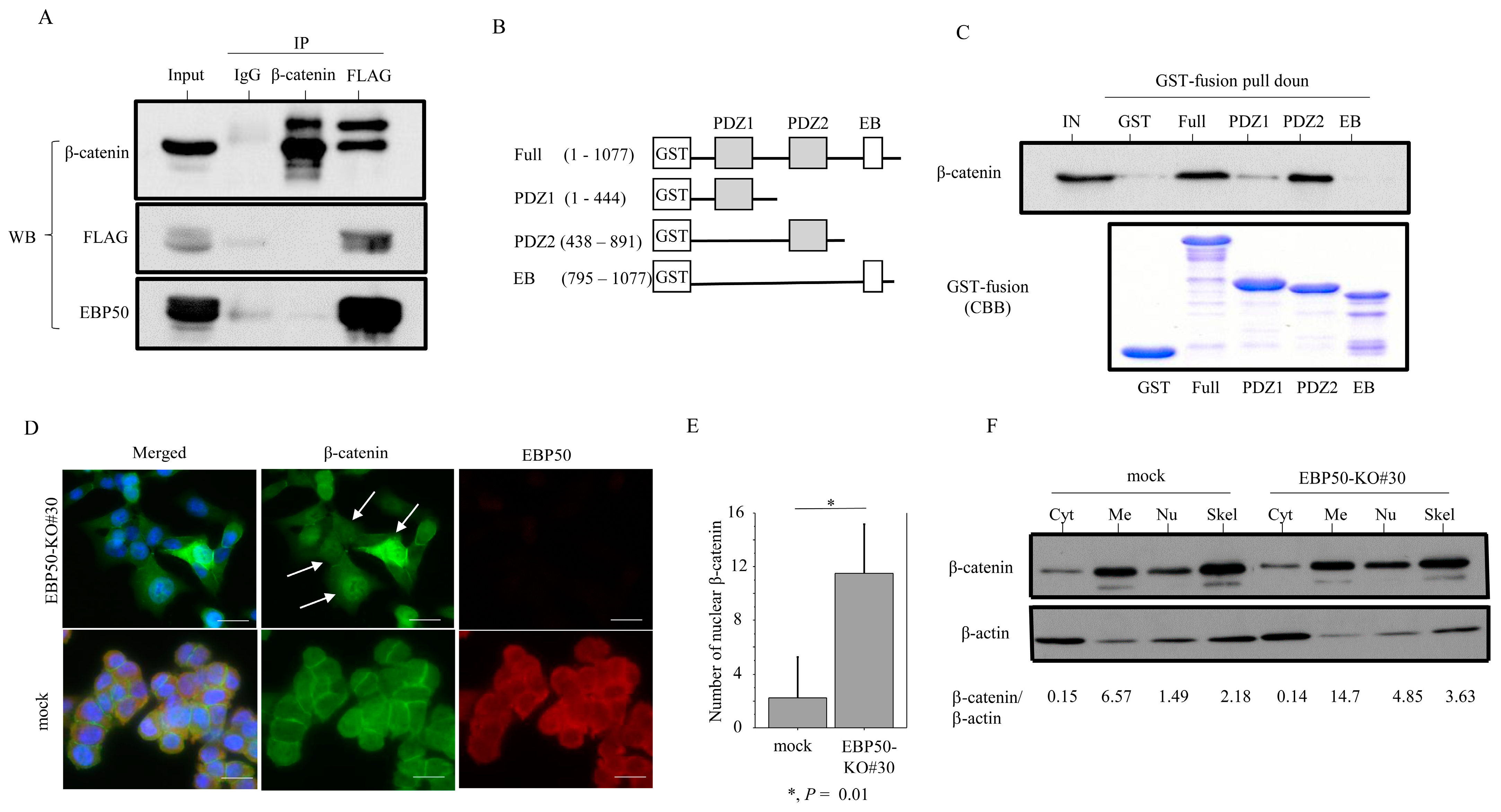
3.4. EBP50 Strongly Interacts with β-Catenin at the Plasma Membrane
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Broad Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Onyoh, E.F.; Hsu, W.-F.; Chang, L.-C.; Lee, Y.-C.; Wu, M.-S.; Chiu, H.-M. The Rise of colorectal cancer in Asia: Epidemiology, screening, and management. Curr. Gastroenterol. Rep. 2019, 21, 36. [Google Scholar] [CrossRef] [PubMed]
- Koo, J.H.; Leong, R.W.; Ching, J.; Yeoh, K.-G.; Wu, D.-C.; Murdani, A.; Cai, Q.; Chiu, H.-M.; Chong, V.H.; Rerknimitr, R.; et al. Knowledge of attitudes toward, and barriers to participation of colorectal cancer screening tests in the Asia-Pacific region: A multicenter study. Gastrointest. Endosc. 2012, 76, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.C.; Ding, H.; Wang, J.; Chan, P.S.; Huang, J. Prevalence and risk factors of colorectal cancer in Asia. Intest. Res. 2019, 17, 317–329. [Google Scholar] [CrossRef]
- Donowitz, M.; Cha, B.; Zachos, N.C.; Brett, C.L.; Sharma, A.; Tse, C.M.; Li, X. NHERF family and NHE3 regulation. J. Physiol. 2005, 567 Pt 1, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Viswanatha, R.; Bretscher, A.; Garbett, D. Dynamics of ezrin and EBP50 in regulating microvilli on the apical aspect of epithelial cells. Biochem. Soc. Trans. 2014, 42, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Mullin, J.M. Epithelial barriers, compartmentation, and cancer. Sci. STKE 2004, 2004, pe2. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.; Molina, J.R.; Hamilton, S.R.; Georgescu, M.M. NHERF1/EBP50 is a new marker in colorectal cancer. Neoplasia 2010, 12, 1013–1022. [Google Scholar] [CrossRef]
- Thiery, J.P. Epithelial-mesenchymal transitions in tumour progression. Nat. Rev. Cancer 2002, 2, 442–454. [Google Scholar] [CrossRef]
- Greenburg, G.; Hay, E.D. Epithelia suspended in collagen gels can lose polarity and express characteristics of migrating mesenchymal cells. J. Cell Biol. 1982, 95, 333–339. [Google Scholar] [CrossRef]
- De Smedt, L.; Palmans, S.; Andel, D.; Govaere, O.; Boeckx, B.; Smeets, D.; Galle, E.; Wouters, J.; Barras, D.; Suffiotti, M.; et al. Expressing profiling of budding cells in colorectal cancer reveals an EMT-like phenotype and molecular subtype switching. Br. J. Cancer 2017, 116, 58–65. [Google Scholar] [CrossRef]
- Claperon, A.; Guedj, N.; Mergey, M.; Vignjevic, D.; Desbois-Mouthon, C.; Boissan, M.; Saubamea, B.; Paradis, V.; Housset, C.; Fouassier, L. Loss of EBP50 stimulates EGFR activity to induce EMT phenotypic features in biliary cancer cells. Oncogene 2012, 31, 1376–1388. [Google Scholar] [CrossRef] [PubMed]
- Shibata, T.; Chuma, M.; Kokubo, A.; Sakamoto, M.; Hirohashi, S. EBP50, a β-catenin-associated protein, enhances Wnt signaling and is over-expressed in hepatocellular carcinoma. Hepatology 2003, 38, 178–186. [Google Scholar] [CrossRef]
- Wheeler, D.S.; Barrick, S.R.; Grubisha, M.J.; Brufsky, A.M.; Friedman, P.A.; Romero, G. Direct interaction between NHERF1 and Frizzled regulates b-catenin signaling. Oncogene 2011, 30, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Vaquero, J.; Ho-Bouldoires, T.H.N.; Claperon, A.; Fouassier, L. Role of the PDZ-scaffold protein NHERF1/EBP50 in cancer biology: From signaling regulation to clinical relevance. Oncogene 2017, 36, 3067–3079. [Google Scholar] [CrossRef] [PubMed]
- Vogelstein, B.; Kinzler, K.W. Cancer genes and the pathways they control. Nat. Med. 2004, 10, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Japanese Society for Cancer of the Colon and Rectum (Ed.) General Rules for Clinical and Pathological Studies on Cancer of the Colon, Rectum and Anus, 3rd English ed.; Japanese Classification of Colorectal, Appendiceal, and Anal Carcinoma: Tokyo, Japan, 2019. [Google Scholar]
- Brierley, J.D.; Gospodarowicz, M.K.; Wittekind, C. (Eds.) TNM Classification of Malignant Tumours; Wiley-Blackwell: Oxford, UK, 2016. [Google Scholar]
- Zengin, M.; Cifci, A. Tumour budding in preoperative biopsy specimens is a useful for identifying high-risk patients in early-stage (pN0) colon cancer. Turk. J. Med. Sci. 2020, 50, 375–385. [Google Scholar] [CrossRef]
- Takahashi, H.; Watanabe, H.; Hashimura, M.; Matsumoto, T.; Yokoi, A.; Nakagawa, M.; Ishibashi, Y.; Ito, T.; Ohhigata, K.; Saegusa, M. A combination of stromal PD-L1 and tumoral nuclear β-catenin expression as an indicator of colorectal carcinoma progression and resistance to chemoradiotherapy in locally advanced rectal carcinoma. J. Pathol. Clin. Res. 2022, 8, 458–469. [Google Scholar] [CrossRef]
- Takahashi, H.; Nakamura, K.; Usami, A.; Tsuruta, T.; Hsahimura, M.; Matsumoto, T.; Saegusa, M. Possible role of β-catenin in resistance to preoperative chemoradiotherapy in locally advanced rectal cancer. Histopathology 2017, 71, 227–237. [Google Scholar] [CrossRef]
- Matsumoto, T.; Yokoi, A.; Konno, R.; Oguri, Y.; Hashimura, M.; Tochimoto, M.; Nakagawa, M.; Jiang, Z.; Ishibashi, Y.; Ito, T.; et al. Cytoplasmic EBP50 and elevated PARP1 are unfavorable prognostic factors in ovarian clear cell carcinoma. Carcinogenesis 2021, 42, 1162–1170. [Google Scholar] [CrossRef]
- Nakagawa, M.; Matsumoto, T.; Yokoi, A.; Hashimura, M.; Oguri, Y.; Konno, R.; Ishibashi, Y.; Ito, T.; Ohhigata, K.; Harada, Y.; et al. Interaction between membranous EBP50 and myosin 9 as a favorable prognostic factor in ovarian clear cell carcinoma. Mol. Oncol. 2023, 17, 2168–2182. [Google Scholar] [CrossRef]
- Saegusa, M.; Hashimura, M.; Kuwata, T.; Okayasu, I. Requirement of Akt/β-catenin pathway for uterine carcinosarcoma genesis, modulating E-cadherin expression through the transactivation of Slug. Am. J. Pathol. 2009, 174, 2107–2115. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.Y.; Hsu, Y.H.; Huang, H.Y.; Shann, Y.-J.; Huang, C.-F.F.; Wei, S.-C.; Chen, C.-L.; Jou, T.-S. Aberrant nuclear localization of EBP50 promotes colorectal carcinogenesis in xenotransplanted mice by modulating TCF-1 and β-catenin interactions. J. Clin. Invest. 2012, 122, 1881–1894. [Google Scholar] [CrossRef] [PubMed]
- Georgescu, M.M.; Gagea, M.; Cote, G. NHERF1/EBP50 suppresses Wnt-b-catenin pathway-driven intestinal neoplasia. Neoplasia 2016, 18, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Malfettone, A.; Silvestris, N.; Paradiso, A.; Mattioli, E.; Simone, G.; Mangia, A. Overexpression of nuclear NHERF1 in advanced colorectal cancer: Association with hypoxic microenvironment and tumor invasive phenotype. Exp. Mol. Pathol. 2012, 92, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Lugli, A.; Kirsch, R.; Ajioka, Y.; Bosman, F.; Cathomas, G.; Dawson, H.; Zimaity, H.E.I.; Flejou, J.-F.; Hansen, T.P.; Hartmann, A.; et al. Recommendations for reporting tumor budding in colorectal cancer based on the International Tumor Budding Consensus Conference (ITBCC) 2016. Mod. Pathol. 2017, 30, 1299–1311. [Google Scholar] [CrossRef]
- Georgescue, M.M.; Cote, G.; Agarwal, N.K.; White, C.L., 3rd. NHERF1/EBP50 controls morphogenesis of 3D colonic glands by stabilizing PTEN and ezrin-radixin-moesin proteins at the apical membrane. Neoplasia 2014, 16, 365–374. [Google Scholar] [CrossRef]
- Giese, A.; Loo, M.A.; Tran, N.; Haskett, D.; Coons, S.W.; Berens, M.E. Dichotomy of astrocytoma migration and proliferation. Int. J. Cancer 1996, 67, 275–282. [Google Scholar] [CrossRef]
- Giese, A.; Bjerkvig, R.; Berens, M.E.; Westphal, M. Cost of migration: Invasion of malignant glioma and implications for treatment. J. Clin. Oncol. 2003, 21, 1624–1636. [Google Scholar] [CrossRef]
- Merzak, A.; McCrea, S.; Koocheckpour, S.; Pilkington, G.J. Control of human glioma cell growth, migration and invasion in vitro by transforming growth factor beta 1. Br. J. Cancer. 1994, 70, 199–203. [Google Scholar] [CrossRef]
- Mani, S.A.; Guo, W.; Liao, M.J.; Eaton, E.N.; Ayyanan, A.; Zhou, A.Y.; Brooks, M.; Reinhard, F.; Zhang, C.C.; Shipitsin, M.; et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008, 133, 704–715. [Google Scholar] [CrossRef]

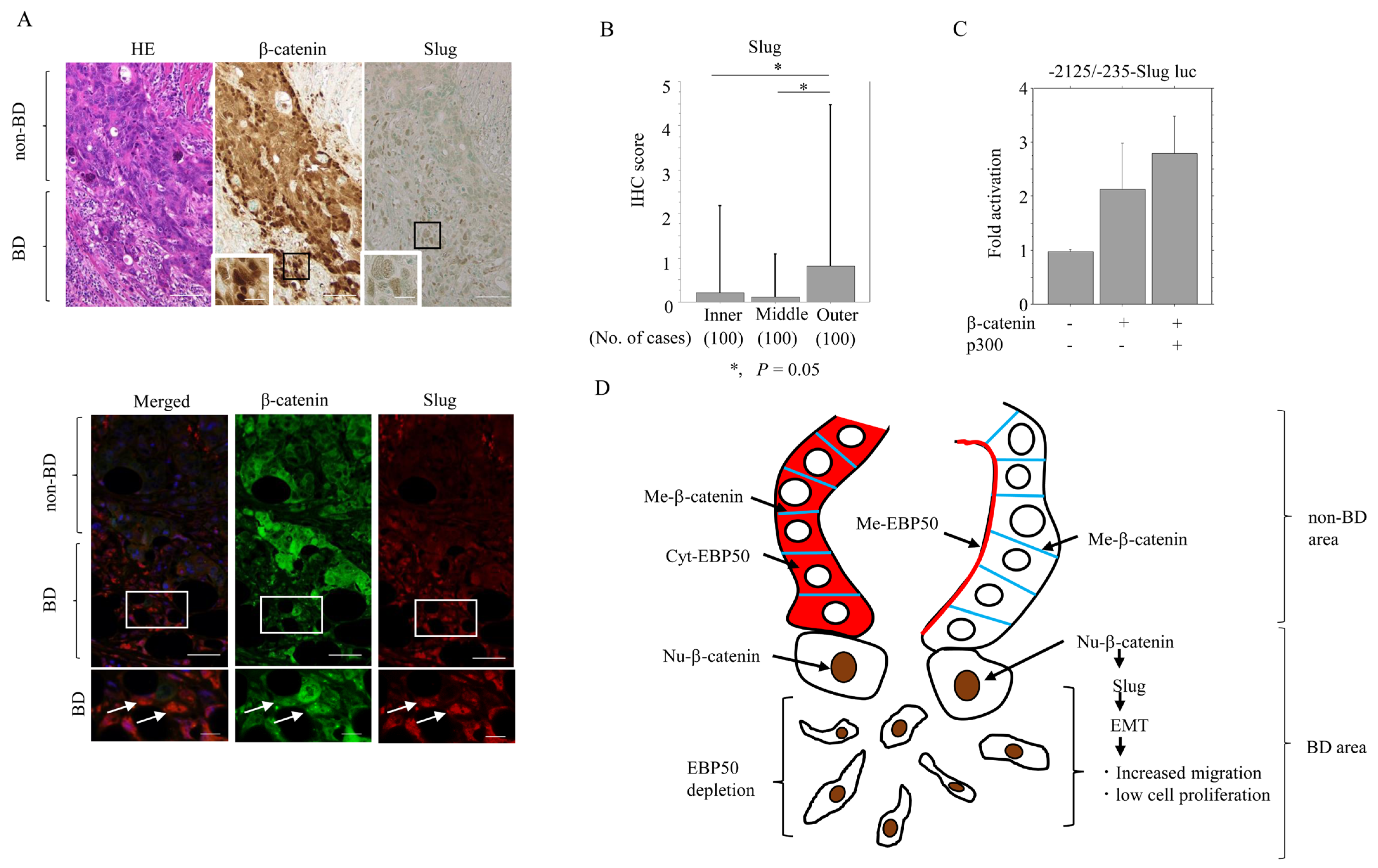
| EBP50 Expression | EBP50 Depletion in Outer Lesion | |||||||
|---|---|---|---|---|---|---|---|---|
| Membrane | Cytoplasm | Severe to Mod | None/Mild | |||||
| n | n (%) | n (%) | p-Value | n | n (%) | n (%) | p-Value | |
| Gender | ||||||||
| Male | 58 | 25 (43.1) | 33 (56.9) | 0.7 | 58 | 35 (60.3) | 23 (39.7) | 0.5 |
| Female | 42 | 16 (38.1) | 26 (61.9) | 42 | 29 (69) | 13(31) | ||
| Age | ||||||||
| ≤68 years | 39 | 17 (43.6) | 22 (56.4) | 0.8 | 39 | 27 (69.2) | 12 (30.8) | 0.4 |
| ≥69 years | 61 | 24 (39.3) | 37 (60.7) | 61 | 37(60.7) | 24 (39.3) | ||
| Tumor location | ||||||||
| Right | 23 | 7 (30.4) | 16 (69.6) | 0.3 | 23 | 17 (73.9) | 6 (26.1) | 0.3 |
| Left | 77 | 34 (44.2) | 43 (55.8) | 77 | 47 (61) | 30 (39) | ||
| Macroscopic finding | ||||||||
| Depression | 94 | 38 (40.4) | 56 (59.6) | 0.9 | 94 | 64 68.1) | 30 (31.9) | 0.003 |
| Elevation | 6 | 3 (50) | 3 (50) | 6 | 0 (0) | 6 (100) | ||
| Hist. differentiation | ||||||||
| tub1 | 47 | 24 (51.1) | 23 (48.9) | 0.1 | 47 | 31 (66) | 16 (34) | 0.1 |
| tub2 | 53 | 17 (32.1) | 36 (67.9) | 53 | 33 (62.3) | 20 (37.7) | ||
| Tumor size | ||||||||
| ≤4.2 cm | 55 | 24 (43.6) | 31 (56.4) | 0.6 | 55 | 34 (61.8) | 21 (38.2) | 0.7 |
| ≥4.3 cm | 45 | 17 (37.8) | 28 (62.2) | 45 | 30 (66.7) | 15 (33.3) | ||
| Depth | ||||||||
| less than SS | 76 | 31 (13.6) | 45 (59.2) | 0.9 | 76 | 44 (57.9) | 32 (42.1) | 0.04 |
| SE | 24 | 10 (41.7) | 14 (58.3) | 24 | 20 (83.3) | 4 (16.7) | ||
| Budding | ||||||||
| BD1 | 41 | 15 (36.6) | 26 (63.4) | 0.5 | 42 | 17 (40.5) | 25 (59.5) | <0.0001 |
| BD2/3 | 59 | 26 (44.1) | 33 (55.9) | 58 | 47 (81) | 11 (19) | ||
| Ly invasion | ||||||||
| Positive | 41 | 10 (24.4) | 31 (75.6) | 0.009 | 41 | 29 (70.7) | 12 (29.3) | 0.3 |
| Negative | 59 | 31 (52.5) | 28 (47.5) | 59 | 35 (59.3) | 24 (40.7) | ||
| V invasion | ||||||||
| Positive | 74 | 29 (39.2) | 45 (60.8) | 0.6 | 74 | 50 | 24 | 0.3 |
| Negative | 26 | 12 (46.2) | 14 (53.8) | 26 | 14 | 12 | ||
| Neural invasion | ||||||||
| Positive | 44 | 14 (31.8) | 30 (68.2) | 0.1 | 44 | 35 (79.5) | 9 (20.5) | 0.007 |
| Negative | 56 | 27 (48.2) | 29 (51.8) | 56 | 29 (51.8) | 27 (48.2) | ||
| LN metastasis | ||||||||
| Positive | 56 | 21 (37.5) | 35 (62.5) | 0.5 | 56 | 38 (67.9) | 18 (32.1) | 0.4 |
| Negative | 44 | 20 (45.5) | 24 (54.5) | 44 | 26 (59.1) | 18 (40.9) | ||
| Distant metastasis | ||||||||
| Positive | 15 | 3 (20) | 12 (80) | 0.1 | 15 | 12 (80) | 3 (20) | 0.2 |
| Negative | 85 | 38 (44.7) | 47 (55.3) | 85 | 52 (61.2) | 33 (38.8) | ||
| Clinical stage | ||||||||
| I/II | 41 | 19 (46.3) | 22 (53.7) | 0.2 | 41 | 23 (56.1) | 18 (43.9) | 0.2 |
| III/IV | 59 | 22 (37.3) | 37 (62.7) | 59 | 41 (69.5) | 18 (30.5) | ||
| EBP50 depletion | ||||||||
| severe to moderate | 64 | 23 (35.9) | 41 (64.1) | 0.1 | NE | NE | NE | |
| none/mild | 36 | 18 (50) | 18 (50) | NE | NE | |||
| EBP50 | Nu-β-Catenin | Ki-67 | |
|---|---|---|---|
| ρ (p) | ρ (p) | ρ (p) | |
| Nu-β-catenin | −0.49 | * | * |
| (<0.0001) | |||
| Ki-67 | 0.59 | −0.33 | * |
| (<0.0001) | (0.0004) | ||
| Slug | 0.33 | 0.44 | 0.37 |
| (0.0005) | (<0.0001) | (<0.0001) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Itou, T.; Ishibashi, Y.; Oguri, Y.; Hashimura, M.; Yokoi, A.; Harada, Y.; Fukagawa, N.; Hayashi, M.; Ono, M.; Kusano, C.; et al. EBP50 Depletion and Nuclear β-Catenin Accumulation Engender Aggressive Behavior of Colorectal Carcinoma through Induction of Tumor Budding. Cancers 2024, 16, 183. https://doi.org/10.3390/cancers16010183
Itou T, Ishibashi Y, Oguri Y, Hashimura M, Yokoi A, Harada Y, Fukagawa N, Hayashi M, Ono M, Kusano C, et al. EBP50 Depletion and Nuclear β-Catenin Accumulation Engender Aggressive Behavior of Colorectal Carcinoma through Induction of Tumor Budding. Cancers. 2024; 16(1):183. https://doi.org/10.3390/cancers16010183
Chicago/Turabian StyleItou, Takashi, Yu Ishibashi, Yasuko Oguri, Miki Hashimura, Ako Yokoi, Yohei Harada, Naomi Fukagawa, Misato Hayashi, Mototsugu Ono, Chika Kusano, and et al. 2024. "EBP50 Depletion and Nuclear β-Catenin Accumulation Engender Aggressive Behavior of Colorectal Carcinoma through Induction of Tumor Budding" Cancers 16, no. 1: 183. https://doi.org/10.3390/cancers16010183
APA StyleItou, T., Ishibashi, Y., Oguri, Y., Hashimura, M., Yokoi, A., Harada, Y., Fukagawa, N., Hayashi, M., Ono, M., Kusano, C., & Saegusa, M. (2024). EBP50 Depletion and Nuclear β-Catenin Accumulation Engender Aggressive Behavior of Colorectal Carcinoma through Induction of Tumor Budding. Cancers, 16(1), 183. https://doi.org/10.3390/cancers16010183






