Addressing Genetic Tumor Heterogeneity, Post-Therapy Metastatic Spread, Cancer Repopulation, and Development of Acquired Tumor Cell Resistance
Abstract
Simple Summary
Abstract
1. Introduction
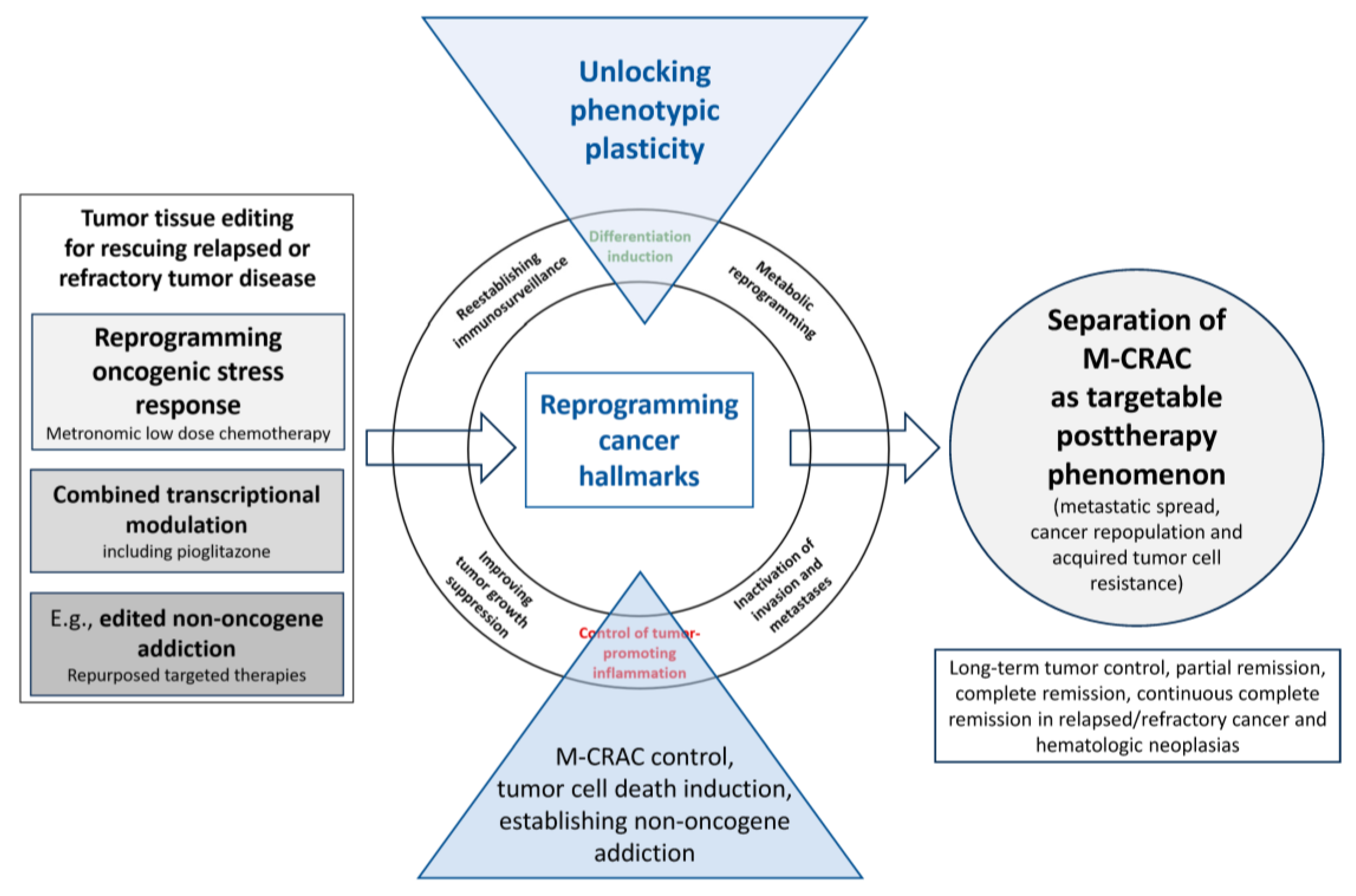
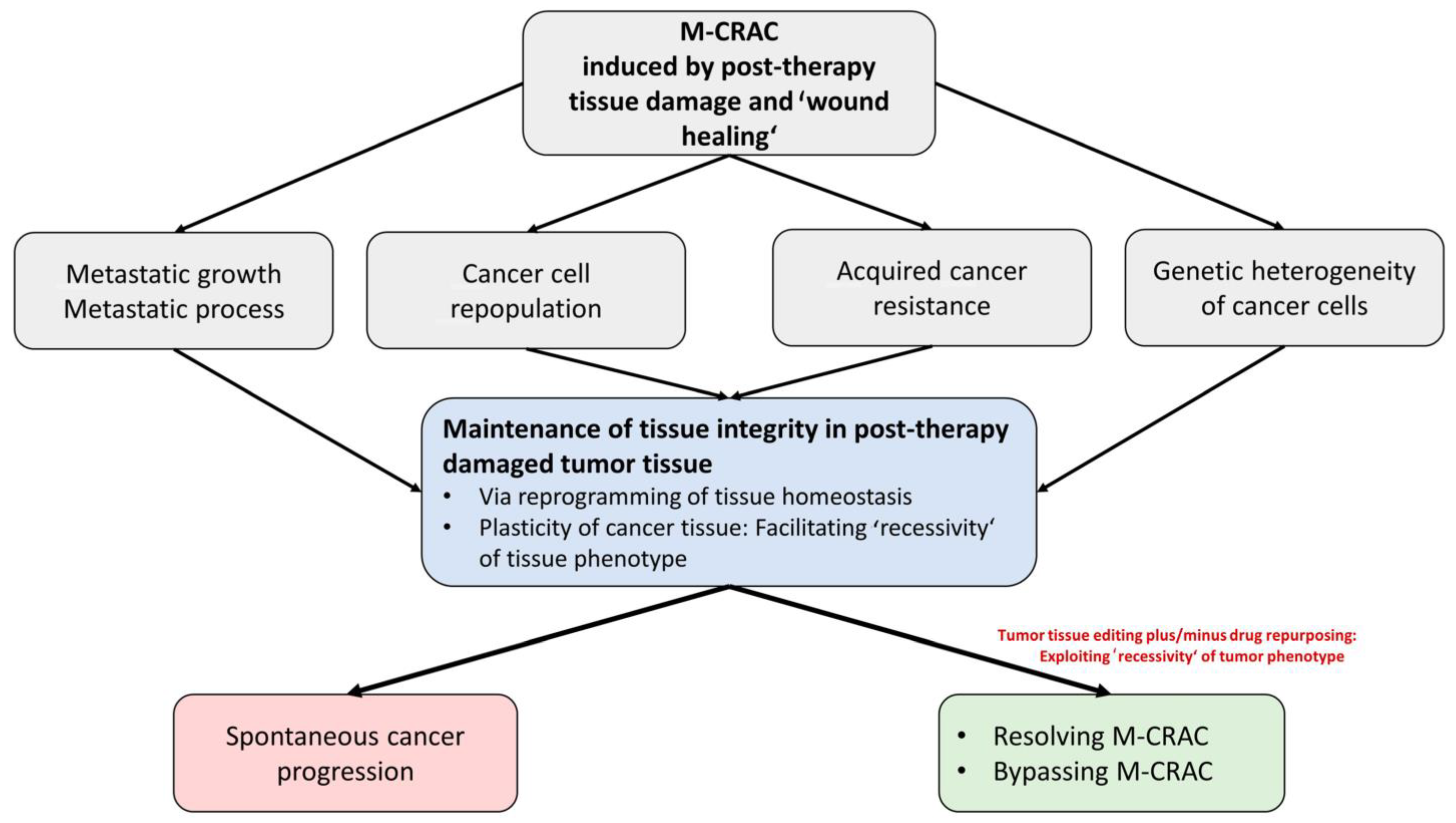
2. M-CRAC as a Target for Tumor Tissue Editing in Refractory or Relapsed Neoplasias
3. Tissue Editing Methods Redirect Cancer-Related Hallmarks into Novel Biologic Hallmarks Facilitating Tumor Response
4. Tissue Editing Approaches: Impact on the Genomic Evolution of Tumors
5. M-CRAC as Driver of Disease Relapse and Chemoresistance: The Therapeutic View
6. Specific Therapeutic Access to M-CRAC with Tumor Tissue Editing Approaches
7. Repurposing Chemotherapy: Metronomic Low-Dose Chemotherapy
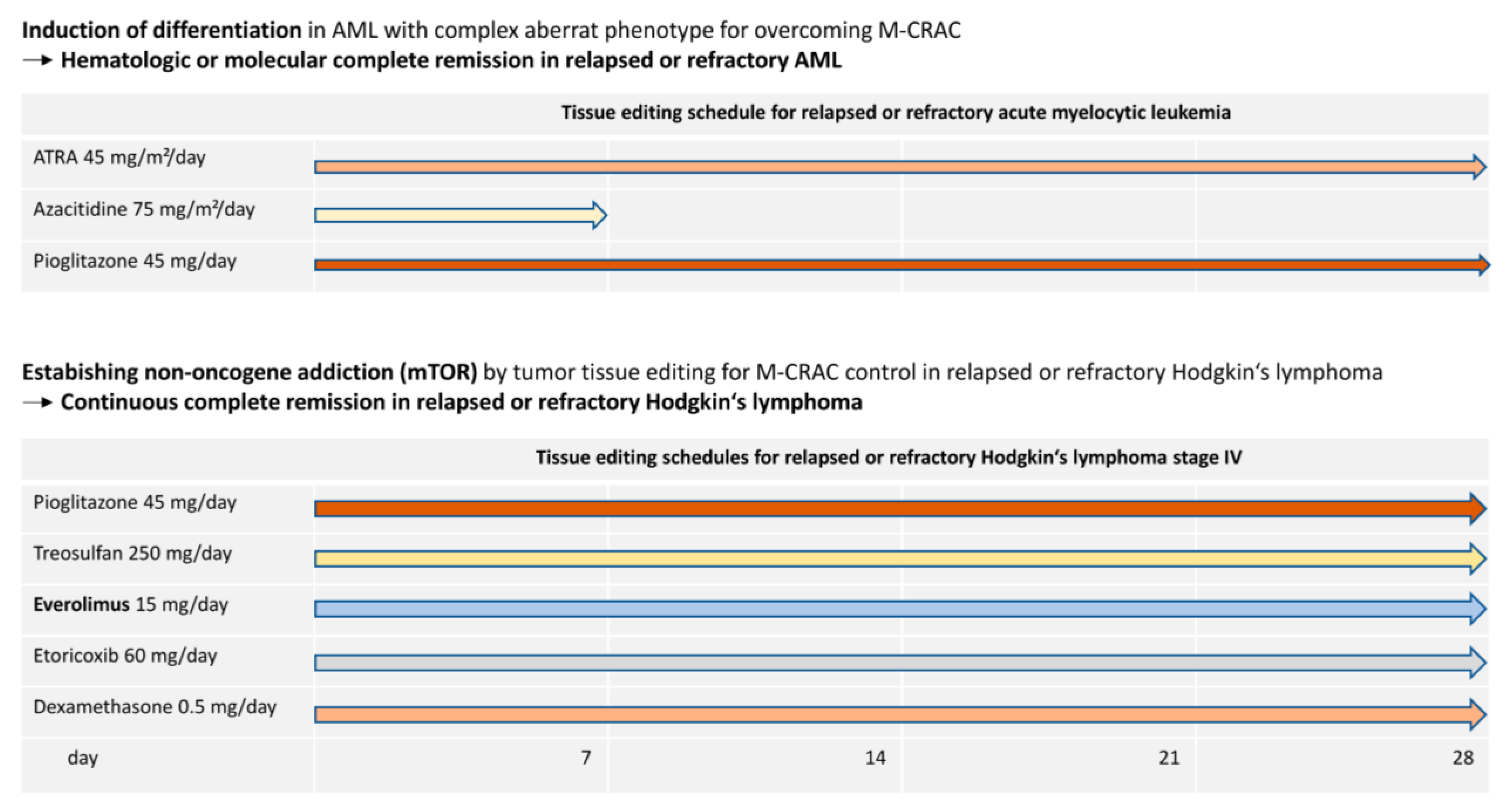
8. Examples of M-CRAC Control and Tissue Editing in the Clinical Setting
9. Addressing M-CRAC Control with a Novel Therapy Model for r/r Neoplasias
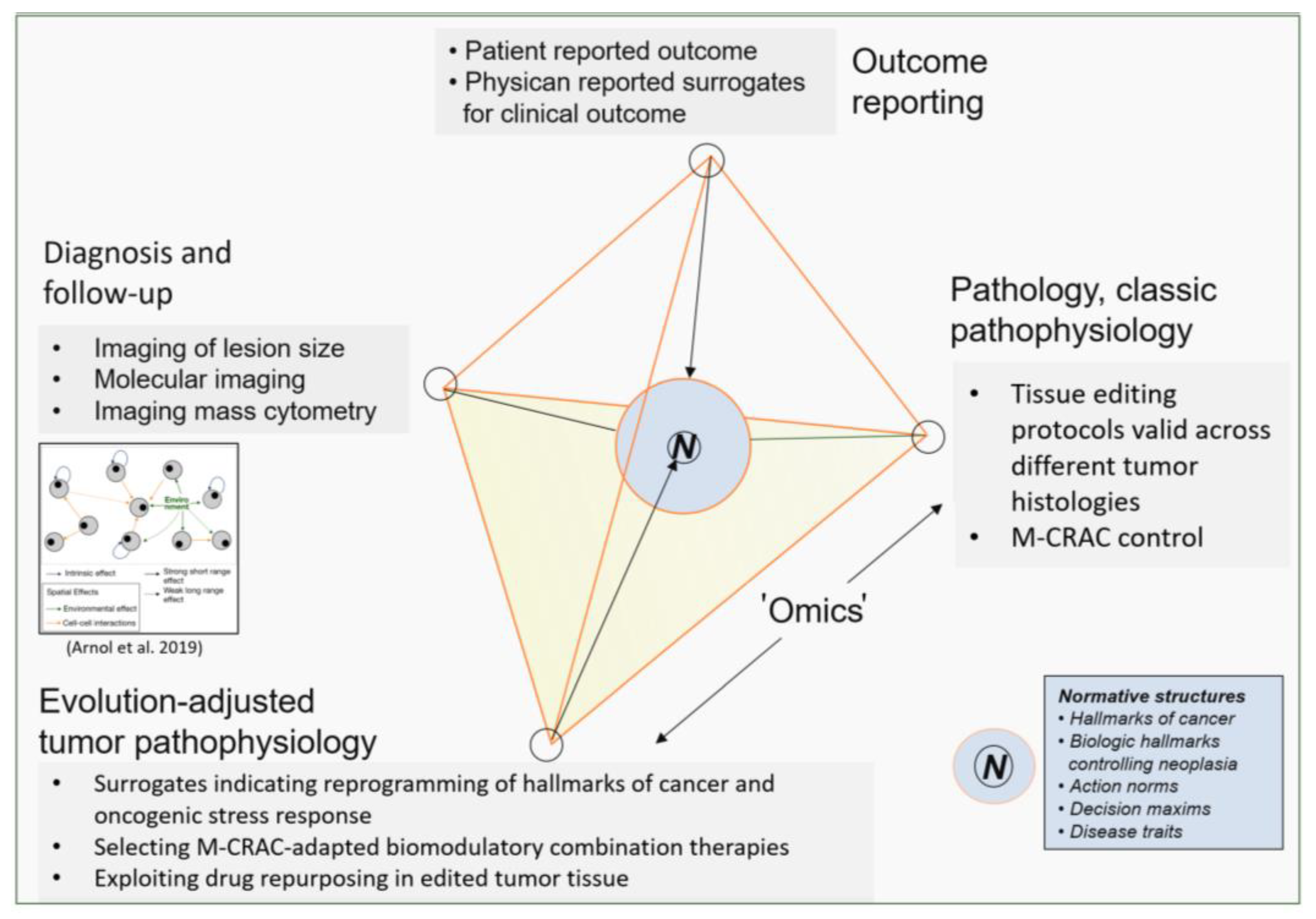
10. Patient Selection Criteria for Treatment with Tumor Tissue Editing Approaches
11. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Christensen, D.S.; Ahrenfeldt, J.; Sokač, M.; Kisistók, J.; Thomsen, M.K.; Maretty, L.; McGranahan, N.; Birkbak, N.J. Treatment Represents a Key Driver of Metastatic Cancer Evolution. Cancer Res. 2022, 82, 2918–2927. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, J.H.; Ballman, K.; Polley, M.-Y.C.; Campbell, J.D.; Fan, C.; Selitsky, S.; Fernandez-Martinez, A.; Parker, J.S.; Hoadley, K.A.; Hu, Z.; et al. CALGB 40603 (Alliance): Long-Term Outcomes and Genomic Correlates of Response and Survival After Neoadjuvant Chemotherapy with or Without Carboplatin and Bevacizumab in Triple-Negative Breast Cancer. JCO 2022, 40, 1323–1334. [Google Scholar] [CrossRef] [PubMed]
- Shaked, Y. The pro-tumorigenic host response to cancer therapies. Nat. Rev. Cancer 2019, 19, 667–685. [Google Scholar] [CrossRef] [PubMed]
- Bethge, W.A.; Martus, P.; Schmitt, M.; Holtick, U.; Subklewe, M.; von Tresckow, B.; Ayuk, F.; Wagner-Drouet, E.M.; Wulf, G.G.; Marks, R.; et al. GLA/DRST real-world outcome analysis of CAR T-cell therapies for large B-cell lymphoma in Germany. Blood 2022, 140, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Gökbuget, N.; Zugmaier, G.; Dombret, H.; Stein, A.; Bonifacio, M.; Graux, C.; Faul, C.; Brüggemann, M.; Taylor, K.; Mergen, N.; et al. Curative outcomes following blinatumomab in adults with minimal residual disease B-cell precursor acute lymphoblastic leukemia. Leuk. Lymphoma 2020, 61, 2665–2673. [Google Scholar] [CrossRef] [PubMed]
- Overmoyer, B. Combination chemotherapy for metastatic breast cancer: Reaching for the cure. JCO 2003, 21, 580–582. [Google Scholar] [CrossRef]
- Westphal, T.; Gampenrieder, S.P.; Rinnerthaler, G.; Greil, R. Cure in metastatic breast cancer. Memo 2018, 11, 172–179. [Google Scholar] [CrossRef]
- Morad, G.; Helmink, B.A.; Sharma, P.; Wargo, J.A. Hallmarks of response, resistance, and toxicity to immune checkpoint blockade. Cell 2021, 184, 5309–5337. [Google Scholar] [CrossRef]
- Heudobler, D.; Ghibelli, L.; Reichle, A. Editorial: Anakoinosis for promoting tumor tissue editing: Novel therapeutic opportunities for establishing clinically relevant tumor control by targeting tumor plasticity. Front. Oncol. 2022, 12, 1005381. [Google Scholar] [CrossRef]
- Corsi, F.; Capradossi, F.; Pelliccia, A.; Briganti, S.; Bruni, E.; Traversa, E.; Torino, F.; Reichle, A.; Ghibelli, L. Apoptosis as Driver of Therapy-Induced Cancer Repopulation and Acquired Cell-Resistance (CRAC): A Simple In Vitro Model of Phoenix Rising in Prostate Cancer. Int. J. Mol. Sci. 2022, 23, 1152. [Google Scholar] [CrossRef]
- Weiss, F.; Lauffenburger, D.; Friedl, P. Towards targeting of shared mechanisms of cancer metastasis and therapy resistance. Nat. Rev. Cancer 2022, 22, 157–173. [Google Scholar] [CrossRef] [PubMed]
- Heudobler, D.; Klobuch, S.; Lüke, F.; Hahn, J.; Grube, M.; Kremers, S.; Südhoff, T.; Westermann, J.; Huetter-Kroenke, M.-L.; Paschka, P.; et al. Low-Dose Azacitidine, Pioglitazone and All-Trans Retinoic Acid Versus Standard-Dose Azacitidine in Patients ≥ 60 Years with Acute Myeloid Leukemia Refractory to Standard Induction Chemotherapy (AMLSG 26-16/AML-ViVA): Results of the Safety Run-in Phase I. Blood 2019, 134, 1382. [Google Scholar] [CrossRef]
- Lüke, F.; Harrer, D.C.; Menhart, K.; Wolff, D.; Holler, E.; Hellwig, D.; Herr, W.; Grube, M.; Vogelhuber, M.; Reichle, A.; et al. Biomodulatory Treatment Regimen, MEPED, Rescues Relapsed and Refractory Classic Hodgkin’s Disease. Front. Pharmacol. 2021, 12, 599561. [Google Scholar] [CrossRef] [PubMed]
- Heudobler, D.; Elger, T.; Mayer, S.; Hart, C.; Vogelhuber, M.; Grube, M.; Hahn, J.; Lüke, F.; Ditz, D.; Günther, A.; et al. Biomodulatory therapy approach with lenalidomide in combination with pioglitazone, dexamethasone, and metronomic low-dose chemotherapy with treosulfan in patients with relapsed/refractory multiple myeloma > second-line. JCO 2019, 37, 8037. [Google Scholar] [CrossRef]
- Fante, M.A.; Felsenstein, M.; Mayer, S.; Gerken, M.; Klinkhammer-Schalke, M.; Herr, W.; Vogelhuber, M.; Reichle, A.; Heudobler, D. All-Oral Low-Dose Chemotherapy TEPIP is Effective and Well-Tolerated in Relapsed/Refractory Patients with Aggressive B-Cell Lymphoma. Front. Oncol. 2022, 12, 852987. [Google Scholar] [CrossRef] [PubMed]
- Harrer, D.C.; Jakob, M.; Vogelhuber, M.; Lüke, F.; Utpatel, K.; Corbacioglu, S.; Herr, W.; Reichle, A.; Heudobler, D. Biomodulatory therapy induces durable remissions in multi-system Langerhans cell histiocytosis. Leuk. Lymphoma 2022, 63, 2858–2868. [Google Scholar] [CrossRef]
- Walter, B.; Schrettenbrunner, I.; Vogelhuber, M.; Grassinger, J.; Bross, K.; Wilke, J.; Suedhoff, T.; Berand, A.; Wieland, W.F.; Rogenhofer, S.; et al. Pioglitazone, etoricoxib, interferon-α, and metronomic capecitabine for metastatic renal cell carcinoma: Final results of a prospective phase II trial. Med. Oncol. 2012, 29, 799–805. [Google Scholar] [CrossRef]
- Vogelhuber, M.; Feyerabend, S.; Stenzl, A.; Suedhoff, T.; Schulze, M.; Huebner, J.; Oberneder, R.; Wieland, W.; Mueller, S.; Eichhorn, F.; et al. Biomodulatory Treatment of Patients with Castration-Resistant Prostate Cancer: A Phase II Study of Imatinib with Pioglitazone, Etoricoxib, Dexamethasone and Low-Dose Treosulfan. Cancer Microenviron. 2015, 8, 33–41. [Google Scholar] [CrossRef]
- Hart, C.; Vogelhuber, M.; Wolff, D.; Klobuch, S.; Ghibelli, L.; Foell, J.; Corbacioglu, S.; Rehe, K.; Haegeman, G.; Thomas, S.; et al. Anakoinosis: Communicative Reprogramming of Tumor Systems—for Rescuing from Chemorefractory Neoplasia. Cancer Microenviron. 2015, 8, 75–92. [Google Scholar] [CrossRef]
- Walter, I.; Schulz, U.; Vogelhuber, M.; Wiedmann, K.; Endlicher, E.; Klebl, F.; Andreesen, R.; Herr, W.; Ghibelli, L.; Hackl, C.; et al. Communicative reprogramming non-curative hepatocellular carcinoma with low-dose metronomic chemotherapy, COX-2 inhibitor and PPAR-gamma agonist: A phase II trial. Med. Oncol. 2017, 34, 192. [Google Scholar] [CrossRef]
- Reichle, A.; Lugner, A.; Ott, C.; Klebl, F.; Vogelhuber, M.; Berand, A.; Andreesen, R. Control of cancer-associated inflammation and survival: Results from a prospective randomized phase II trial in gastric cancer. JCO 2009, 27, e15584. [Google Scholar] [CrossRef]
- Hart, C.; Vogelhuber, M.; Hafner, C.; Landthaler, M.; Berneburg, M.; Haferkamp, S.; Herr, W.; Reichle, A. Biomodulatory metronomic therapy in stage IV melanoma is well-tolerated and may induce prolonged progression-free survival, a phase I trial. J. Eur. Acad. Dermatol. Venereol. 2016, 30, e119–e121. [Google Scholar] [CrossRef] [PubMed]
- Heudobler, D.; Schulz, C.; Fischer, J.R.; Staib, P.; Wehler, T.; Südhoff, T.; Schichtl, T.; Wilke, J.; Hahn, J.; Lüke, F.; et al. A Randomized Phase II Trial Comparing the Efficacy and Safety of Pioglitazone, Clarithromycin and Metronomic Low-Dose Chemotherapy with Single-Agent Nivolumab Therapy in Patients with Advanced Non-small Cell Lung Cancer Treated in Second or Further Line (ModuLung). Front. Pharmacol. 2021, 12, 599598. [Google Scholar] [CrossRef] [PubMed]
- Vogt, T.; Hafner, C.; Bross, K.; Bataille, F.; Jauch, K.-W.; Berand, A.; Landthaler, M.; Andreesen, R.; Reichle, A. Antiangiogenetic therapy with pioglitazone, rofecoxib, and metronomic trofosfamide in patients with advanced malignant vascular tumors. Cancer 2003, 98, 2251–2256. [Google Scholar] [CrossRef]
- Reichle, A.; Vogt, T. Systems biology: A therapeutic target for tumor therapy. Cancer Microenviron. 2008, 1, 159–170. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Reichle, A.; Klebl, F.; Bross, K.; Kullmann, F.; Wild, P.; Berand, A.; Krause, S.W.; Schölmerich, J.; Andreesen, R. Pioglitazone and Rofecoxib Combined with Angiostatically Scheduled Capecitabine in Far-Advanced Hepatobiliary Carcinoma. In From Molecular to Modular Tumor Therapy; Reichle, A., Ed.; Springer Netherlands: Dordrecht, The Netherlands, 2010; pp. 341–352. ISBN 978-90-481-9530-5. [Google Scholar][Green Version]
- Ugocsai, P.; Wolff, D.; Menhart, K.; Hellwig, D.; Holler, E.; Herr, W.; Reichle, A. Biomodulatory metronomic therapy induces PET-negative remission in chemo- and brentuximab-refractory Hodgkin lymphoma. Br. J. Haematol. 2016, 172, 290–293. [Google Scholar] [CrossRef] [PubMed]
- Reichle, A.; Vogt, T.; Kunz-Schughart, L.; Bretschneider, T.; Bachthaler, M.; Bross, K.; Freund, S.; Andreesen, R. Anti-inflammatory and angiostatic therapy in chemorefractory multisystem Langerhans’ cell histiocytosis of adults. Br. J. Haematol. 2005, 128, 730–732. [Google Scholar] [CrossRef] [PubMed]
- Petrella, G.; Corsi, F.; Ciufolini, G.; Germini, S.; Capradossi, F.; Pelliccia, A.; Torino, F.; Ghibelli, L.; Cicero, D.O. Metabolic Reprogramming of Castration-Resistant Prostate Cancer Cells as a Response to Chemotherapy. Metabolites 2022, 13, 65. [Google Scholar] [CrossRef]
- Walter, B.; Rogenhofer, S.; Vogelhuber, M.; Berand, A.; Wieland, W.F.; Andreesen, R.; Reichle, A. Modular therapy approach in metastatic castration-refractory prostate cancer. World J. Urol. 2010, 28, 745–750. [Google Scholar] [CrossRef]
- Hau, P.; Kunz-Schughart, L.; Bogdahn, U.; Baumgart, U.; Hirschmann, B.; Weimann, E.; Muhleisen, H.; Ruemmele, P.; Steinbrecher, A.; Reichle, A. Low-dose chemotherapy in combination with COX-2 inhibitors and PPAR-gamma agonists in recurrent high-grade gliomas—a phase II study. Oncology 2007, 73, 21–25. [Google Scholar] [CrossRef]
- Nakade, S.; Yamamoto, T.; Sakuma, T. Cancer induction and suppression with transcriptional control and epigenome editing technologies. J. Hum. Genet. 2018, 63, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Frezza, V.; Chellini, L.; Del Verme, A.; Paronetto, M.P. RNA Editing in Cancer Progression. Cancers 2023, 15, 5277. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, A.A.; Lim, W.A.; Qi, L.S. Beyond editing: Repurposing CRISPR-Cas9 for precision genome regulation and interrogation. Nat. Rev. Mol. Cell Biol. 2016, 17, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Voigt, P.; Reinberg, D. Epigenome editing. Nat. Biotechnol. 2013, 31, 1097–1099. [Google Scholar] [CrossRef] [PubMed]
- Reichle, A.; Hildebrandt, G.C. Principles of modular tumor therapy. Cancer Microenviron. 2009, 2, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Lüke, F.; Harrer, D.C.; Pantziarka, P.; Pukrop, T.; Ghibelli, L.; Gerner, C.; Reichle, A.; Heudobler, D. Drug Repurposing by Tumor Tissue Editing. Front. Oncol. 2022, 12, 900985. [Google Scholar] [CrossRef] [PubMed]
- Heudobler, D.; Reichle, A.; Ghibelli, L. Editorial: Anakoinosis: An Innovative Anticancer Therapy Targeting the Aberrant Cancer Tissue Homeostasis. Front. Pharmacol. 2021, 12, 779021. [Google Scholar] [CrossRef]
- Heudobler, D.; Rechenmacher, M.; Lüke, F.; Vogelhuber, M.; Klobuch, S.; Thomas, S.; Pukrop, T.; Hackl, C.; Herr, W.; Ghibelli, L.; et al. Clinical Efficacy of a Novel Therapeutic Principle, Anakoinosis. Front. Pharmacol. 2018, 9, 1357. [Google Scholar] [CrossRef]
- Heudobler, D.; Lüke, F.; Vogelhuber, M.; Klobuch, S.; Pukrop, T.; Herr, W.; Gerner, C.; Pantziarka, P.; Ghibelli, L.; Reichle, A. Anakoinosis: Correcting Aberrant Homeostasis of Cancer Tissue-Going Beyond Apoptosis Induction. Front. Oncol. 2019, 9, 1408. [Google Scholar] [CrossRef]
- Reichle, A.; Vogt, T.; Coras, B.; Terheyden, P.; Neuber, K.; Trefzer, U.; Schultz, E.; Berand, A.; Bröcker, E.B.; Landthaler, M.; et al. Targeted combined anti-inflammatory and angiostatic therapy in advanced melanoma: A randomized phase II trial. Melanoma Res. 2007, 17, 360–364. [Google Scholar] [CrossRef]
- Vogt, T.; Coras, B.; Hafner, C.; Landthaler, M.; Reichle, A. Antiangiogenic therapy in metastatic prostate carcinoma complicated by cutaneous lupus erythematodes. Lancet Oncol. 2006, 7, 695–697. [Google Scholar] [CrossRef] [PubMed]
- Reichle, A.; Grassinger, J.; Bross, K.; Wilke, J.; Suedhoff, T.; Walter, B.; Wieland, W.-F.; Berand, A.; Andreesen, R. C-reactive Protein in Patients with Metastatic Clear Cell Renal Carcinoma: An Important Biomarker for Tumor-associated Inflammation. Biomark. Insights 2007, 1, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.; Schelker, R.; Klobuch, S.; Zaiss, S.; Troppmann, M.; Rehli, M.; Haferlach, T.; Herr, W.; Reichle, A. Biomodulatory therapy induces complete molecular remission in chemorefractory acute myeloid leukemia. Haematologica 2015, 100, e4–e6. [Google Scholar] [CrossRef] [PubMed]
- Heudobler, D.; Luke, F.; Hahn, J.; Grube, M.; Schlosser, P.; Kremers, S.; Sudhoff, T.; Westermann, J.; Hutter-Kronke, M.L.; Schlenk, R.F.; et al. Low-dose azacitidine, pioglitazone and all-trans retinoic acid is safe in patients aged ≥60 years with acute myeloid leukemia refractory to standard induction chemotherapy (AMLSG 26-16/AML-ViVA): Results of the safety run-in phase. Haematologica 2023. [CrossRef] [PubMed]
- Heudobler, D.; Klobuch, S.; Thomas, S.; Hahn, J.; Herr, W.; Reichle, A. Cutaneous Leukemic Infiltrates Successfully Treated with Biomodulatory Therapy in a Rare Case of Therapy-Related High Risk MDS/AML. Front. Pharmacol. 2018, 9, 1279. [Google Scholar] [CrossRef] [PubMed]
- Kattner, A.-S.; Holler, E.; Herr, W.; Reichle, A.; Wolff, D.; Heudobler, D. Successful Treatment of Early Relapsed High-Risk AML After Allogeneic Hematopoietic Stem Cell Transplantation with Biomodulatory Therapy. Front. Oncol. 2020, 10, 443. [Google Scholar] [CrossRef] [PubMed]
- Arnol, D.; Schapiro, D.; Bodenmiller, B.; Saez-Rodriguez, J.; Stegle, O. Modeling Cell-Cell Interactions from Spatial Molecular Data with Spatial Variance Component Analysis. Cell Rep. 2019, 29, 202–211.e6. [Google Scholar] [CrossRef]
- Ishay-Ronen, D.; Diepenbruck, M.; Kalathur, R.K.R.; Sugiyama, N.; Tiede, S.; Ivanek, R.; Bantug, G.; Morini, M.F.; Wang, J.; Hess, C.; et al. Gain Fat-Lose Metastasis: Converting Invasive Breast Cancer Cells into Adipocytes Inhibits Cancer Metastasis. Cancer Cell 2019, 35, 17–32.e6. [Google Scholar] [CrossRef]
- Klobuch, S.; Steinberg, T.; Bruni, E.; Mirbeth, C.; Heilmeier, B.; Ghibelli, L.; Herr, W.; Reichle, A.; Thomas, S. Biomodulatory Treatment with Azacitidine, All-trans Retinoic Acid and Pioglitazone Induces Differentiation of Primary AML Blasts into Neutrophil Like Cells Capable of ROS Production and Phagocytosis. Front. Pharmacol. 2018, 9, 1380. [Google Scholar] [CrossRef]
- Weber, R.J.; Desai, T.A.; Gartner, Z.J. Non-autonomous cell proliferation in the mammary gland and cancer. Curr. Opin. Cell Biol. 2017, 45, 55–61. [Google Scholar] [CrossRef]
- Thies, K.A.; Lefler, J.E.; Leone, G.; Ostrowski, M.C. PTEN in the Stroma. Cold Spring Harb. Perspect. Med. 2019, 9, a036111. [Google Scholar] [CrossRef] [PubMed]
- Berger, A.H.; Knudson, A.G.; Pandolfi, P.P. A continuum model for tumour suppression. Nature 2011, 476, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Jaaks, P.; Coker, E.A.; Vis, D.J.; Edwards, O.; Carpenter, E.F.; Leto, S.M.; Dwane, L.; Sassi, F.; Lightfoot, H.; Barthorpe, S.; et al. Effective drug combinations in breast, colon and pancreatic cancer cells. Nature 2022, 603, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Heudobler, D.; Rechenmacher, M.; Lüke, F.; Vogelhuber, M.; Pukrop, T.; Herr, W.; Ghibelli, L.; Gerner, C.; Reichle, A. Peroxisome Proliferator-Activated Receptors (PPAR)γ Agonists as Master Modulators of Tumor Tissue. Int. J. Mol. Sci. 2018, 19, 3540. [Google Scholar] [CrossRef] [PubMed]
- Jabs, W.J.; Busse, M.; Krüger, S.; Jocham, D.; Steinhoff, J.; Doehn, C. Expression of C-reactive protein by renal cell carcinomas and unaffected surrounding renal tissue. Kidney Int. 2005, 68, 2103–2110. [Google Scholar] [CrossRef] [PubMed]
- Patil, V.M.; Noronha, V.; Menon, N.; Rai, R.; Bhattacharjee, A.; Singh, A.; Nawale, K.; Jogdhankar, S.; Tambe, R.; Dhumal, S.; et al. Low-Dose Immunotherapy in Head and Neck Cancer: A Randomized Study. JCO 2023, 41, 222–232. [Google Scholar] [CrossRef]
- Chen, Y.-L.; Chang, M.-C.; Cheng, W.-F. Metronomic chemotherapy and immunotherapy in cancer treatment. Cancer Lett. 2017, 400, 282–292. [Google Scholar] [CrossRef]
- Cazzaniga, M.E.; Cordani, N.; Capici, S.; Cogliati, V.; Riva, F.; Cerrito, M.G. Metronomic Chemotherapy. Cancers 2021, 13, 2236. [Google Scholar] [CrossRef]
- Joaquin Garcia, A.; Rediti, M.; Venet, D.; Majjaj, S.; Kammler, R.; Munzone, E.; Gianni, L.; Thürlimann, B.; Laáng, I.; Colleoni, M.; et al. Differential Benefit of Metronomic Chemotherapy Among Triple-Negative Breast Cancer Subtypes Treated in the IBCSG Trial 22-00. Clin. Cancer Res. 2023, 29, 4908–4919. [Google Scholar] [CrossRef]
- Sambath, J.; Noronha, V.; Manda, S.S.; Mishra, R.; Chandrani, P.; Patil, V.; Menon, N.; Chougule, A.; Ramachandran, V.; Limaye, S.; et al. Whole exome sequencing uncovers HRAS mutations as potential mediators of resistance to metronomic chemotherapy. Gene 2024, 893, 147952. [Google Scholar] [CrossRef]
- Vo, J.N.; Wu, Y.-M.; Mishler, J.; Hall, S.; Mannan, R.; Wang, L.; Ning, Y.; Zhou, J.; Hopkins, A.C.; Estill, J.C.; et al. The genetic heterogeneity and drug resistance mechanisms of relapsed refractory multiple myeloma. Nat. Commun. 2022, 13, 3750. [Google Scholar] [CrossRef] [PubMed]
- Haertle, L.; Barrio, S.; Munawar, U.; Han, S.; Zhou, X.; Vogt, C.; Fernández, R.A.; Bittrich, M.; Ruiz-Heredia, Y.; Da Viá, M.; et al. Cereblon enhancer methylation and IMiD resistance in multiple myeloma. Blood 2021, 138, 1721–1726. [Google Scholar] [CrossRef] [PubMed]
- Schürch, C.M.; Rasche, L.; Frauenfeld, L.; Weinhold, N.; Fend, F. A review on tumor heterogeneity and evolution in multiple myeloma: Pathological, radiological, molecular genetics, and clinical integration. Virchows Arch. 2020, 476, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Larrayoz, M.; Garcia-Barchino, M.J.; Celay, J.; Etxebeste, A.; Jimenez, M.; Perez, C.; Ordoñez, R.; Cobaleda, C.; Botta, C.; Fresquet, V.; et al. Preclinical models for prediction of immunotherapy outcomes and immune evasion mechanisms in genetically heterogeneous multiple myeloma. Nat. Med. 2023, 29, 632–645. [Google Scholar] [CrossRef] [PubMed]
- Klein, C.A. Cancer progression and the invisible phase of metastatic colonization. Nat. Rev. Cancer 2020, 20, 681–694. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.-Y.; Zhai, H.-R.; Hou, Q.-Y.; Su, J.; Liu, S.-Y.; Yan, H.-H.; Li, Y.-S.; Chen, Z.-Y.; Zhong, W.-Z.; Wu, Y.-L. Mixed Responses to Systemic Therapy Revealed Potential Genetic Heterogeneity and Poor Survival in Patients with Non-Small Cell Lung Cancer. Oncologist 2017, 22, 61–69. [Google Scholar] [CrossRef]
- Morana, O.; Wood, W.; Gregory, C.D. The Apoptosis Paradox in Cancer. Int. J. Mol. Sci. 2022, 23, 1328. [Google Scholar] [CrossRef]
- Wild, C.P. The exposome: From concept to utility. Int. J. Epidemiol. 2012, 41, 24–32. [Google Scholar] [CrossRef]
- Miller, G.W.; Jones, D.P. The nature of nurture: Refining the definition of the exposome. Toxicol. Sci. 2014, 137, 1–2. [Google Scholar] [CrossRef]
- Danenberg, E.; Bardwell, H.; Zanotelli, V.R.T.; Provenzano, E.; Chin, S.-F.; Rueda, O.M.; Green, A.; Rakha, E.; Aparicio, S.; Ellis, I.O.; et al. Breast tumor microenvironment structures are associated with genomic features and clinical outcome. Nat. Genet. 2022, 54, 660–669. [Google Scholar] [CrossRef]
- Dvorak, H.F. Tumors: Wounds that do not heal. Similarities between tumor stroma generation and wound healing. N. Engl. J. Med. 1986, 315, 1650–1659. [Google Scholar] [CrossRef] [PubMed]
- Zimmerlin, L.; Park, T.S.; Zambidis, E.T.; Donnenberg, V.S.; Donnenberg, A.D. Mesenchymal stem cell secretome and regenerative therapy after cancer. Biochimie 2013, 95, 2235–2245. [Google Scholar] [CrossRef] [PubMed]
- Stein, J.E.; Lipson, E.J.; Cottrell, T.R.; Forde, P.M.; Anders, R.A.; Cimino-Mathews, A.; Thompson, E.D.; Allaf, M.E.; Yarchoan, M.; Feliciano, J.; et al. Pan-Tumor Pathologic Scoring of Response to PD-(L)1 Blockade. Clin. Cancer Res. 2020, 26, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Cottrell, T.R.; Thompson, E.D.; Forde, P.M.; Stein, J.E.; Duffield, A.S.; Anagnostou, V.; Rekhtman, N.; Anders, R.A.; Cuda, J.D.; Illei, P.B.; et al. Pathologic features of response to neoadjuvant anti-PD-1 in resected non-small-cell lung carcinoma: A proposal for quantitative immune-related pathologic response criteria (irPRC). Ann. Oncol. 2018, 29, 1853–1860. [Google Scholar] [CrossRef] [PubMed]
- Goff, P.H.; Riolobos, L.; LaFleur, B.J.; Spraker, M.B.; Seo, Y.D.; Smythe, K.S.; Campbell, J.S.; Pierce, R.H.; Zhang, Y.; He, Q.; et al. Neoadjuvant Therapy Induces a Potent Immune Response to Sarcoma, Dominated by Myeloid and B Cells. Clin. Cancer Res. 2022, 28, 1701–1711. [Google Scholar] [CrossRef]
- Rawson, R.V.; Adhikari, C.; Bierman, C.; Lo, S.N.; Shklovskaya, E.; Rozeman, E.A.; Menzies, A.M.; van Akkooi, A.C.J.; Shannon, K.F.; Gonzalez, M.; et al. Pathological response and tumour bed histopathological features correlate with survival following neoadjuvant immunotherapy in stage III melanoma. Ann. Oncol. 2021, 32, 766–777. [Google Scholar] [CrossRef]
- Labrie, M.; Brugge, J.S.; Mills, G.B.; Zervantonakis, I.K. Therapy resistance: Opportunities created by adaptive responses to targeted therapies in cancer. Nat. Rev. Cancer 2022, 22, 323–339. [Google Scholar] [CrossRef]
- Khalaf, K.; Hana, D.; Chou, J.T.-T.; Singh, C.; Mackiewicz, A.; Kaczmarek, M. Aspects of the Tumor Microenvironment Involved in Immune Resistance and Drug Resistance. Front. Immunol. 2021, 12, 656364. [Google Scholar] [CrossRef]
- Jing, X.; Yang, F.; Shao, C.; Wei, K.; Xie, M.; Shen, H.; Shu, Y. Role of hypoxia in cancer therapy by regulating the tumor microenvironment. Mol. Cancer 2019, 18, 157. [Google Scholar] [CrossRef]
- McAleese, C.E.; Choudhury, C.; Butcher, N.J.; Minchin, R.F. Hypoxia-mediated drug resistance in breast cancers. Cancer Lett. 2021, 502, 189–199. [Google Scholar] [CrossRef]
- Buyuk, B.; Jin, S.; Ye, K. Epithelial-to-Mesenchymal Transition Signaling Pathways Responsible for Breast Cancer Metastasis. Cell. Mol. Bioeng. 2022, 15, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Savage, P.; Stebbing, J.; Bower, M.; Crook, T. Why does cytotoxic chemotherapy cure only some cancers? Nat. Clin. Pract. Oncol. 2009, 6, 43–52. [Google Scholar] [CrossRef] [PubMed]
- D’Alterio, C.; Scala, S.; Sozzi, G.; Roz, L.; Bertolini, G. Paradoxical effects of chemotherapy on tumor relapse and metastasis promotion. Semin. Cancer Biol. 2020, 60, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Norton, L. Evolving concepts in the systemic drug therapy of breast cancer. Semin. Oncol. 1997, 24, S10-3–S10-10. [Google Scholar]
- Welch, D.R.; Hurst, D.R. Defining the Hallmarks of Metastasis. Cancer Res. 2019, 79, 3011–3027. [Google Scholar] [CrossRef]
- Friedl, P.; Alexander, S. Cancer invasion and the microenvironment: Plasticity and reciprocity. Cell 2011, 147, 992–1009. [Google Scholar] [CrossRef]
- Quail, D.F.; Joyce, J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013, 19, 1423–1437. [Google Scholar] [CrossRef]
- Hamidi, H.; Ivaska, J. Every step of the way: Integrins in cancer progression and metastasis. Nat. Rev. Cancer 2018, 18, 533–548. [Google Scholar] [CrossRef]
- Obenauf, A.C.; Zou, Y.; Ji, A.L.; Vanharanta, S.; Shu, W.; Shi, H.; Kong, X.; Bosenberg, M.C.; Wiesner, T.; Rosen, N.; et al. Therapy-induced tumour secretomes promote resistance and tumour progression. Nature 2015, 520, 368–372. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.-Q.; Yang, Z.-L.; Wang, L.; Zhang, J.-C.; Sun, Y.-F.; Li, Z.-Y.; Qin, L. NCAPH regulates gastric cancer progression through DNA damage response. Neoplasma 2022, 69, 283–291. [Google Scholar] [CrossRef]
- Konen, J.M.; Rodriguez, B.L.; Padhye, A.; Ochieng, J.K.; Gibson, L.; Diao, L.; Fowlkes, N.W.; Fradette, J.J.; Peng, D.H.; Cardnell, R.J.; et al. Dual Inhibition of MEK and AXL Targets Tumor Cell Heterogeneity and Prevents Resistant Outgrowth Mediated by the Epithelial-to-Mesenchymal Transition in NSCLC. Cancer Res. 2021, 81, 1398–1412. [Google Scholar] [CrossRef] [PubMed]
- Thomalla, D.; Beckmann, L.; Grimm, C.; Oliverio, M.; Meder, L.; Herling, C.D.; Nieper, P.; Feldmann, T.; Merkel, O.; Lorsy, E.; et al. Deregulation and epigenetic modification of BCL2-family genes cause resistance to venetoclax in hematologic malignancies. Blood 2022, 140, 2113–2126. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Metzger, E.; Schüle, R.; Kirfel, J.; Buettner, R. Epigenetic regulation of cancer growth by histone demethylases. Int. J. Cancer 2010, 127, 1991–1998. [Google Scholar] [CrossRef] [PubMed]
- Pan, G.; Liu, Y.; Shang, L.; Zhou, F.; Yang, S. EMT-associated microRNAs and their roles in cancer stemness and drug resistance. Cancer Commun. 2021, 41, 199–217. [Google Scholar] [CrossRef] [PubMed]
- Bakir, B.; Chiarella, A.M.; Pitarresi, J.R.; Rustgi, A.K. EMT, MET, Plasticity, and Tumor Metastasis. Trends Cell Biol. 2020, 30, 764–776. [Google Scholar] [CrossRef] [PubMed]
- Blau, O.; Baldus, C.D.; Hofmann, W.-K.; Thiel, G.; Nolte, F.; Burmeister, T.; Türkmen, S.; Benlasfer, O.; Schümann, E.; Sindram, A.; et al. Mesenchymal stromal cells of myelodysplastic syndrome and acute myeloid leukemia patients have distinct genetic abnormalities compared with leukemic blasts. Blood 2011, 118, 5583–5592. [Google Scholar] [CrossRef] [PubMed]
- Rasche, L.; Chavan, S.S.; Stephens, O.W.; Patel, P.H.; Tytarenko, R.; Ashby, C.; Bauer, M.; Stein, C.; Deshpande, S.; Wardell, C.; et al. Spatial genomic heterogeneity in multiple myeloma revealed by multi-region sequencing. Nat. Commun. 2017, 8, 268. [Google Scholar] [CrossRef]
- Afify, S.M.; Hassan, G.; Seno, A.; Seno, M. Cancer-inducing niche: The force of chronic inflammation. Br. J. Cancer 2022, 127, 193–201. [Google Scholar] [CrossRef]
- Teresi, R.E.; Waite, K.A. PPARγ, PTEN, and the Fight against Cancer. PPAR Res. 2008, 2008, 932632. [Google Scholar] [CrossRef]
- Álvarez-Garcia, V.; Tawil, Y.; Wise, H.M.; Leslie, N.R. Mechanisms of PTEN loss in cancer: It’s all about diversity. Semin. Cancer Biol. 2019, 59, 66–79. [Google Scholar] [CrossRef]
- Aramini, B.; Masciale, V.; Grisendi, G.; Bertolini, F.; Maur, M.; Guaitoli, G.; Chrystel, I.; Morandi, U.; Stella, F.; Dominici, M.; et al. Dissecting Tumor Growth: The Role of Cancer Stem Cells in Drug Resistance and Recurrence. Cancers 2022, 14, 976. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, T.; Ramachandran, V.; Fournier, K.F.; Wang, H.; Marquis, L.; Abbruzzese, J.L.; Gallick, G.E.; Logsdon, C.D.; McConkey, D.J.; Choi, W. Epithelial to mesenchymal transition contributes to drug resistance in pancreatic cancer. Cancer Res. 2009, 69, 5820–5828. [Google Scholar] [CrossRef] [PubMed]
- Berger, A.H.; Pandolfi, P.P. Haplo-insufficiency: A driving force in cancer. J. Pathol. 2011, 223, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.S.; Yip, Y.S.; Lim, E.K.Y.; Wahli, W.; Tan, N.S. PPARs and Tumor Microenvironment: The Emerging Roles of the Metabolic Master Regulators in Tumor Stromal-Epithelial Crosstalk and Carcinogenesis. Cancers 2021, 13, 2153. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, P.S.; Chamoto, K.; Kumar, A.; Honjo, T. PPAR-Induced Fatty Acid Oxidation in T Cells Increases the Number of Tumor-Reactive CD8+ T Cells and Facilitates Anti-PD-1 Therapy. Cancer Immunol. Res. 2018, 6, 1375–1387. [Google Scholar] [CrossRef]
- Denison, T.A.; Bae, Y.H. Tumor heterogeneity and its implication for drug delivery. J. Control. Release 2012, 164, 187–191. [Google Scholar] [CrossRef]
- Du, L.; Cheng, Q.; Zheng, H.; Liu, J.; Liu, L.; Chen, Q. Targeting stemness of cancer stem cells to fight colorectal cancers. Semin. Cancer Biol. 2022, 82, 150–161. [Google Scholar] [CrossRef]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef]
- Peng, D.; Fu, M.; Wang, M.; Wei, Y.; Wei, X. Targeting TGF-β signal transduction for fibrosis and cancer therapy. Mol. Cancer 2022, 21, 104. [Google Scholar] [CrossRef]
- Sanford-Crane, H.; Abrego, J.; Sherman, M.H. Fibroblasts as Modulators of Local and Systemic Cancer Metabolism. Cancers 2019, 11, 619. [Google Scholar] [CrossRef]
- Alieva, M.; van Rheenen, J.; Broekman, M.L.D. Potential impact of invasive surgical procedures on primary tumor growth and metastasis. Clin. Exp. Metastasis 2018, 35, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zeh, H.J.; Kang, R.; Kroemer, G.; Tang, D. Cell death in pancreatic cancer: From pathogenesis to therapy. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 804–823. [Google Scholar] [CrossRef] [PubMed]
- Kasprzak, A. The Role of Tumor Microenvironment Cells in Colorectal Cancer (CRC) Cachexia. Int. J. Mol. Sci. 2021, 22, 1565. [Google Scholar] [CrossRef] [PubMed]
- Labi, V.; Erlacher, M. How cell death shapes cancer. Cell Death Dis. 2015, 6, e1675. [Google Scholar] [CrossRef] [PubMed]
- Haj-Shomaly, J.; Vorontsova, A.; Barenholz-Cohen, T.; Levi-Galibov, O.; Devarasetty, M.; Timaner, M.; Raviv, Z.; Cooper, T.J.; Soker, S.; Hasson, P.; et al. T Cells Promote Metastasis by Regulating Extracellular Matrix Remodeling following Chemotherapy. Cancer Res. 2022, 82, 278–291. [Google Scholar] [CrossRef] [PubMed]
- Ferri-Borgogno, S.; Zhu, Y.; Sheng, J.; Burks, J.K.; Gomez, J.A.; Wong, K.K.; Wong, S.T.C.; Mok, S.C. Spatial Transcriptomics Depict Ligand-Receptor Cross-talk Heterogeneity at the Tumor-Stroma Interface in Long-Term Ovarian Cancer Survivors. Cancer Res. 2023, 83, 1503–1516. [Google Scholar] [CrossRef] [PubMed]
- Ishay-Ronen, D.; Christofori, G. Targeting Cancer Cell Metastasis by Converting Cancer Cells into Fat. Cancer Res. 2019, 79, 5471–5475. [Google Scholar] [CrossRef]
- Bar-Hai, N.; Ishay-Ronen, D. Engaging plasticity: Differentiation therapy in solid tumors. Front. Pharmacol. 2022, 13, 944773. [Google Scholar] [CrossRef]
- Deyell, M.; Garris, C.S.; Laughney, A.M. Cancer metastasis as a non-healing wound. Br. J. Cancer 2021, 124, 1491–1502. [Google Scholar] [CrossRef]
- Meng, F.; Henson, R.; Patel, T. Chemotherapeutic stress selectively activates NF-kappa B-dependent AKT and VEGF expression in liver cancer-derived endothelial cells. Am. J. Physiol. Cell Physiol. 2007, 293, C749-60. [Google Scholar] [CrossRef]
- From Molecular to Modular Tumor Therapy; Reichle, A., Ed.; Springer: Dordrecht, The Netherlands, 2010; ISBN 978-90-481-9530-5. [Google Scholar]
- Matesanz, S.; Blanco-Sánchez, M.; Ramos-Muñoz, M.; de La Cruz, M.; Benavides, R.; Escudero, A. Phenotypic integration does not constrain phenotypic plasticity: Differential plasticity of traits is associated to their integration across environments. New Phytol. 2021, 231, 2359–2370. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Lai, Y.; Zhang, R.; Daoud, A.; Feng, Q.; Zhou, J.; Shang, J. Low Dose Cyclophosphamide Modulates Tumor Microenvironment by TGF-β Signaling Pathway. Int. J. Mol. Sci. 2020, 21, 957. [Google Scholar] [CrossRef] [PubMed]
- Doloff, J.C.; Waxman, D.J. Transcriptional profiling provides insights into metronomic cyclophosphamide-activated, innate immune-dependent regression of brain tumor xenografts. BMC Cancer 2015, 15, 375. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, R.; Girotti, A.; Hileeto, D.; Arias, F.J. Metronomic Anti-Cancer Therapy: A Multimodal Therapy Governed by the Tumor Microenvironment. Cancers 2021, 13, 5414. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, Y.; Weeraratna, A.T. Fibroblasts in cancer: Unity in heterogeneity. Cell 2023, 186, 1580–1609. [Google Scholar] [CrossRef] [PubMed]
- Bondarenko, M.; Le Grand, M.; Shaked, Y.; Raviv, Z.; Chapuisat, G.; Carrère, C.; Montero, M.-P.; Rossi, M.; Pasquier, E.; Carré, M.; et al. Metronomic Chemotherapy Modulates Clonal Interactions to Prevent Drug Resistance in Non-Small Cell Lung Cancer. Cancers 2021, 13, 2239. [Google Scholar] [CrossRef]
- Ho-Xuan, H.; Lehmann, G.; Glazar, P.; Gypas, F.; Eichner, N.; Heizler, K.; Schlitt, H.J.; Zavolan, M.; Rajewsky, N.; Meister, G.; et al. Gene Expression Signatures of a Preclinical Mouse Model during Colorectal Cancer Progression under Low-Dose Metronomic Chemotherapy. Cancers 2020, 13, 49. [Google Scholar] [CrossRef]
- Tran, A.P.; Ali Al-Radhawi, M.; Kareva, I.; Wu, J.; Waxman, D.J.; Sontag, E.D. Delicate Balances in Cancer Chemotherapy: Modeling Immune Recruitment and Emergence of Systemic Drug Resistance. Front. Immunol. 2020, 11, 1376. [Google Scholar] [CrossRef]
- Mitra Ghosh, T.; White, J.; Davis, J.; Mazumder, S.; Kansom, T.; Skarupa, E.; Barnett, G.S.; Piazza, G.A.; Bird, R.C.; Mitra, A.K.; et al. Identification and Characterization of Key Differentially Expressed Genes Associated with Metronomic Dosing of Topotecan in Human Prostate Cancer. Front. Pharmacol. 2021, 12, 736951. [Google Scholar] [CrossRef]
- Nagel, R.; Semenova, E.A.; Berns, A. Drugging the addict: Non-oncogene addiction as a target for cancer therapy. EMBO Rep. 2016, 17, 1516–1531. [Google Scholar] [CrossRef]
- Lines, C.L.; McGrath, M.J.; Dorwart, T.; Conn, C.S. The integrated stress response in cancer progression: A force for plasticity and resistance. Front. Oncol. 2023, 13, 1206561. [Google Scholar] [CrossRef] [PubMed]
- Benzekry, S.; Pasquier, E.; Barbolosi, D.; Lacarelle, B.; Barlési, F.; André, N.; Ciccolini, J. Metronomic reloaded: Theoretical models bringing chemotherapy into the era of precision medicine. Semin. Cancer Biol. 2015, 35, 53–61. [Google Scholar] [CrossRef] [PubMed]
- André, N.; Banavali, S.; Snihur, Y.; Pasquier, E. Has the time come for metronomics in low-income and middle-income countries? Lancet Oncol. 2013, 14, e239–e248. [Google Scholar] [CrossRef] [PubMed]
- Ergun, Y. Common Sense Oncology: Including everyone. Lancet Oncol. 2023, 24, e403. [Google Scholar] [CrossRef] [PubMed]
- Georgakis, G.V.; Li, Y.; Rassidakis, G.Z.; Medeiros, L.J.; Mills, G.B.; Younes, A. Inhibition of the phosphatidylinositol-3 kinase/Akt promotes G1 cell cycle arrest and apoptosis in Hodgkin lymphoma. Br. J. Haematol. 2006, 132, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.; Lupinacci, L.; Perazzo, F.; Bas, C.; Carranza, O.; Puparelli, C.; Kowalyszyn, R.; Magri, I.; Varela, M.; Richardet, E.; et al. Efficacy and Safety of Nivolumab in Previously Treated Patients with Non-Small-cell Lung Cancer: Real World Experience in Argentina. Clin. Lung Cancer 2020, 21, e380–e387. [Google Scholar] [CrossRef] [PubMed]
- Youn, B.; Trikalinos, N.A.; Mor, V.; Wilson, I.B.; Dahabreh, I.J. Real-world use and survival outcomes of immune checkpoint inhibitors in older adults with non-small cell lung cancer. Cancer 2020, 126, 978–985. [Google Scholar] [CrossRef]
- Johnston, P.B.; Inwards, D.J.; Colgan, J.P.; Laplant, B.R.; Kabat, B.F.; Habermann, T.M.; Micallef, I.N.; Porrata, L.F.; Ansell, S.M.; Reeder, C.B.; et al. A Phase II trial of the oral mTOR inhibitor everolimus in relapsed Hodgkin lymphoma. Am. J. Hematol. 2010, 85, 320–324. [Google Scholar] [CrossRef]
- Ballav, S.; Biswas, B.; Sahu, V.K.; Ranjan, A.; Basu, S. PPAR-γ Partial Agonists in Disease-Fate Decision with Special Reference to Cancer. Cells 2022, 11, 3215. [Google Scholar] [CrossRef]
- Gillessen, S.; Hüttmann, A.; Vucinic, V.; Müller, H.; Plütschow, A.; Viardot, A.; Topp, M.S.; Kobe, C.; Böll, B.; Eichenauer, D.A.; et al. Reinduction therapy with everolimus in combination with dexamethasone, high-dose cytarabin and cisplatinum in patients with relapsed or refractory classical Hodgkin lymphoma: An experimental phase I/II multicentre trial of the German Hodgkin Study Group (GHSG HD-R3i). Br. J. Haematol. 2022, 196, 606–616. [Google Scholar] [CrossRef]
- Brown, R.E. The NF-kappaB pathway and the successful application of anti-inflammatory and angiostatic therapy in Langerhans’ cell histiocytosis. Br. J. Haematol. 2005, 130, 147–148. [Google Scholar] [CrossRef] [PubMed]
- Singhal, A.; Kumar, S. Neutrophil and remnant clearance in immunity and inflammation. Immunology 2022, 165, 22–43. [Google Scholar] [CrossRef] [PubMed]
- Schlenk, R.F.; Weber, D.; Krzykalla, J.; Kindler, T.; Wulf, G.; Hertenstein, B.; Salih, H.R.; Südhoff, T.; Krauter, J.; Martens, U.; et al. Randomized phase-III study of low-dose cytarabine and etoposide + /- all-trans retinoic acid in older unfit patients with NPM1-mutated acute myeloid leukemia. Sci. Rep. 2023, 13, 14809. [Google Scholar] [CrossRef]
- Ye, L.; Jin, F.; Kumar, S.K.; Dai, Y. The mechanisms and therapeutic targets of ferroptosis in cancer. Expert Opin. Ther. Targets 2021, 25, 965–986. [Google Scholar] [CrossRef] [PubMed]
- La Rojo de Vega, M. A holistic view of cancer. Cancer Cell 2023, 41, 373. [Google Scholar] [CrossRef] [PubMed]
- Meier-Menches, S.M.; Neuditschko, B.; Janker, L.; Gerner, M.C.; Schmetterer, K.G.; Reichle, A.; Gerner, C. A Proteomic Platform Enables to Test for AML Normalization In Vitro. Front. Chem. 2022, 10, 826346. [Google Scholar] [CrossRef] [PubMed]
- Muqaku, B.; Eisinger, M.; Meier, S.M.; Tahir, A.; Pukrop, T.; Haferkamp, S.; Slany, A.; Reichle, A.; Gerner, C. Multi-omics Analysis of Serum Samples Demonstrates Reprogramming of Organ Functions Via Systemic Calcium Mobilization and Platelet Activation in Metastatic Melanoma. Mol. Cell. Proteom. 2017, 16, 86–99. [Google Scholar] [CrossRef]
- Giesen, C.; Wang, H.A.O.; Schapiro, D.; Zivanovic, N.; Jacobs, A.; Hattendorf, B.; Schüffler, P.J.; Grolimund, D.; Buhmann, J.M.; Brandt, S.; et al. Highly multiplexed imaging of tumor tissues with subcellular resolution by mass cytometry. Nat. Methods 2014, 11, 417–422. [Google Scholar] [CrossRef]
- Tietscher, S.; Wagner, J.; Anzeneder, T.; Langwieder, C.; Rees, M.; Sobottka, B.; de Souza, N.; Bodenmiller, B. A comprehensive single-cell map of T cell exhaustion-associated immune environments in human breast cancer. Nat. Commun. 2023, 14, 98. [Google Scholar] [CrossRef]
- Weiss, F.; Atlasy, N.; van Reijmersdal, V.; Stunnenberg, H.; Hulsbergen-Veelken, C.; Friedl, P. 3D spheroid culture to examine adaptive therapy response in invading tumor cells. In Vitro Model. 2022, 1, 463–471. [Google Scholar] [CrossRef]
- Jackson, G.H.; Davies, F.E.; Pawlyn, C.; Cairns, D.A.; Striha, A.; Collett, C.; Hockaday, A.; Jones, J.R.; Kishore, B.; Garg, M.; et al. Lenalidomide maintenance versus observation for patients with newly diagnosed multiple myeloma (Myeloma XI): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2019, 20, 57–73. [Google Scholar] [CrossRef] [PubMed]
- De Castro, G.; Kudaba, I.; Wu, Y.-L.; Lopes, G.; Kowalski, D.M.; Turna, H.Z.; Caglevic, C.; Zhang, L.; Karaszewska, B.; Laktionov, K.K.; et al. Five-Year Outcomes with Pembrolizumab Versus Chemotherapy as First-Line Therapy in Patients with Non-Small-Cell Lung Cancer and Programmed Death Ligand-1 Tumor Proportion Score ≥1% in the KEYNOTE-042 Study. JCO 2023, 41, 1986–1991. [Google Scholar] [CrossRef] [PubMed]
- Duvivier, H.L.; Rothe, M.; Mangat, P.K.; Garrett-Mayer, E.; Ahn, E.R.; Al Baghdadi, T.; Alva, A.S.; Dublis, S.A.; Cannon, T.L.; Calfa, C.J.; et al. Pembrolizumab in Patients with Tumors with High Tumor Mutational Burden: Results from the Targeted Agent and Profiling Utilization Registry Study. JCO 2023, 41, JCO2300702. [Google Scholar] [CrossRef] [PubMed]
- Blazquez, R.; Sparrer, D.; Wendl, C.; Evert, M.; Riemenschneider, M.J.; Krahn, M.P.; Erez, N.; Proescholdt, M.; Pukrop, T. The macro-metastasis/organ parenchyma interface (MMPI)—A hitherto unnoticed area. Semin. Cancer Biol. 2020, 60, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Szatmari, I.; Töröcsik, D.; Agostini, M.; Nagy, T.; Gurnell, M.; Barta, E.; Chatterjee, K.; Nagy, L. PPARγ regulates the function of human dendritic cells primarily by altering lipid metabolism. Blood 2007, 110, 3271–3280. [Google Scholar] [CrossRef]
- Luo, J.; Solimini, N.L.; Elledge, S.J. Principles of Cancer Therapy: Oncogene and Non-oncogene Addiction. Cell 2009, 136, 823–837. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-R.; Schultheis, A.M.; Yu, H.; Mandelker, D.; Ladanyi, M.; Büttner, R. Precision medicine in non-small cell lung cancer: Current applications and future directions. Semin. Cancer Biol. 2022, 84, 184–198. [Google Scholar] [CrossRef]
- Szeitz, B.; Megyesfalvi, Z.; Woldmar, N.; Valkó, Z.; Schwendenwein, A.; Bárány, N.; Paku, S.; László, V.; Kiss, H.; Bugyik, E.; et al. In-depth proteomic analysis reveals unique subtype-specific signatures in human small-cell lung cancer. Clin. Transl. Med. 2022, 12, e1060. [Google Scholar] [CrossRef]
- Pich, O.; Bailey, C.; Watkins, T.B.K.; Zaccaria, S.; Jamal-Hanjani, M.; Swanton, C. The translational challenges of precision oncology. Cancer Cell 2022, 40, 458–478. [Google Scholar] [CrossRef]
- Liu, R.; Rizzo, S.; Waliany, S.; Garmhausen, M.R.; Pal, N.; Huang, Z.; Chaudhary, N.; Wang, L.; Harbron, C.; Neal, J.; et al. Systematic pan-cancer analysis of mutation-treatment interactions using large real-world clinicogenomics data. Nat. Med. 2022, 28, 1656–1661. [Google Scholar] [CrossRef]
| Transcriptional Regulation | MCT, Targeted Therapy | r/r Neoplasia [12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30] | Reprogramming Cancer Hallmarks | Best Response | Reference Citation |
|---|---|---|---|---|---|
| Pioglitazone, rofecoxib | MCT | Hepatocellular carcinoma | Inflammation control | PR | [20] |
| Pioglitazone, rofecoxib | MCT | Cholangiocellular carcinoma | n.d. | cCR | [26] |
| Pioglitazone, rofecoxib | MCT | High-grade gliomas | n.d. | SD | [31] |
| Pioglitazone, rofecoxib | MCT | Angiosarcoma | n.d. | cCR | [24] |
| Rofecoxib plus/minus pioglitazone | MCT | Metastatic gastric cancer | ® n.d. | PR | [21] |
| Rofecoxib plus/minus pioglitazone Pioglitazone, etoricoxib | MCT MCT + temsirolimus | r/r metastatic melanoma uveal melanoma | ® Inflammation control | PR SD | [22,41] |
| Pioglitazone, etoricoxib | MCT, clarithromycin vs. nivolumab | r/r Non-small cell lung cancer | ® Enhancing immune surveillance | PR | [23] |
| Pioglitazone, dexamethasone, etoricoxib | MCT MCT + imatinib | Castration-refractory prostate cancer | n.d. | PR | [18,30,42] |
| Pioglitazone, dexamethasone, etoricoxib | MCT, everolimus | r/r Hodgkin’s lymphoma | Inflammation control | cCR | [13,27] |
| Pioglitazone, dexamethasone, etoricoxib | MCT | r/r Multisystem Langerhans cell histiocytosis | Inflammation control | cCR | [16,28] |
| Pioglitazone, rofecoxib Pioglitazone, rofecoxib, + interferon-α | MCT MCT | r/r Renal clear cell carcinoma r/r Renal clear cell carcinoma | No sufficient inflammation control Inflammation control | SD cCR | [17,25,43] |
| Pioglitazone, dexamethasone | MCT, lenalidomide | r/r Multiple myeloma | n.d. | PR | [14] |
| Pioglitazone, all-trans retinoic acid | Azacytidine | r/r Non-promyelocytic acute myelocytic leukemia | Differentiation induction | CR | [44,45,46,47] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harrer, D.C.; Lüke, F.; Pukrop, T.; Ghibelli, L.; Reichle, A.; Heudobler, D. Addressing Genetic Tumor Heterogeneity, Post-Therapy Metastatic Spread, Cancer Repopulation, and Development of Acquired Tumor Cell Resistance. Cancers 2024, 16, 180. https://doi.org/10.3390/cancers16010180
Harrer DC, Lüke F, Pukrop T, Ghibelli L, Reichle A, Heudobler D. Addressing Genetic Tumor Heterogeneity, Post-Therapy Metastatic Spread, Cancer Repopulation, and Development of Acquired Tumor Cell Resistance. Cancers. 2024; 16(1):180. https://doi.org/10.3390/cancers16010180
Chicago/Turabian StyleHarrer, Dennis Christoph, Florian Lüke, Tobias Pukrop, Lina Ghibelli, Albrecht Reichle, and Daniel Heudobler. 2024. "Addressing Genetic Tumor Heterogeneity, Post-Therapy Metastatic Spread, Cancer Repopulation, and Development of Acquired Tumor Cell Resistance" Cancers 16, no. 1: 180. https://doi.org/10.3390/cancers16010180
APA StyleHarrer, D. C., Lüke, F., Pukrop, T., Ghibelli, L., Reichle, A., & Heudobler, D. (2024). Addressing Genetic Tumor Heterogeneity, Post-Therapy Metastatic Spread, Cancer Repopulation, and Development of Acquired Tumor Cell Resistance. Cancers, 16(1), 180. https://doi.org/10.3390/cancers16010180







