Topical Immunotherapy for Actinic Keratosis and Field Cancerization
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
3. Current Therapies for Actinic Keratosis and Field Cancerization with Primary Immunomodulatory Effect
3.1. Imiquimod
3.2. Diclofenac Disodium
4. Current Therapies for Actinic Keratosis and Field Cancerization with Secondary Immunomodulatory Effect
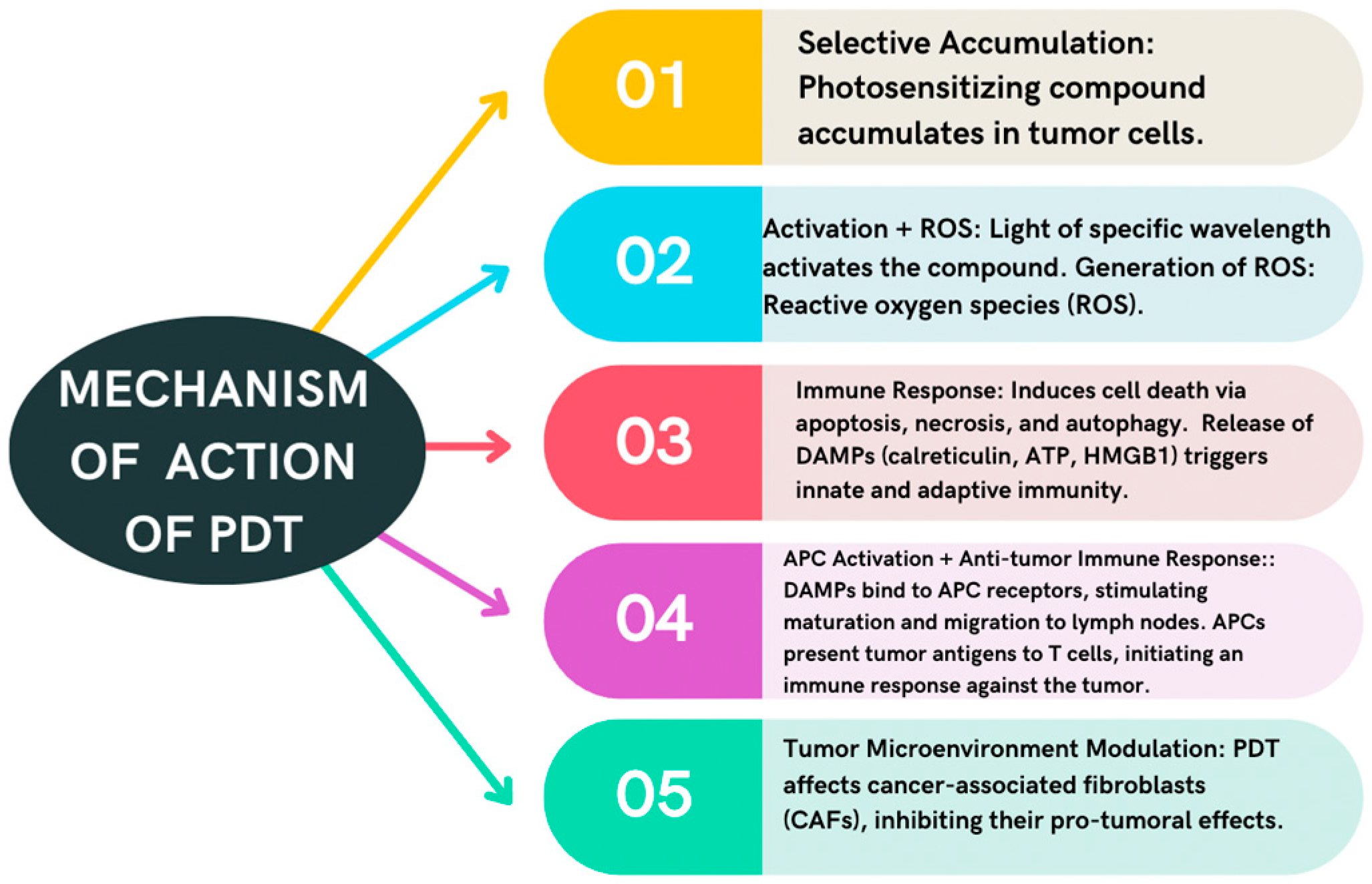
4.1. Photodynamic Therapy
4.2. 5-Fluorouracil
5. Other Therapies Used Off-Label for Actinic Keratosis and Cancerization Field with Immunomodulatory Effect
5.1. Vitamin D
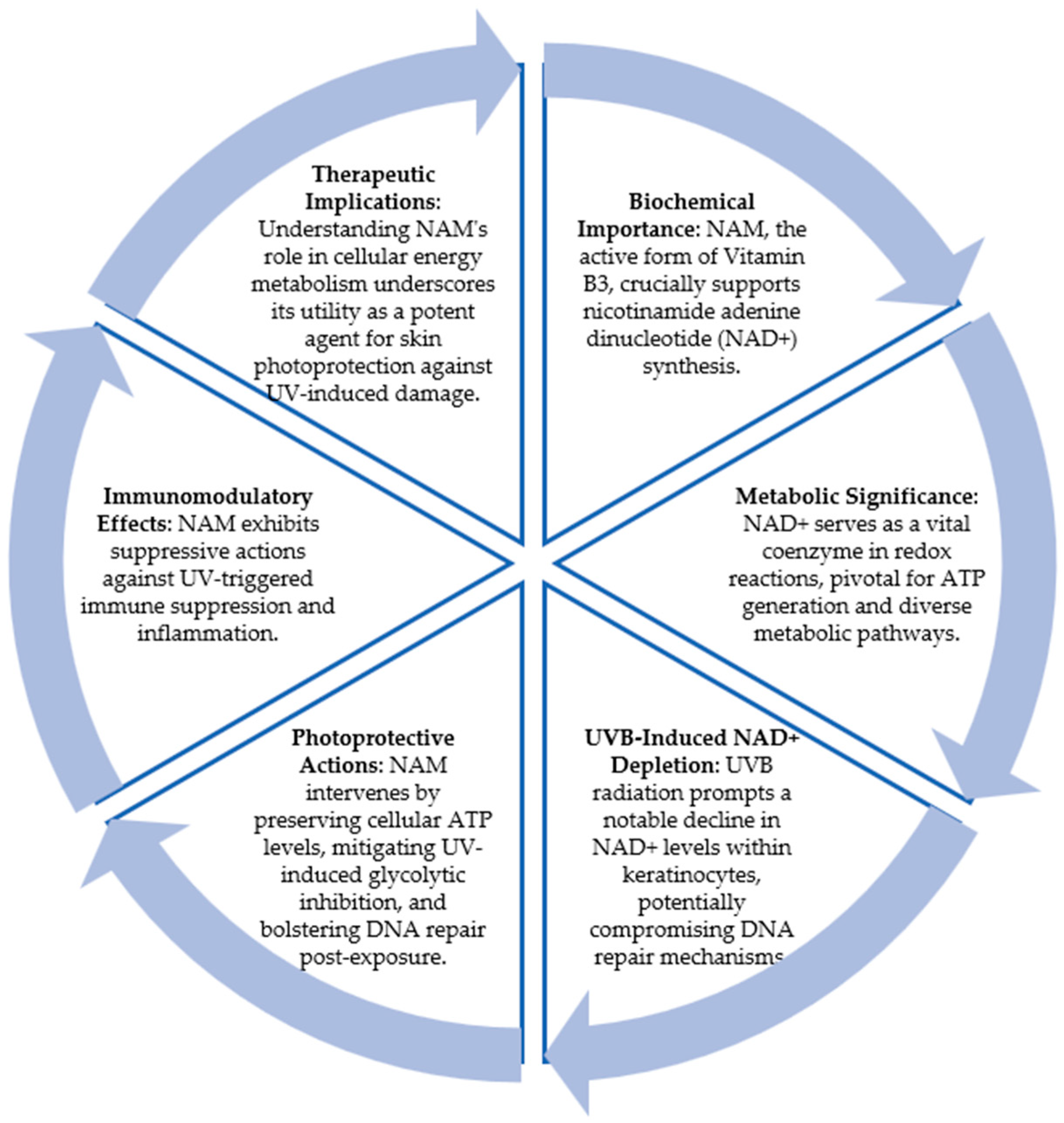
5.2. Nicotinamide
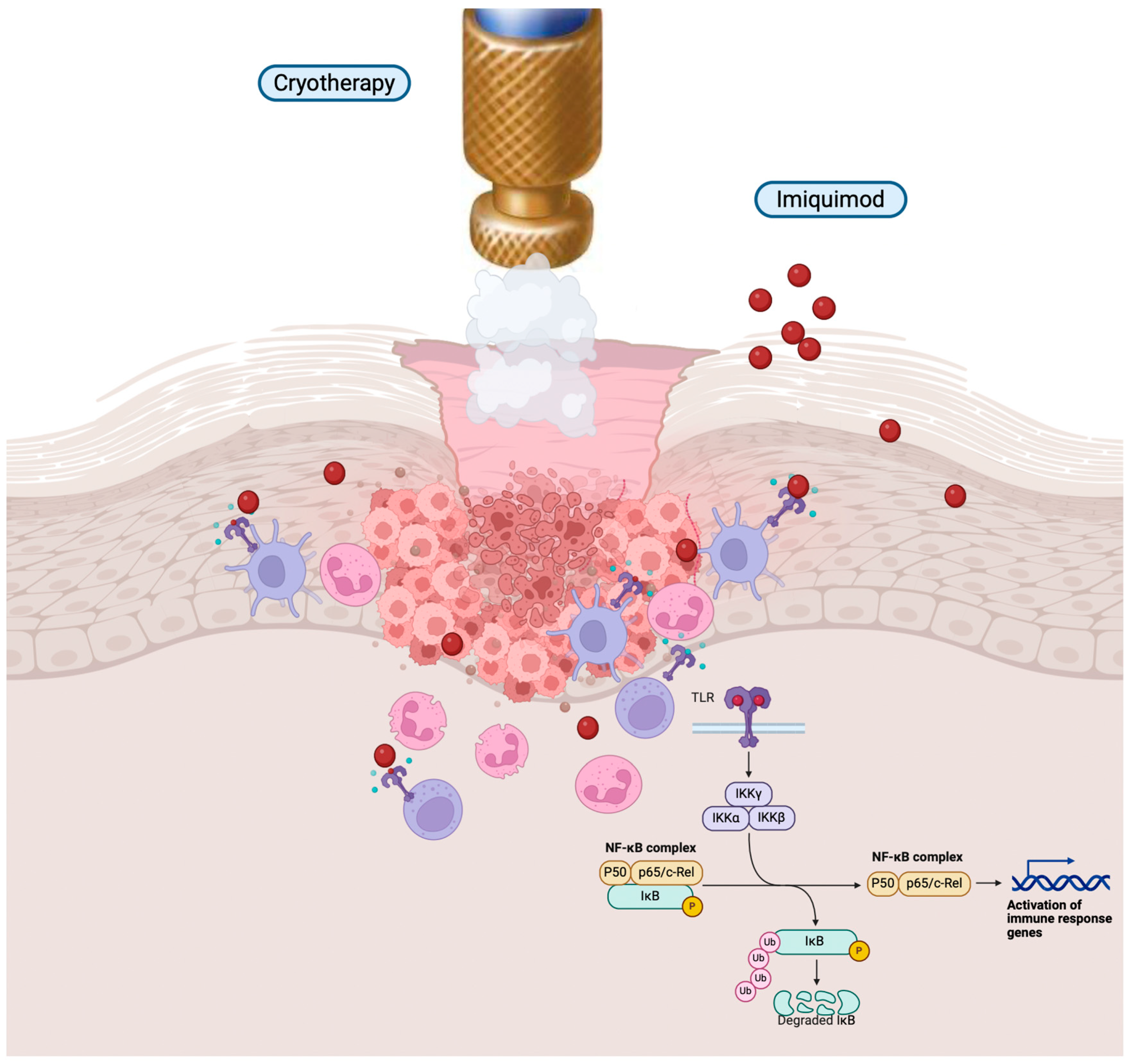
6. Topical Immunotherapy Associated with Cryotherapy: Cryoimmunotherapy
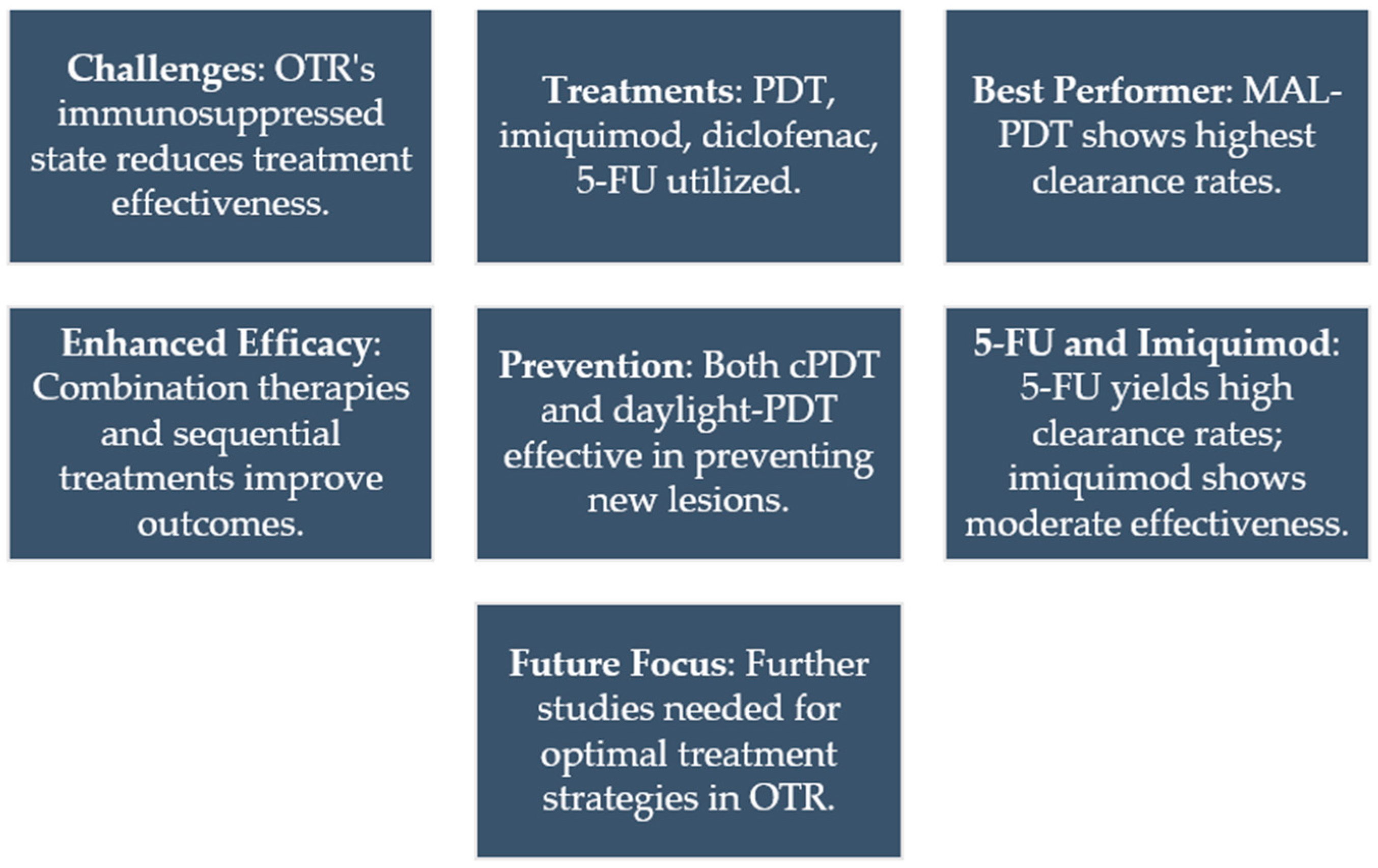
7. Topical Immunotherapy for Actinic Keratosis in Organ Transplant Recipients
8. Future Perspectives in Immunotherapy for Actinic Keratoses
9. Conclusions
Funding
Conflicts of Interest
References
- Ferrándiz, C.; Plazas, M.J.; Sabaté, M.; Palomino, R. Prevalence of Actinic Keratosis among Dermatology Outpatients in Spain. Actas Dermosifiloiogr. 2016, 107, 674–680. [Google Scholar] [CrossRef]
- Berman, B.; Cockerell, C.J. Pathobiology of Actinic Keratosis: Ultraviolet-Dependent Keratinocyte Proliferation. J. Am. Acad. Dermatol. 2013, 68, S10–S19. [Google Scholar] [CrossRef]
- Balcere, A.; Konrāde-Jilmaza, L.; Pauliņa, L.A.; Čēma, I.; Krūmiņa, A. Clinical Characteristics of Actinic Keratosis Associated with the Risk of Progression to Invasive Squamous Cell Carcinoma: A Systematic Review. J. Clin. Med. 2022, 11, 5899. [Google Scholar] [CrossRef] [PubMed]
- Piquero-Casals, J.; Morgado-Carrasco, D.; Gilaberte, Y.; Del Rio, R.; Macaya-Pascual, A.; Granger, C.; López-Estebaranz, J.L. Management Pearls on the Treatment of Actinic Keratoses and Field Cancerization. Dermatol. Ther. 2020, 10, 903–915. [Google Scholar] [CrossRef] [PubMed]
- Ríos-Viñuela, E.; Álvarez, P.; Lavernia, J.; Serra-Guillén, C.; Requena, C.; Bernia, E.; Diago, A.; Llombart, B.; Sanmartín, O. Cemiplimab in Advanced Cutaneous Squamous Cell Carcinoma: Real-World Experience in a Monographic Oncology Center. Actas Dermosifiliogr. 2022, 113, 610–615. [Google Scholar] [CrossRef]
- Yusuf, N. Immunomodulation of Skin Cancer. Int. J. Mol. Sci. 2023, 24, 10462. [Google Scholar] [CrossRef] [PubMed]
- Dianzani, C.; Conforti, C.; Giuffrida, R.; Corneli, P.; di Meo, N.; Farinazzo, E.; Moret, A.; Magaton Rizzi, G.; Zalaudek, I. Current Therapies for Actinic Keratosis. Int. J. Dermatol. 2020, 59, 677–684. [Google Scholar] [CrossRef]
- Scarfì, F.; Patrizi, A.; Veronesi, G.; Lambertini, M.; Tartari, F.; Mussi, M.; Melotti, B.; Dika, E. The Role of Topical Imiquimod in Melanoma Cutaneous Metastases: A Critical Review of the Literature. Dermatol. Ther. 2020, 33, e14165. [Google Scholar] [CrossRef]
- Wenzel, J.; Uerlich, M.; Haller, O.; Bieber, T.; Tueting, T. Enhanced Type I Interferon Signaling and Recruitment of Chemokine Receptor CXCR3-Expressing Lymphocytes into the Skin Following Treatment with the TLR7-Agonist Imiquimod. J. Cutan. Pathol. 2005, 32, 257–262. [Google Scholar] [CrossRef]
- Cantisani, C.; Lazic, T.; Richetta, A.G.; Clerico, R.; Mattozzi, C.; Calvieri, S. Imiquimod 5% Cream Use in Dermatology, Side Effects and Recent Patents. Recent. Pat. Inflamm. Allergy Drug Discov. 2012, 6, 65–69. [Google Scholar] [CrossRef]
- Arcuri, D.; Ramchatesingh, B.; Lagacé, F.; Iannattone, L.; Netchiporouk, E.; Lefrançois, P.; Litvinov, I.V. Pharmacological Agents Used in the Prevention and Treatment of Actinic Keratosis: A Review. Int. J. Mol. Sci. 2023, 24, 4989. [Google Scholar] [CrossRef] [PubMed]
- Jansen, M.H.E.; Kessels, J.P.H.M.; Nelemans, P.J.; Kouloubis, N.; Arits, A.H.M.M.; van Pelt, H.P.A.; Quaedvlieg, P.J.F.; Essers, B.A.B.; Steijlen, P.M.; Kelleners-Smeets, N.W.J.; et al. Randomized Trial of Four Treatment Approaches for Actinic Keratosis. N. Engl. J. Med. 2019, 380, 935–946. [Google Scholar] [CrossRef] [PubMed]
- Worley, B.; Harikumar, V.; Reynolds, K.; Dirr, M.K.A.; Christensen, R.E.; Anvery, N.; Yi, M.D.; Poon, E.; Alam, M. Treatment of Actinic Keratosis: A Systematic Review. Arch. Dermatol. Res. 2022, 315, 1099–1108. [Google Scholar] [CrossRef] [PubMed]
- Steeb, T.; Wessely, A.; Petzold, A.; Brinker, T.J.; Schmitz, L.; Schöffski, O.; Berking, C.; Heppt, M.V. Long-Term Recurrence Rates of Actinic Keratosis: A Systematic Review and Pooled Analysis of Randomized Controlled Trials. J. Am. Acad. Dermatol. 2022, 86, 1116–1119. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xie, Y.; Wang, L. Rare Cutaneous Side Effects of Imiquimod: A Review on Its Mechanisms, Diagnosis, and Management. Dermatol. Ther. 2023, 13, 1909–1934. [Google Scholar] [CrossRef] [PubMed]
- Jetter, N.; Chandan, N.; Wang, S.; Tsoukas, M. Field Cancerization Therapies for Management of Actinic Keratosis: A Narrative Review. Am. J. Clin. Dermatol. 2018, 19, 543–557. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Liang, X.; Ye, L.; Wang, Y. No Chemopreventive Effect of Nonsteroidal Anti-Inflammatory Drugs on Nonmelanoma Skin Cancer: Evidence from Meta-Analysis. PLoS ONE 2014, 9, e96887. [Google Scholar] [CrossRef]
- Garofalo, V.; Ventura, A.; Mazzilli, S.; DIluvio, L.; Bianchi, L.; Toti, L.; Tisone, G.; Milani, M.; Campione, E. Treatment of Multiple Actinic Keratosis and Field of Cancerization with Topical Piroxicam 0.8% and Sunscreen 50+ in Organ Transplant Recipients: A Series of 10 Cases. Case Rep. Dermatol. 2017, 9, 211–216. [Google Scholar] [CrossRef]
- Thomas, G.J.; Herranz, P.; Cruz, S.B.; Parodi, A. Treatment of Actinic Keratosis through Inhibition of Cyclooxygenase-2: Potential Mechanism of Action of Diclofenac Sodium 3% in Hyaluronic Acid 2.5. Dermatol. Ther. 2019, 32, e12800. [Google Scholar] [CrossRef]
- Singer, K.; Dettmer, K.; Unger, P.; Schönhammer, G.; Renner, K.; Peter, K.; Siska, P.J.; Berneburg, M.; Herr, W.; Oefner, P.J.; et al. Topical Diclofenac Reprograms Metabolism and Immune Cell Infiltration in Actinic Keratosis. Front. Oncol. 2019, 9, 455682. [Google Scholar] [CrossRef]
- Maltusch, A.; Röwert-Huber, J.; Matthies, C.; Lange-Asschenfeldt, S.; Stockfleth, E. Modes of Action of Diclofenac 3%/Hyaluronic Acid 2.5% in the Treatment of Actinic Keratosis. J. Dtsch. Dermatol. Ges. 2011, 9, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, J.R.; Armstrong, A.W.; Kircik, L.H. Individual Article: Safety and Tolerability of Topical Agents for Actinic Keratosis: A Systematic Review of Phase 3 Clinical Trials. J. Drugs Dermatol. 2021, 20, 4–14. [Google Scholar]
- Steinbauer, J.M.; Schreml, S.; Kohl, E.A.; Karrer, S.; Landthaler, M.; Szeimies, R.-M. Photodynamic Therapy in Dermatology. J. Dtsch. Dermatol. Ges. 2010, 8, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Juarranz, A.; Jaén, P.; Sanz-Rodríguez, F.; Cuevas, J.; González, S. Photodynamic Therapy of Cancer. Basic Principles and Applications. Clin. Transl. Oncol. 2008, 10, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic Therapy of Cancer: An Update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef] [PubMed]
- van Straten, D.; Mashayekhi, V.; de Bruijn, H.S.; Oliveira, S.; Robinson, D.J. Oncologic Photodynamic Therapy: Basic Principles, Current Clinical Status and Future Directions. Cancers 2017, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Cerro, P.A.; Mascaraque, M.; Gallego-Rentero, M.; Almenara-Blasco, M.; Nicolás-Morala, J.; Santiago, J.L.; González, S.; Gracia-Cazaña, T.; Juarranz, Á.; Gilaberte, Y. Tumor Microenvironment in Non-Melanoma Skin Cancer Resistance to Photodynamic Therapy. Front. Oncol. 2022, 12, 970279. [Google Scholar] [CrossRef]
- Garg, A.D.; Nowis, D.; Golab, J.; Vandenabeele, P.; Krysko, D.V.; Agostinis, P. Immunogenic Cell Death, DAMPs and Anticancer Therapeutics: An Emerging Amalgamation. Biochim. Biophys. Acta 2010, 1805, 53–71. [Google Scholar] [CrossRef]
- Eisen, D.B.; Asgari, M.M.; Bennett, D.D.; Connolly, S.M.; Dellavalle, R.P.; Freeman, E.E.; Goldenberg, G.; Leffell, D.J.; Peschin, S.; Sligh, J.E.; et al. Guidelines of Care for the Management of Actinic Keratosis. J. Am. Acad. Dermatol. 2021, 85, 209–233. [Google Scholar] [CrossRef]
- Sinclair, R.; Baker, C.; Spelman, L.; Supranowicz, M.; MacMahon, B. A Review of Actinic Keratosis, Skin Field Cancerisation and the Efficacy of Topical Therapies. Australas. J. Dermatol. 2021, 62, 119–123. [Google Scholar] [CrossRef]
- Bento, C.D.O.; Pantaleão, L.; de Souza, M.B.; Vilar, E.A.G.; Luiz, R.R.; Filho, P.J.S.; Gismondi, R.A.O.C.; Issa, M.C.A. Comparison of Clinical and Histologic Findings in Daylight Photodynamic Therapy for Skin Field Cancerization: A Randomized Controlled Four-Arm Study on Physical Methods-Assisted Delivery of Methyl Aminolevulinate. Photodiagn. Photodyn. Ther. 2021, 35, 102404. [Google Scholar] [CrossRef]
- Morton, C.A.; Szeimies, R.-M.; Basset-Séguin, N.; Calzavara-Pinton, P.G.; Gilaberte, Y.; Haedersdal, M.; Hofbauer, G.F.L.; Hunger, R.E.; Karrer, S.; Piaserico, S.; et al. European Dermatology Forum Guidelines on Topical Photodynamic Therapy 2019 Part 2: Emerging Indications—Field Cancerization, Photorejuvenation and Inflammatory/Infective Dermatoses. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 17–29. [Google Scholar] [CrossRef]
- Serra-Guillén, C.; Nagore, E.; Bancalari, E.; Kindem, S.; Sanmartín, O.; Llombart, B.; Requena, C.; Serra-Guillén, I.; Calomarde, L.; Diago, A.; et al. A Randomized Intraindividual Comparative Study of Methyl-5-Aminolaevulinate vs. 5-Aminolaevulinic Acid Nanoemulsion (BF-200 ALA) in Photodynamic Therapy for Actinic Keratosis of the Face and Scalp. Br. J. Dermatol. 2018, 179, 1410–1411. [Google Scholar] [CrossRef]
- Wiegell, S.R.; Wulf, H.C.; Szeimies, R.-M.; Basset-Seguin, N.; Bissonnette, R.; Gerritsen, M.-J.P.; Gilaberte, Y.; Calzavara-Pinton, P.; Morton, C.A.; Sidoroff, A.; et al. Daylight Photodynamic Therapy for Actinic Keratosis: An International Consensus: International Society for Photodynamic Therapy in Dermatology. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 673–679. [Google Scholar] [CrossRef]
- Wiegell, S.R.; Haedersdal, M.; Philipsen, P.A.; Eriksen, P.; Enk, C.D.; Wulf, H.C. Continuous Activation of PpIX by Daylight Is as Effective as and Less Painful than Conventional Photodynamic Therapy for Actinic Keratoses; a Randomized, Controlled, Single-Blinded Study. Br. J. Dermatol. 2008, 158, 740–746. [Google Scholar] [CrossRef]
- de Oliveira, E.C.V.; da Motta, V.R.V.; Pantoja, P.C.; Ilha, C.S.d.O.; Magalhães, R.F.; Galadari, H.; Leonardi, G.R. Actinic Keratosis—Review for Clinical Practice. Int. J. Dermatol. 2019, 58, 400–407. [Google Scholar] [CrossRef]
- Draghiciu, O.; Lubbers, J.; Nijman, H.W.; Daemen, T. Myeloid Derived Suppressor Cells-An Overview of Combat Strategies to Increase Immunotherapy Efficacy. Oncoimmunology 2015, 4, 954829. [Google Scholar] [CrossRef]
- Apetoh, L.; Vegran, F.; Ladoire, S.; Ghiringhelli, F. Restoration of Antitumor Immunity through Selective Inhibition of Myeloid Derived Suppressor Cells by Anticancer Therapies. Curr. Mol. Med. 2011, 11, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.; Heusinkveld, L.E.; Cheng, C.E.; Lefatshe, L.; Silva, P.; Hasan, T.; Maytin, E.V. Combination of 5-Fluorouracil with Photodynamic Therapy: Enhancement of Innate and Adaptive Immune Responses in a Murine Model of Actinic Keratosis. Photochem. Photobiol. 2023, 99, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Steeb, T.; Wessely, A.; Petzold, A.; Brinker, T.J.; Schmitz, L.; Leiter, U.; Garbe, C.; Schöffski, O.; Berking, C.; Heppt, M.V. Evaluation of Long-Term Clearance Rates of Interventions for Actinic Keratosis: A Systematic Review and Network Meta-Analysis. JAMA Dermatol. 2021, 157, 1066–1077. [Google Scholar] [CrossRef] [PubMed]
- Krawtchenko, N.; Roewert-Huber, J.; Ulrich, M.; Mann, I.; Sterry, W.; Stockfleth, E. A Randomised Study of Topical 5% Imiquimod vs. Topical 5-fluorouracil vs. Cryosurgery in Immunocompetent Patients with Actinic Keratoses: A Comparison of Clinical and Histological Outcomes Including 1-year Follow-up. Br. J. Dermatol. 2007, 157, 34–40. [Google Scholar] [CrossRef]
- Lampley, N.; Rigo, R.; Schlesinger, T.; Rossi, A.M. Field Therapy for Actinic Keratosis: A Structured Review of the Literature on Efficacy, Cost, and Adherence. Dermatol. Surg. 2023, 49, 124–129. [Google Scholar] [CrossRef]
- Wu, Y.; Tang, N.; Cai, L.; Li, Q. Relative Efficacy of 5-Fluorouracil Compared with Other Treatments among Patients with Actinic Keratosis: A Network Meta-Analysis. Dermatol. Ther. 2019, 32, e12822. [Google Scholar] [CrossRef]
- Ezzedine, K.; Painchault, C.; Brignone, M. Systematic Literature Review and Network Meta-Analysis of the Efficacy and Acceptability of Interventions in Actinic Keratoses. Acta Derm. Venereol. 2021, 101, adv00358. [Google Scholar] [CrossRef] [PubMed]
- Demehri, S.; Turkoz, A.; Manivasagam, S.; Yockey, L.J.; Turkoz, M.; Kopan, R. Elevated Epidermal Thymic Stromal Lymphopoietin Levels Establish an Antitumor Environment in the Skin. Cancer Cell 2012, 22, 494–505. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, T.J.; Tabacchi, M.; Eliane, J.-P.; Tuchayi, S.M.; Manivasagam, S.; Mirzaalian, H.; Turkoz, A.; Kopan, R.; Schaffer, A.; Saavedra, A.P.; et al. Randomized Trial of Calcipotriol Combined with 5-Fluorouracil for Skin Cancer Precursor Immunotherapy. J. Clin. Investig. 2016, 127, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, A.R.; Tabacchi, M.; Ngo, K.H.; Wallendorf, M.; Rosman, I.S.; Cornelius, L.A.; Demehri, S. Skin Cancer Precursor Immunotherapy for Squamous Cell Carcinoma Prevention. J. Clin. Investig. 2019, 4, 125476. [Google Scholar] [CrossRef] [PubMed]
- Azin, M.; Mahon, A.B.; Isaacman, S.; Seaman, J.E.; Allen, I.E.; Szarek, M.; Cornelius, L.A.; Demehri, S. Topical Calcipotriol Plus 5-Fluorouracil Immunotherapy for Actinic Keratosis Treatment. JID Innov. Skin. Sci. Mol. Popul. Health 2022, 2, 100104. [Google Scholar] [CrossRef] [PubMed]
- Piaserico, S.; Piccioni, A.; Gutiérrez Garcìa-Rodrigo, C.; Sacco, G.; Pellegrini, C.; Fargnoli, M.C. Sequential Treatment with Calcitriol and Methyl Aminolevulinate-Daylight Photodynamic Therapy for Patients with Multiple Actinic Keratoses of the Upper Extremities. Photodiagn. Photodyn. Ther. 2021, 34, 102325. [Google Scholar] [CrossRef] [PubMed]
- Borgia, F.; Riso, G.; Catalano, F.; Coppola, M.; Giuffrida, R.; Cannavò, S.P. Topical Tacalcitol as Neoadjuvant for Photodynamic Therapy of Acral Actinic Keratoses: An Intra-Patient Randomized Study. Photodiagn. Photodyn. Ther. 2020, 31, 101803. [Google Scholar] [CrossRef]
- Surjana, D.; Halliday, G.M.; Damian, D.L. Role of nicotinamide in DNA damage, mutagenesis, and DNA repair. J. Nucleic Acids 2010, 2010, 1–13. [Google Scholar] [CrossRef]
- Park, J.; Halliday, G.M.; Surjana, D.; Damian, D.L. Nicotinamide Prevents Ultraviolet Radiation-Induced Cellular Energy Loss. Photochem. Photobiol. 2010, 86, 942–948. [Google Scholar] [CrossRef]
- Monfrecola, G.; Gaudiello, F.; Cirillo, T.; Fabbrocini, G.; Balato, A.; Lembo, S. Nicotinamide Downregulates Gene Expression of Interleukin-6, Interleukin-10, Monocyte Chemoattractant Protein-1, and Tumour Necrosis Factor-α Gene Expression in HaCaT Keratinocytes after Ultraviolet B Irradiation. Clin. Exp. Dermatol. 2013, 38, 185–188. [Google Scholar] [CrossRef]
- Snaidr, V.A.; Damian, D.L.; Halliday, G.M. Nicotinamide for Photoprotection and Skin Cancer Chemoprevention: A Review of Efficacy and Safety. Exp. Dermatol. 2019, 28, 15–22. [Google Scholar] [CrossRef]
- Mainville, L.; Smilga, A.S.; Fortin, P.R. Effect of Nicotinamide in Skin Cancer and Actinic Keratoses Chemoprophylaxis, and Adverse Effects Related to Nicotinamide: A Systematic Review and Meta-Analysis. J. Cutan. Med. Surg. 2022, 26, 297–308. [Google Scholar] [CrossRef]
- Allen, N.C.; Martin, A.J.; Snaidr, V.A.; Eggins, R.; Chong, A.H.; Fernandéz-Peñas, P.; Gin, D.; Sidhu, S.; Paddon, V.L.; Banney, L.A.; et al. Nicotinamide for Skin-Cancer Chemoprevention in Transplant Recipients. N. Engl. J. Med. 2023, 388, 804–812. [Google Scholar] [CrossRef] [PubMed]
- Sivapirabu, G.; Yiasemides, E.; Halliday, G.M.; Park, J.; Damian, D.L. Topical Nicotinamide Modulates Cellular Energy Metabolism and Provides Broad-Spectrum Protection against Ultraviolet Radiation-Induced Immunosuppression in Humans. Br. J. Dermatol. 2009, 161, 1357–1364. [Google Scholar] [CrossRef] [PubMed]
- Moloney, F.; Vestergaard, M.; Radojkovic, B.; Damian, D. Randomized, Double-Blinded, Placebo Controlled Study to Assess the Effect of Topical 1% Nicotinamide on Actinic Keratoses. Br. J. 2010, 162, 1138–1139. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; West, C.E.; Roh, Y.S.; Sutaria, N.; Kwatra, S.G.; Kwatra, M.M. Mouse Models for Actinic Keratoses. J. Pharmacol. Toxicol. Methods 2021, 110, 107071. [Google Scholar] [CrossRef] [PubMed]
- Salman, S.; Sarsik, S.M.; El-Qushayri, A.E.; Sakr, S.; Ghozy, S.; Salem, M.L. Safety and Efficacy of the Combination of Cryotherapy and Photodynamic Modalities with Imiquimod in Patients with Actinic Keratosis: A Systematic Review and Meta-Analysis. Ital. J. Dermatol. Venerol. 2023, 158, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.K.; Thomas, D.R.; Poulin, Y.; Maddin, F. Efficacy of Imiqui- Mod as an Adjunct to Cryotherapy for Actinic Keratoses. J. Cutan. Med. Surg. 2011, 11, 195–201. [Google Scholar] [CrossRef]
- Tanaka, N.; Ohata, C.; Ishii, N.; Imamura, K.; Ueda, A.; Furumura, M.; Yasumoto, S.; Kawakami, T.; Tsuruta, D.; Hashimoto, T. Comparative Study for the Effect of Photodynamic Therapy, Imiquimod Im- Munotherapy and Combination of Both Therapies on 40 Lesions of Actinic Keratosis in Japanese Patients. J. Dermatol. 2013, 40, 962–967. [Google Scholar] [CrossRef]
- Serra-Guillén, C.; Nagore, E.; Hueso, L.; Traves, V.; Messeguer, F.; Sanmartín, O.; Llombart, B.; Requena, C.; Botella-Estrada, R.; Guillén, C. A Randomized Pilot Comparative Study of Topical Methyl Ami- Nolevulinate Photodynamic Therapy versus Imiquimod 5% versus Se-Quential Application of Both Therapies in Immunocompetent Patients with Actinic Keratosis: Clinical and Histologic Outcomes. J. Am. Acad. Dermatol. 2012, 66, 131–137. [Google Scholar] [CrossRef]
- Lee, J.; Jorizzo, J.; Lebwoh, M.; Markowitz, O.; Levy, S. Cryosurgery Followed by Imiquimod 3.75% to Treat Actinic Keratosis. J. Am. Acad. Dermatol. 2011, 64, AB2. [Google Scholar] [CrossRef]
- Goldenberg, G.; Linkner, R.V.; Singer, G.; Frankel, A. An Investigator- Initiated Study to Assess the Safety and Efficacy of Imiquimod 3.75% Cream When Used After Cryotherapy in the Treatment of Hypertrophic Actinic Keratoses on Dorsal Hands and Forearms. J. Clin. Aesthet. Dermatol. 2013, 6, 36–43. [Google Scholar] [PubMed]
- Pasquali, P.; Segurado-Miravalles, G.; González, S. Sequential Treatment of Actinic Keratosis with Cryotherapy and Ingenol Mebutate: Reflectance Confocal Microscopy Monitoring of Efficacy and Local Skin Reaction. Int. J. Dermatol. 2018, 57, 1178–1181. [Google Scholar] [CrossRef] [PubMed]
- Dragieva, G.; Prinz, B.M.; Hafner, J.; Dummer, R.; Burg, G.; Binswanger, U.; Kempf, W. A Randomized Controlled Clinical Trial of Topical Photodynamic Therapy with Methyl Aminolaevulinate in the Treatment of Actinic Keratoses in Transplant Recipients. Br. J. Dermatol. 2004, 151, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Piaserico, S.; Belloni Fortina, A.; Rigotti, P.; Rossi, B.; Baldan, N.; Alaibac, M.; Marchini, F. Topical Photodynamic Therapy of Actinic Keratosis in Renal Transplant Recipients. Transplant. Proc. 2007, 39, 1847–1850. [Google Scholar] [CrossRef]
- Dragieva, G.; Hafner, J.; Dummer, R.; Schmid-Grendelmeier, P.; Roos, M.; Prinz, B.M.; Burg, G.; Binswanger, U.; Kempf, W. Topical Photodynamic Therapy in the Treatment of Actinic Keratoses and Bowen’s Disease in Transplant Recipients. Transplantation 2004, 77, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Hasson, A.; Navarrete-Dechent, C.; Nicklas, C.; Cruz, C. Topical Photodynamic Therapy with Methylaminolevulinate for the Treatment of Actinic Keratosis and Reduction of Photodamage in Organ Transplant Recipients: A Case-Series of 16 Patients. Indian J. Dermatol. Venereol. Leprol. 2013, 78, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Perrett, C.M.; McGregor, J.M.; Warwick, J.; Karran, P.; Leigh, I.; Proby, C.; Harwood, C. Treatment of Post-Transplant Premalignant Skin Disease: A Randomized Intrapatient Comparative Study of 5-Fluorouracil Cream and Topical Photodynamic Therapy. Br. J. Dermatol. 2007, 156, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Togsverd-Bo, K.; Lei, U.; Erlendsson, A.; Taudorf, E.; Philipsen, P.; Wulf, H.; Skov, L.; Haedersdal, M. Combination of Ablative Fractional Laser and Daylight-Mediated Photodynamic Therapy for Actinic Keratosis in Organ Transplant Recipients—A Randomized Controlled Trial. Br. J. Dermatol. 2005, 172, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Jambusaria-Pahlajani, A.; Ortman, S.; Schmults, C.D.; Liang, C. Sequential Curettage, 5-Fluorouracil, and Photodynamic Therapy for Field Cancerization of the Scalp and Face in Solid Organ Transplant Recipients. Dermatol. Surg. 2016, 42 (Suppl. 1), 66–72. [Google Scholar] [CrossRef] [PubMed]
- Bencini, P.L.; Galimberti, M.G.; Pellacani, G.; Longo, C. Application of Photodynamic Therapy Combined with Pre-Illumination Microneedling in the Treatment of Actinic Keratosis in Organ Transplant Recipients. Br. J. Dermatol. 2012, 167, 1193–1194. [Google Scholar] [CrossRef] [PubMed]
- Wulf, H.C.; Pavel, S.; Stender, I.; Bakker-Wensveen, C.A. Topical Photodynamic Therapy for Prevention of New Skin Lesions in Renal Transplant Recipients. Acta Derm. Venereol. 2006, 86, 25–28. [Google Scholar] [CrossRef] [PubMed]
- de Graaf, Y.G.; Kennedy, C.; Wolterbeek, R.; Collen, A.F.; Willemze, R.; Bouwes Bavinck, J.N. Photodynamic therapy does not prevent cutaneous squamous-cell carcinoma in organ-transplant recipients: Results of a randomized-controlled trial. J. Investig. Dermatol. 2006, 10, 170. [Google Scholar] [CrossRef]
- Wennberg, A.-M.; Stenquist, B.; Stockfleth, E.; Keohane, S.; Lear, J.T.; Jemec, G.; Mork, C.; Christensen, E.; Kapp, A.; Solvsten, H.; et al. Photodynamic Therapy with Methyl Aminolevulinate for Prevention of New Skin Lesions in Transplant Recipients: A Randomized Study. Transplantation 2008, 11, 171. [Google Scholar] [CrossRef]
- Togsverd-Bo, K.; Omland, S.H.; Wulf, H.C.; Sørensen, S.S.; Haedersdal, M. Primary Prevention of Skin Dysplasia in Renal Transplant Recipients with Photodynamic Therapy: A Randomized Controlled Trial. Am. J. Transplant. 2015, 15, 2986–2990. [Google Scholar] [CrossRef]
- Togsverd-Bo, K.; Halldin, C.; Sandberg, C.; Gonzalez, H.; Wennberg, A.; Sørensen, S.; Wulf, H.; Hædersdal, M. Photodynamic therapy is more effective than imiquimod for actinic keratosis in organ transplant recipients: A randomized intraindividual controlled trial. Br. J. Dermatol. 2018, 178, 903–909. [Google Scholar] [CrossRef]
- Willey, A.; Mehta, S.; Lee, P.K. Reduction in the Incidence of Squamous Cell Carcinoma in Solid Organ Transplant Recipients Treated with Cyclic Photodynamic Therapy. Dermatol. Surg. 2010, 14, 174. [Google Scholar] [CrossRef] [PubMed]
- Bernad, I.; Aguado, L.; Núñez-Córdoba, J.M.; Redondo, P. Daylight Photodynamic Therapy for Prevention of New Actinic Keratosis and Keratinocyte Carcinomas in Organ Transplants. A Cryotherapy-Controlled Randomized Clinical Trial. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 1464–1470. [Google Scholar] [CrossRef]
- Ingham, A.I.; Weightman, W. The Efficacy and Safety of Topical 5% 5-Fluorouracil in Renal Transplant Recipients for the Treatment of Actinic Keratoses. Australas J. Dermatol. 2014, 55, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, C.; Hackethal, M.; Ulrich, M.; Howorka, A.; Forschner, T.; Sterry, W.; Stockfleth, E. Treatment of Multiple Actinic Keratoses with Topical Diclofenac 3% Gel in Organ Transplant Recipients: A Series of Six Cases. Br. J. Dermatol. 2007, 156, 40–42. [Google Scholar] [CrossRef] [PubMed]
- Brown, V.L.; Atkins, C.L.; Ghali, L.; Cerio, R.; Harwood, C.A.; Proby, C.M. Safety and Efficacy of 5% Imiquimod Cream for the Treatment of Skin Dysplasia in High-Risk Renal Transplant Recipients. Arch. Dermatol. 2005, 141, 985–993. [Google Scholar]
- Santos-Juanes, J.; Esteve, A.; Mas-Vidal, A.; Coto-Segura, P.; Salgueiro, E.; Gómez, E.; Osuna, C.G. Acute Renal Failure Caused by Imiquimod 5% Cream in a Renal Transplant Patient: Review of the Literature on Side Effects of Imiquimod. Dermatology 2011, 222, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, C.; Johannsen, A.; Röwert-Huber, J.; Ulrich, M.; Sterry, W.; Stockfleth, E. Results of a randomized, placebo-controlled safety and efficacy study of topical diclofenac 3% gel in organ transplant patients with multiple actinic keratoses. Eur. J. Dermatol. 2010, 20, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Daxenberger, F.; Deußing, M.; Eijkenboom, Q.; Gust, C.; Thamm, J.; Hartmann, D.; French, L.E.; Welzel, J.; Schuh, S.; Sattler, E.C. Innovation in Actinic Keratosis Assessment: Artificial Intelligence-Based Approach to LC-OCT PRO Score Evaluation. Cancers 2023, 15, 4457. [Google Scholar] [CrossRef] [PubMed]
- Orte Cano, C.; Suppa, M.; del Marmol, V. Where Artificial Intelligence Can Take Us in the Management and Understanding of Cancerization Fields. Cancers 2023, 15, 5264. [Google Scholar] [CrossRef]
- Del Regno, L.; Catapano, S.; Di Stefani, A.; Cappilli, S.; Peris, K.A. Review of Existing Therapies for Actinic Keratosis: Current Status and Future Directions. Am. J. Clin. Dermatol. 2022, 23, 339–352. [Google Scholar] [CrossRef]
- Cramer, P.; Stockfleth, E. Actinic Keratosis: Where Do We Stand and Where Is the Future Going to Take Us? Expert Opin. Emerg. Drugs 2020, 25, 49–58. [Google Scholar] [CrossRef]
- Lee, M.; Park, C.S.; Lee, Y.R.; Im, S.A.; Song, S.; Lee, C.K. Resiquimod, a TLR7/8 Agonist, Promotes Differentiation of Myeloid-Derived Suppressor Cells into Macrophages and Dendritic Cells. Arch. Pharmacal Res. 2014, 37, 1234–1240. [Google Scholar] [CrossRef] [PubMed]
- Bubna, A.K. Imiquimod—Its Role in the Treatment of Cutaneous Malignancies. Indian. J. Pharmacol. 2015, 47, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Szeimies, R.M.; Bichel, J.; Ortonne, J.P.; Stockfleth, E.; Lee, J.; Meng, T.C. A Phase II Dose-Ranging Study of Topical Resiquimod to Treat Actinic Keratosis. Br. J. Dermatol. 2008, 159, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Stockfleth, E.; Hofbauer, G.F.L.; Reinhold, U.; Popp, G.; Hengge, U.R.; Szeimies, R.M.; Brüning, H.; Anliker, M.; Hunger, T.; Dummer, R.; et al. Topical resiquimod dosing regimens in patients with multiple actinic keratoses: A multicentre, partly placebo-controlled, double-blind clinical trial. Br. J. Dermatol. 2019, 180, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Rajaratnam, R. Resiquimod for Actinic Keratosis: Is This a New Treatment Option? Br. J. Dermatol. 2019, 180, 254–255. [Google Scholar] [CrossRef] [PubMed]
- Neagu, N.; Dianzani, C.; Venuti, A.; Bonin, S.; Voidăzan, S.; Zalaudek, I.; Conforti, C. The Role of HPV in Keratinocyte Skin Cancer Development: A Systematic Review. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 40–46. [Google Scholar] [CrossRef]
- Galati, L.; Brancaccio, R.N.; Robitaille, A.; Cuenin, C.; Luzi, F.; Fiorucci, G.; Chiantore, M.V.; Marascio, N.; Matera, G.; Liberto, M.C.; et al. Detection of Human Papillomaviruses in Paired Healthy Skin and Actinic Keratosis by next Generation Sequencing. Papillomavirus Res. 2020, 9, 100196. [Google Scholar] [CrossRef]
- Wenande, E.; Bech-Thomsen, N.; Togsverd-Bo, K.; Haedersdal, M. Off-Label 9-Valent Human Papillomavirus Vaccination for Actinic Keratosis: A Case Series. Case Rep. Dermatol. 2013, 13, 457–463. [Google Scholar] [CrossRef]
- Bertelsen, M.; Stahlhut, M.; Grue-Sørensen, G.; Liang, X.; Christensen, G.B.; Skak, K.; Engell, K.M.; Högberg, T. Ingenol Disoxate: A Novel 4-Isoxazolecarboxylate Ester of Ingenol with Improved Properties for Treatment of Actinic Keratosis and Other Non-Melanoma Skin Cancers. Dermatol. Ther. 2016, 6, 599–626. [Google Scholar] [CrossRef]
- Weiss, J.; Ulrich, M.; Bukhalo, M.; Østerdal, M.L.; Bang, B.; Hanke, C.W. A Seamless Phase I/II Dose-Finding Trial Assessing Ingenol Disoxate (LEO 43204) for Field Treatment of Actinic Keratosis on the Scalp. Br. J. Dermatol. 2017, 176, 1456–1464. [Google Scholar] [CrossRef]
- Bourcier, M.; Stein Gold, L.; Guenther, L.; Andreassen, C.M.; Selmer, J.; Goldenberg, G. A Dose-Finding Trial with a Novel Ingenol Derivative (Ingenol Disoxate: LEO 43204) for Field Treatment of Actinic Keratosis on Full Face or 250 cm2 on the Chest. J. Dermatol. Treat. 2017, 28, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Berman, B.; Bukhalo, M.; Hanke, C.W.; Jarner, M.F.; Larsson, T.; Siegel, D.M.; Skov, T.; Szeimies, R.M. Efficacy and Safety of Ingenol Disoxate Gel in Field Treatment of Actinic Keratosis on Full Face, Scalp or Large Area (250 cm2). J. Clin. Aesthet. Dermatol. 2017, 10, 26–32. [Google Scholar] [PubMed]
- Berman, B.; Tyring, S.; Nahm, W.K.; Østerdal, M.L.; Petersen, A.H.; Siegel, D.M. Three-Day Field Treatment with Ingenol Disoxate (LEO 43204) for Actinic Keratosis: Cosmetic Outcomes and Patient Satisfaction from a Phase II Trial. J. Clin. Aesthet. Dermatol. 2017, 10, 26–32. [Google Scholar] [PubMed]
- Jedlowski, P.M. Ingenol Mebutate Is Associated With Increased Reporting Odds for Squamous Cell Carcinoma in Actinic Keratosis Patients, a Pharmacovigilance Study of the FDA Adverse Event Reporting System (FAERS). J. Cutan. Med. Surg. 2023, 27, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Spitz, K.E.; Chu, L.; Swerlick, R.A. Atezolizumab (Programmed Cell Death-Ligand 1 Antibody)-Induced Inflammation of Actinic Keratosis: A Case Report. SAGE Open Med. Case Rep. 2022, 11, 2050313X221131863. [Google Scholar] [CrossRef]
- Ansary, T.M.; Hossain, M.D.R.; Komine, M.; Ohtsuki, M. Immunotherapy for the Treatment of Squamous Cell Carcinoma: Potential Benefits and Challenges. Int. J. Mol. Sci. 2023, 15, 8530. [Google Scholar] [CrossRef]
- Stravodimou, A.; Tzelepi, V.; Balasis, S.; Georgiou, S.; Papadaki, H.; Mouzaki, A.; Melachrinou, M.; Kourea, E.P. PD-L1 Expression, T-lymphocyte Subpopulations and Langerhans Cells in Cutaneous Squamous Cell Carcinoma and Precursor Lesions. Anticancer Res. 2021, 41, 3439–3448. [Google Scholar] [CrossRef]
- Mahen, K.K.; Markley, L.; Bogart, J.; Klatka, H.; Krishna, V.; Maytin, E.V.; Stark, G.R.; McDonald, C. Topical N-Phosphonacetyl-l-Aspartate Is a Dual Action Candidate for Treating Non-Melanoma Skin Cancer. Exp. Dermatol. 2023, 32, 1485–1497. [Google Scholar] [CrossRef]
| Primary Immunomodulatory Effect | Secondary Immunomodulatory Effect | Immunomodulatory Effect under Research | Topical Immunotherapy Associated with Cryotherapy | Organ Transplant Recipients | Future Perspectives |
|---|---|---|---|---|---|
|
|
|
|
|
|
| FDA Approval | Imiquimod approved for genital warts, AK, and superficial BCC. |
| TLR Activation | Binds to TLR7 and TLR8, initiating NF-κB pathway via MyD88. |
| Cytokine Release | Increases proinflammatory cytokines (TNF-α, IFN-α, IL-6, IL-8, IL-12) and chemokines (CCL2, CCL3, CCL4). |
| Innate Immunity Enhancement | Amplifies innate immunity. |
| Th1 Phenotype Induction | Promotes T cell conversion to Th1 phenotype, stimulating IFN-γ secretion. |
| pDC Activation | Stimulates plasmacytoid dendritic cells (pDCs) expressing TLR7/9. |
| Type I Interferon Production | Triggers robust production of IFN-α and IFN-β. |
| Immune Response Amplification | Enhances both innate and acquired immune responses. Principio del formulario Final del formulario |
| Name of the Treatment | Mechanism of Action | Posology | Adverse Effects |
|---|---|---|---|
| 5-fluorouracil | Pyrimidine analogue | 5% 2 times daily for 2–4 weeks | Scaling Pruritus |
| Inhibition of thymidylate synthetase | 4% 1 time daily for 4 weeks | Erythema Burning | |
| Induces cell apoptosis | 0.5% 2 times daily for 4–6 weeks | Crusting | |
| Imiquimod | Activates toll-like receptor 7 | 2.5%, 3.75%, and 5% three times a week for 12–16 weeks | Local reactions Erythema |
| Production of TNF-α, IFN-γ, IFN-α, and IL-12 Induces cell apoptosis | Systemic flu-like symptoms | ||
| Diclofenac disodium | Inhibition of COX-1 and COX-2 | 3% 2 times a day for 60–90 days | Irritation Itching Erythema |
| Inhibition of angiogenesis and cell proliferation Induces cell apoptosis | |||
| Photodynamic therapy | Production of ROS and releases DAMPs Modulates CAFs | Daylight: One session curettage of the lesions followed by application of the photosensitizer, 30 min incubation, and sun exposure for 2 h. Conventional: Two sessions curettage of the lesions followed by application of the photosensitizer, 3 h incubation, and subsequent lighting. | Pain Burning Erythema Irritation Pruritus |
| Induces cell apoptosis, necrosis, and autophagy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernal Masferrer, L.; Gracia Cazaña, T.; Bernad Alonso, I.; Álvarez-Salafranca, M.; Almenara Blasco, M.; Gallego Rentero, M.; Juarranz de la Fuente, Á.; Gilaberte, Y. Topical Immunotherapy for Actinic Keratosis and Field Cancerization. Cancers 2024, 16, 1133. https://doi.org/10.3390/cancers16061133
Bernal Masferrer L, Gracia Cazaña T, Bernad Alonso I, Álvarez-Salafranca M, Almenara Blasco M, Gallego Rentero M, Juarranz de la Fuente Á, Gilaberte Y. Topical Immunotherapy for Actinic Keratosis and Field Cancerization. Cancers. 2024; 16(6):1133. https://doi.org/10.3390/cancers16061133
Chicago/Turabian StyleBernal Masferrer, Laura, Tamara Gracia Cazaña, Isabel Bernad Alonso, Marcial Álvarez-Salafranca, Manuel Almenara Blasco, María Gallego Rentero, Ángeles Juarranz de la Fuente, and Yolanda Gilaberte. 2024. "Topical Immunotherapy for Actinic Keratosis and Field Cancerization" Cancers 16, no. 6: 1133. https://doi.org/10.3390/cancers16061133
APA StyleBernal Masferrer, L., Gracia Cazaña, T., Bernad Alonso, I., Álvarez-Salafranca, M., Almenara Blasco, M., Gallego Rentero, M., Juarranz de la Fuente, Á., & Gilaberte, Y. (2024). Topical Immunotherapy for Actinic Keratosis and Field Cancerization. Cancers, 16(6), 1133. https://doi.org/10.3390/cancers16061133








