Ferroptosis Inducers Upregulate PD-L1 in Recurrent Triple-Negative Breast Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Datasets of Cell Line Models Stimulated with Ferroptosis Inducers
2.1.1. MDA-MB-231 RNA-Sequencing Transcriptome
2.1.2. HCC38 Microarray Transcriptome
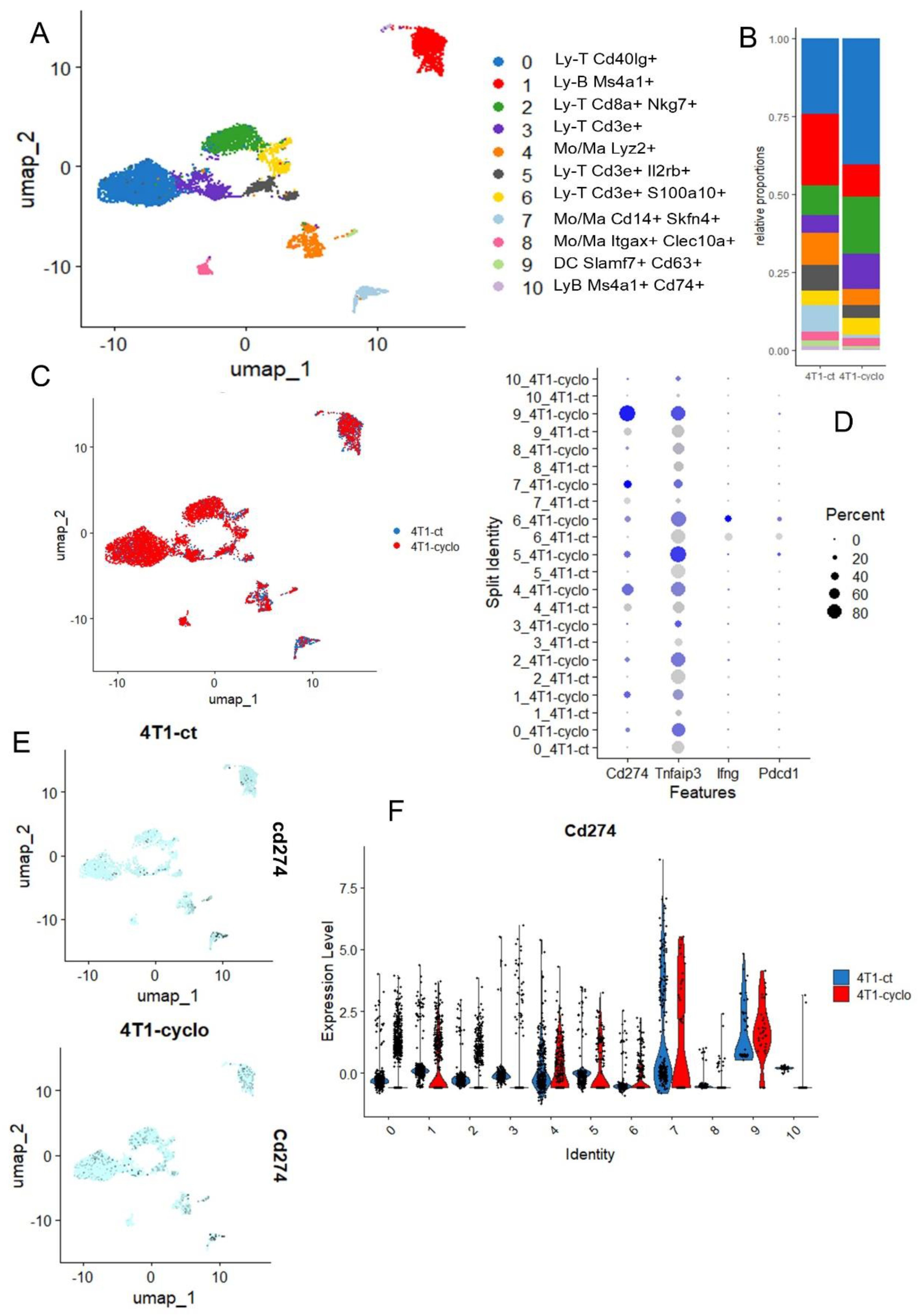
2.1.3. Single Cell Transcriptome for Immune Microenvironment of 4T1-Transplanted Tumors
2.1.4. Proteomic Validation of PDL1 Regulation under Ferroptosis Inducer
2.2. Cohorts of Breast Cancer Tumor Samples
2.2.1. Training Cohort: TCGA Firehose Invasive Breast Cancer Cohort
2.2.2. Validation Cohort: METABRIC Breast Cancer Cohort
2.3. Transcriptome Immune Modulation Scoring
2.4. Ferroptosis Visualization in Transcriptome Enrichment
2.5. CD274 Ferroptosis-Driver Gene Score Quantification
2.6. Immunohistochemistry
2.7. Single Cell Transcriptome Analysis
2.8. Flow Cytometry
3. Results
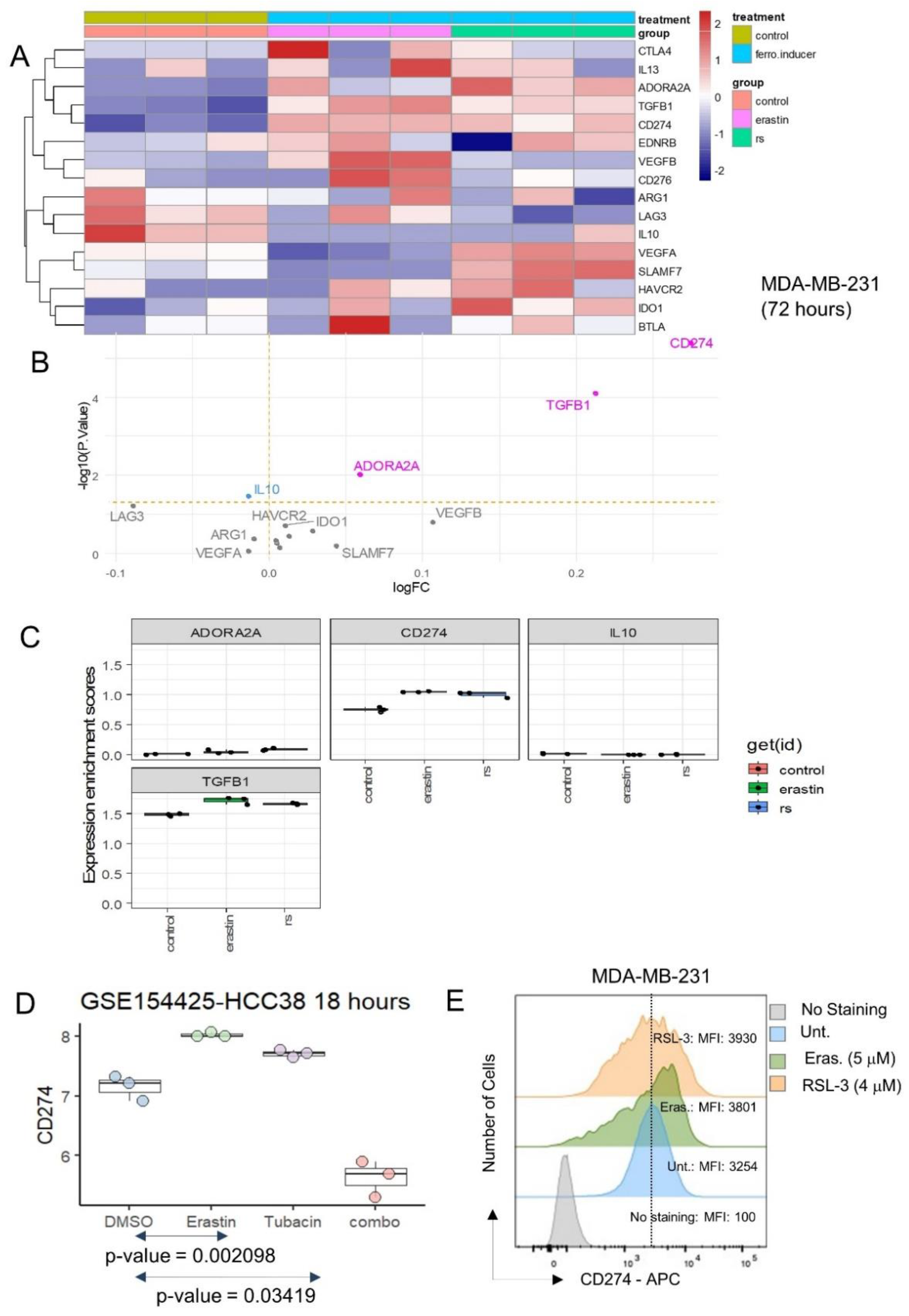
3.1. CD274 (PD-L1) Is Up-Regulated by Ferroptosis Inducers in Triple-Negative Breast Cancer Cells
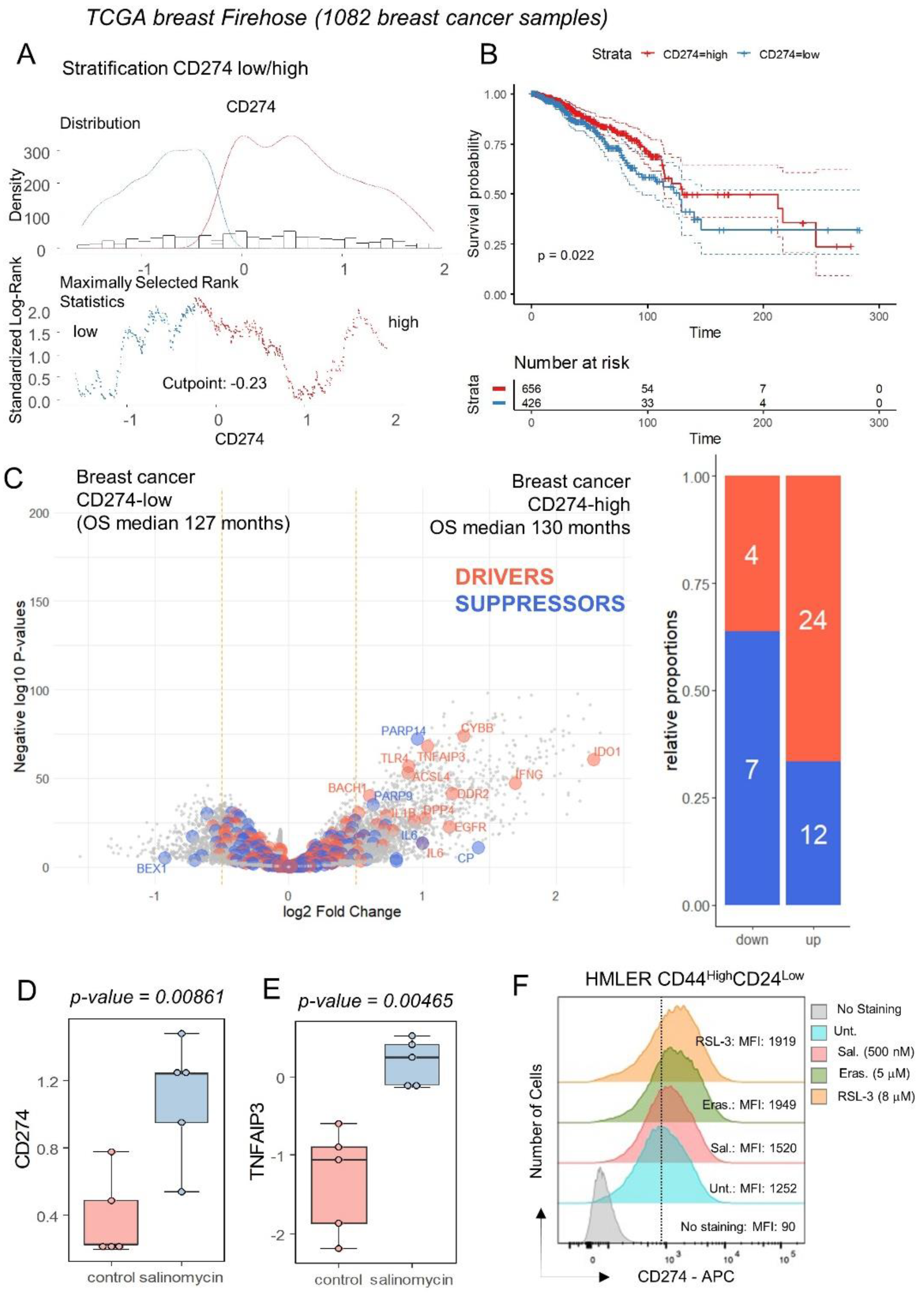
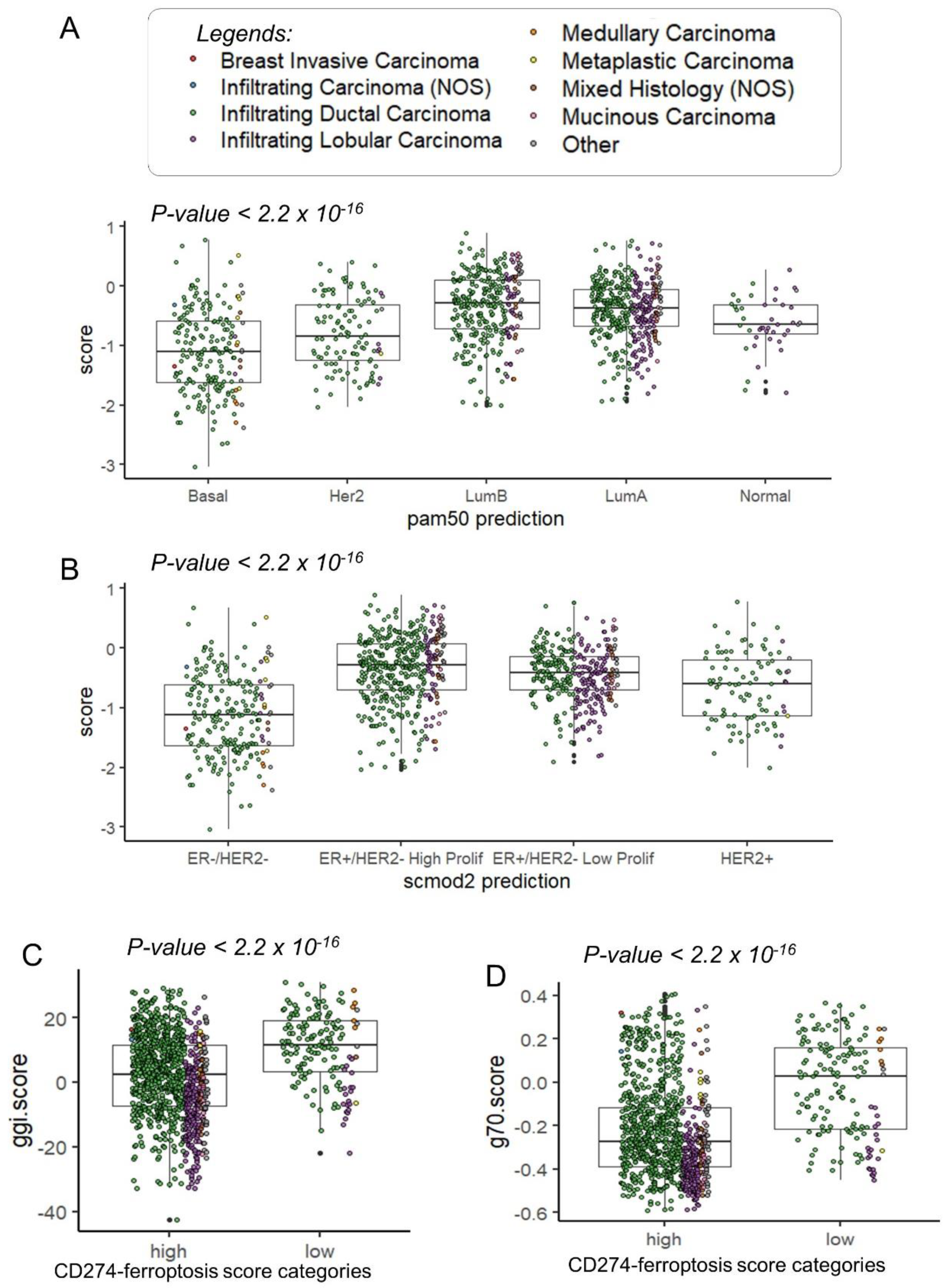
3.2. Breast Tumors with High Expression of CD274 Harbored Upregulation of Ferroptosis Drivers
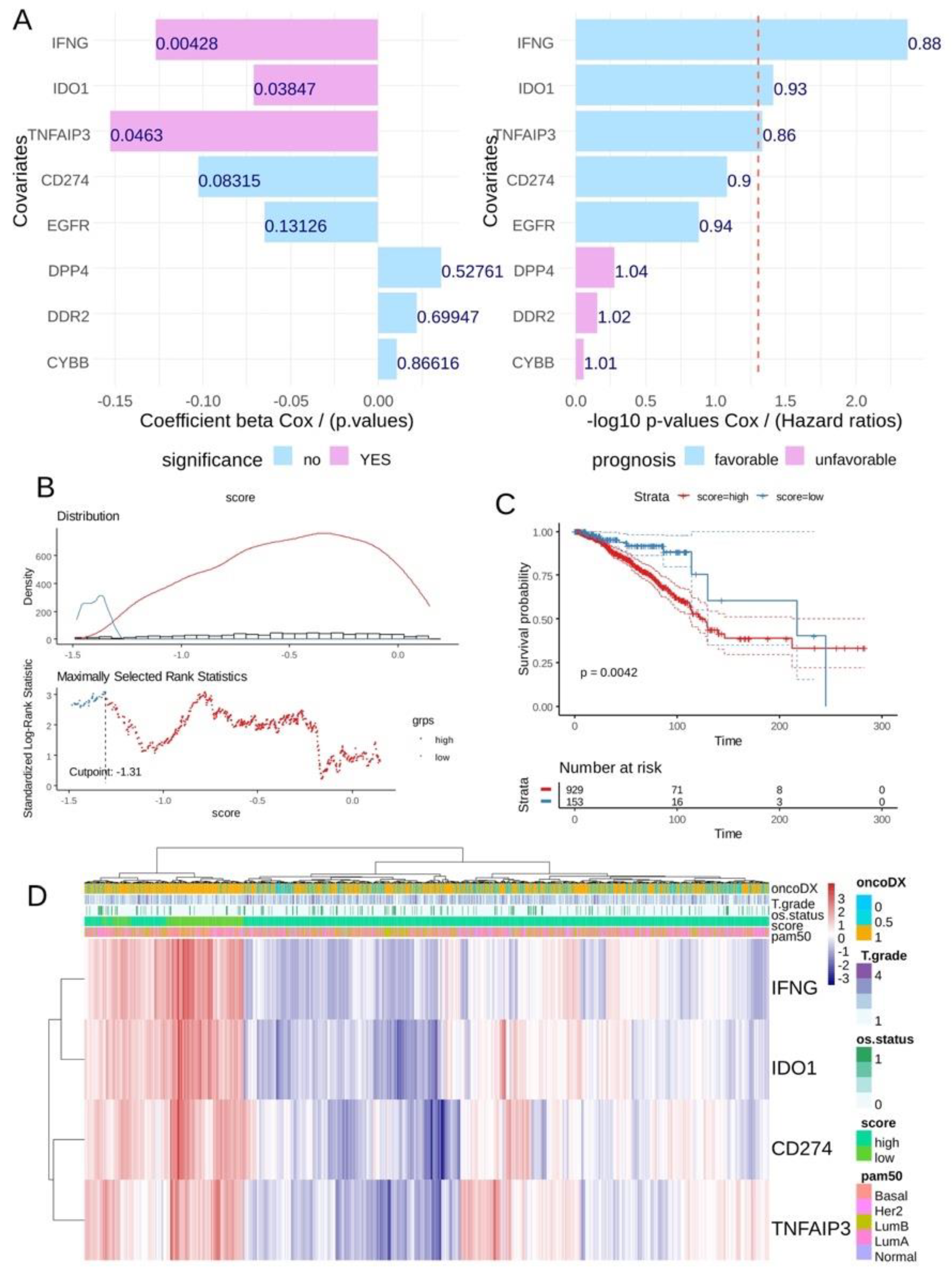
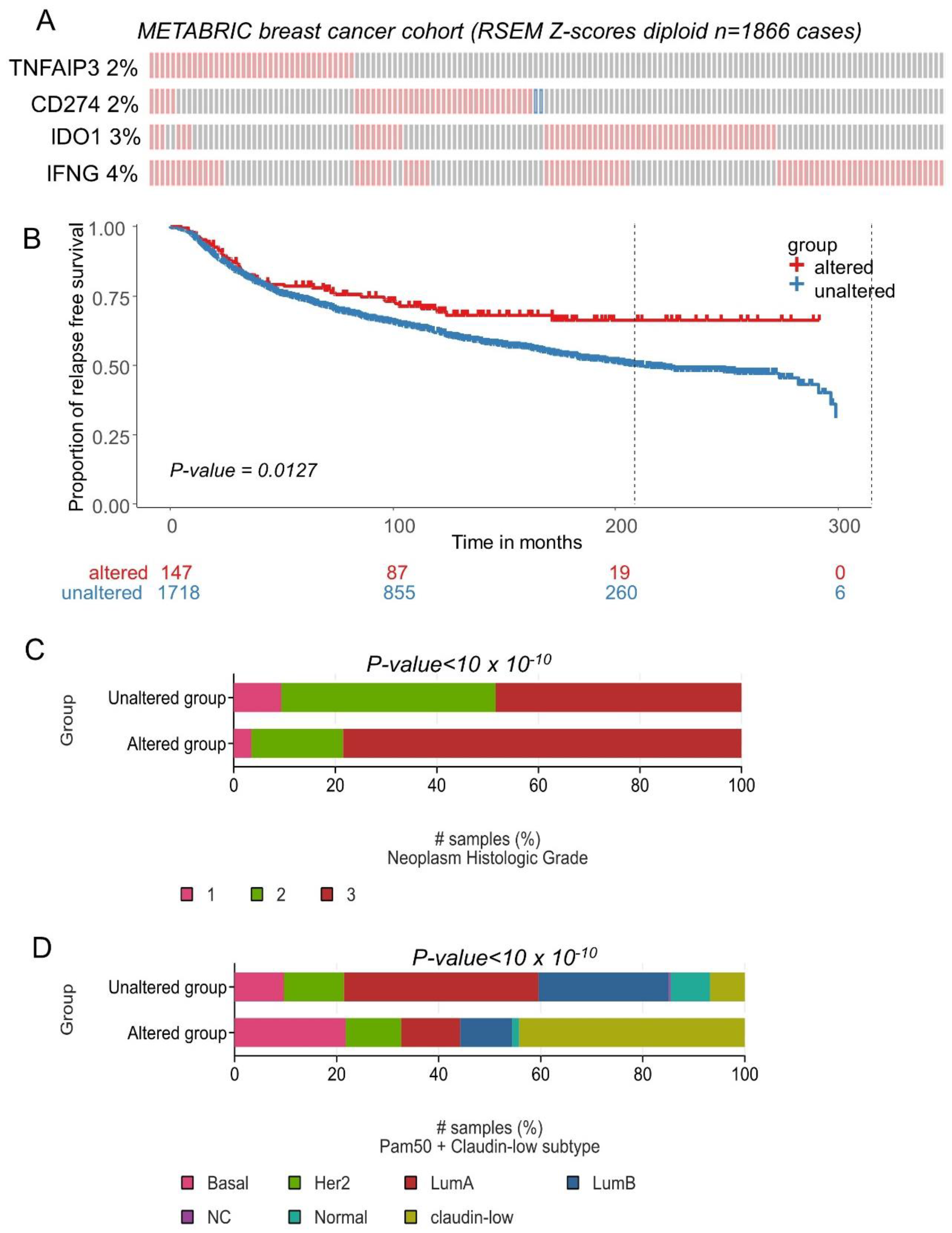
3.3. CD274 Ferroptosis-Driver Score Predicts Recurrence of Breast Cancer
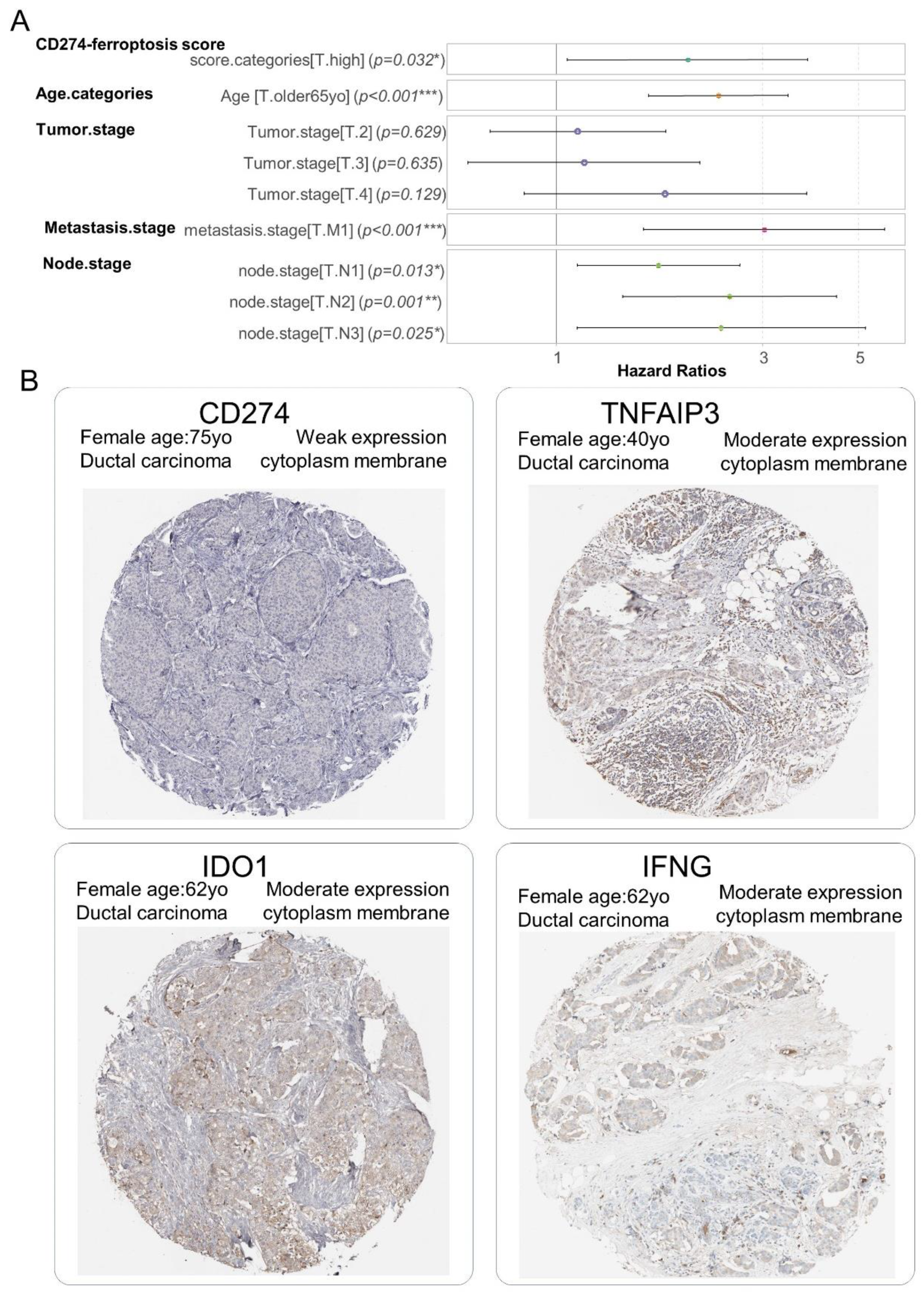
3.4. CD274 Ferroptosis-Driver Score Is an Independent Prognosis Marker in Breast Cancer Overall Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sotiriou, C.; Neo, S.-Y.; McShane, L.M.; Korn, E.L.; Long, P.M.; Jazaeri, A.; Martiat, P.; Fox, S.B.; Harris, A.L.; Liu, E.T. Breast Cancer Classification and Prognosis Based on Gene Expression Profiles from a Population-Based Study. Proc. Natl. Acad. Sci. USA 2003, 100, 10393–10398. [Google Scholar] [CrossRef] [PubMed]
- Perou, C.M.; Sørlie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular Portraits of Human Breast Tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef]
- Sorlie, T.; Tibshirani, R.; Parker, J.; Hastie, T.; Marron, J.S.; Nobel, A.; Deng, S.; Johnsen, H.; Pesich, R.; Geisler, S.; et al. Repeated Observation of Breast Tumor Subtypes in Independent Gene Expression Data Sets. Proc. Natl. Acad. Sci. USA 2003, 100, 8418–8423. [Google Scholar] [CrossRef] [PubMed]
- Prat, A.; Pineda, E.; Adamo, B.; Galván, P.; Fernández, A.; Gaba, L.; Díez, M.; Viladot, M.; Arance, A.; Muñoz, M. Clinical Implications of the Intrinsic Molecular Subtypes of Breast Cancer. Breast 2015, 24 (Suppl. 2), S26–S35. [Google Scholar] [CrossRef] [PubMed]
- Hudis, C.A.; Gianni, L. Triple-Negative Breast Cancer: An Unmet Medical Need. Oncologist 2011, 16, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.T.; Zackrisson, S.; Senkus, E.; ESMO Guidelines Committee. Early Breast Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2019, 30, 1194–1220. [Google Scholar] [CrossRef] [PubMed]
- Farkona, S.; Diamandis, E.P.; Blasutig, I.M. Cancer Immunotherapy: The Beginning of the End of Cancer? BMC Med. 2016, 14, 73. [Google Scholar] [CrossRef]
- Schmid, P.; Cortes, J.; Pusztai, L.; McArthur, H.; Kümmel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; Harbeck, N.; et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N. Engl. J. Med. 2020, 382, 810–821. [Google Scholar] [CrossRef]
- Yu, H.; Guo, P.; Xie, X.; Wang, Y.; Chen, G. Ferroptosis, a New Form of Cell Death, and Its Relationships with Tumourous Diseases. J. Cell Mol. Med. 2017, 21, 648–657. [Google Scholar] [CrossRef]
- Shi, Y.; Jin, J.; Ji, W.; Guan, X. Therapeutic Landscape in Mutational Triple Negative Breast Cancer. Mol. Cancer 2018, 17, 99. [Google Scholar] [CrossRef]
- Kastenhuber, E.R.; Lowe, S.W. Putting P53 in Context. Cell 2017, 170, 1062–1078. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Wang, W.; Zhang, W. Ferroptosis and the Bidirectional Regulatory Factor P53. Cell Death Discov. 2023, 9, 197. [Google Scholar] [CrossRef] [PubMed]
- Mou, Y.; Wang, J.; Wu, J.; He, D.; Zhang, C.; Duan, C.; Li, B. Ferroptosis, a New Form of Cell Death: Opportunities and Challenges in Cancer. J. Hematol. Oncol. 2019, 12, 34. [Google Scholar] [CrossRef] [PubMed]
- Wuguo, T.; Jianjie, Z.; Donglin, L. Correlation between Ferroptosis-Related Genes and PDL-1 Immunotherapy in Triple-Negative Breast Cancer. Asian J. Surg. 2023, 46, 4595–4597. [Google Scholar] [CrossRef]
- Li, P.; Lin, Q.; Sun, S.; Yang, N.; Xia, Y.; Cao, S.; Zhang, W.; Li, Q.; Guo, H.; Zhu, M.; et al. Inhibition of Cannabinoid Receptor Type 1 Sensitizes Triple-Negative Breast Cancer Cells to Ferroptosis via Regulating Fatty Acid Metabolism. Cell Death Dis. 2022, 13, 808. [Google Scholar] [CrossRef]
- Barrett, T.; Wilhite, S.E.; Ledoux, P.; Evangelista, C.; Kim, I.F.; Tomashevsky, M.; Marshall, K.A.; Phillippy, K.H.; Sherman, P.M.; Holko, M.; et al. NCBI GEO: Archive for Functional Genomics Data Sets—Update. Nucleic Acids Res. 2013, 41, D991–D995. [Google Scholar] [CrossRef] [PubMed]
- Alothaim, T.; Charbonneau, M.; Tang, X. HDAC6 Inhibitors Sensitize Non-Mesenchymal Triple-Negative Breast Cancer Cells to Cysteine Deprivation. Sci. Rep. 2021, 11, 10956. [Google Scholar] [CrossRef] [PubMed]
- Carpen, L.; Falvo, P.; Orecchioni, S.; Mitola, G.; Hillje, R.; Mazzara, S.; Mancuso, P.; Pileri, S.; Raveane, A.; Bertolini, F. A Single-Cell Transcriptomic Landscape of Innate and Adaptive Intratumoral Immunity in Triple Negative Breast Cancer during Chemo- and Immunotherapies. Cell Death Discov. 2022, 8, 106. [Google Scholar] [CrossRef]
- Cosialls, E.; Pacreau, E.; Duruel, C.; Ceccacci, S.; Elhage, R.; Desterke, C.; Roger, K.; Guerrera, C.; Ducloux, R.; Souquere, S.; et al. mTOR Inhibition Suppresses Salinomycin-Induced Ferroptosis in Breast Cancer Stem Cells by Ironing out Mitochondrial Dysfunctions. Cell Death Dis. 2023, 14, 744. [Google Scholar] [CrossRef]
- Liu, J.; Lichtenberg, T.; Hoadley, K.A.; Poisson, L.M.; Lazar, A.J.; Cherniack, A.D.; Kovatich, A.J.; Benz, C.C.; Levine, D.A.; Lee, A.V.; et al. An Integrated TCGA Pan-Cancer Clinical Data Resource to Drive High-Quality Survival Outcome Analytics. Cell 2018, 173, 400–416.e11. [Google Scholar] [CrossRef]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative Analysis of Complex Cancer Genomics and Clinical Profiles Using the cBioPortal. Sci. Signal 2013, 6, pl1. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor Package for Differential Expression Analysis of Digital Gene Expression Data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Law, C.W.; Chen, Y.; Shi, W.; Smyth, G.K. Voom: Precision Weights Unlock Linear Model Analysis Tools for RNA-Seq Read Counts. Genome Biol. 2014, 15, R29. [Google Scholar] [CrossRef] [PubMed]
- Gendoo, D.M.A.; Ratanasirigulchai, N.; Schröder, M.S.; Paré, L.; Parker, J.S.; Prat, A.; Haibe-Kains, B. Genefu: An R/Bioconductor Package for Computation of Gene Expression-Based Signatures in Breast Cancer. Bioinformatics 2016, 32, 1097–1099. [Google Scholar] [CrossRef]
- Parker, J.S.; Mullins, M.; Cheang, M.C.U.; Leung, S.; Voduc, D.; Vickery, T.; Davies, S.; Fauron, C.; He, X.; Hu, Z.; et al. Supervised Risk Predictor of Breast Cancer Based on Intrinsic Subtypes. J. Clin. Oncol. 2009, 27, 1160–1167. [Google Scholar] [CrossRef] [PubMed]
- Wirapati, P.; Sotiriou, C.; Kunkel, S.; Farmer, P.; Pradervand, S.; Haibe-Kains, B.; Desmedt, C.; Ignatiadis, M.; Sengstag, T.; Schütz, F.; et al. Meta-Analysis of Gene Expression Profiles in Breast Cancer: Toward a Unified Understanding of Breast Cancer Subtyping and Prognosis Signatures. Breast Cancer Res. 2008, 10, R65. [Google Scholar] [CrossRef] [PubMed]
- Prat, A.; Parker, J.S.; Karginova, O.; Fan, C.; Livasy, C.; Herschkowitz, J.I.; He, X.; Perou, C.M. Phenotypic and Molecular Characterization of the Claudin-Low Intrinsic Subtype of Breast Cancer. Breast Cancer Res. 2010, 12, R68. [Google Scholar] [CrossRef] [PubMed]
- van ’t Veer, L.J.; Dai, H.; van de Vijver, M.J.; He, Y.D.; Hart, A.A.M.; Mao, M.; Peterse, H.L.; van der Kooy, K.; Marton, M.J.; Witteveen, A.T.; et al. Gene Expression Profiling Predicts Clinical Outcome of Breast Cancer. Nature 2002, 415, 530–536. [Google Scholar] [CrossRef]
- Sotiriou, C.; Wirapati, P.; Loi, S.; Harris, A.; Fox, S.; Smeds, J.; Nordgren, H.; Farmer, P.; Praz, V.; Haibe-Kains, B.; et al. Gene Expression Profiling in Breast Cancer: Understanding the Molecular Basis of Histologic Grade to Improve Prognosis. JNCI J. Natl. Cancer Inst. 2006, 98, 262–272. [Google Scholar] [CrossRef]
- Desterke, C. Tcga.Breast a R Package Composed of Eset (RNAseq)—TCGA Breast Cancer with Clinical Scores Computed, 2023.
- Curtis, C.; Shah, S.P.; Chin, S.-F.; Turashvili, G.; Rueda, O.M.; Dunning, M.J.; Speed, D.; Lynch, A.G.; Samarajiwa, S.; Yuan, Y.; et al. The Genomic and Transcriptomic Architecture of 2,000 Breast Tumours Reveals Novel Subgroups. Nature 2012, 486, 346–352. [Google Scholar] [CrossRef]
- Pereira, B.; Chin, S.-F.; Rueda, O.M.; Vollan, H.-K.M.; Provenzano, E.; Bardwell, H.A.; Pugh, M.; Jones, L.; Russell, R.; Sammut, S.-J.; et al. The Somatic Mutation Profiles of 2,433 Breast Cancers Refines Their Genomic and Transcriptomic Landscapes. Nat. Commun. 2016, 7, 11479. [Google Scholar] [CrossRef] [PubMed]
- Rueda, O.M.; Sammut, S.-J.; Seoane, J.A.; Chin, S.-F.; Caswell-Jin, J.L.; Callari, M.; Batra, R.; Pereira, B.; Bruna, A.; Ali, H.R.; et al. Dynamics of Breast-Cancer Relapse Reveal Late-Recurring ER-Positive Genomic Subgroups. Nature 2019, 567, 399–404. [Google Scholar] [CrossRef]
- Thorsson, V.; Gibbs, D.L.; Brown, S.D.; Wolf, D.; Bortone, D.S.; Ou Yang, T.-H.; Porta-Pardo, E.; Gao, G.F.; Plaisier, C.L.; Eddy, J.A.; et al. The Immune Landscape of Cancer. Immunity 2018, 48, 812–830.e14. [Google Scholar] [CrossRef] [PubMed]
- Hänzelmann, S.; Castelo, R.; Guinney, J. GSVA: Gene Set Variation Analysis for Microarray and RNA-Seq Data. BMC Bioinform. 2013, 14, 7. [Google Scholar] [CrossRef] [PubMed]
- Desterke, C. Immunemod: R-Package Which Allows to Compute Immune Score Based on Expression of Molecules Grouped in Nine Distinct Classes of Immune Modulation Functions, 2023.
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. Limma Powers Differential Expression Analyses for RNA-Sequencing and Microarray Studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Zhou, N.; Yuan, X.; Du, Q.; Zhang, Z.; Shi, X.; Bao, J.; Ning, Y.; Peng, L. FerrDb V2: Update of the Manually Curated Database of Ferroptosis Regulators and Ferroptosis-Disease Associations. Nucleic Acids Res. 2023, 51, D571–D582. [Google Scholar] [CrossRef]
- Desterke, C. Ferroviz: A R-Package to Perform Visualization of Ferroptosis Related Genes in a Transcriptome Differential Expressed Gene Analysis Output from Limma Algorithm, 2023.
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2009. [Google Scholar]
- Uhlén, M.; Björling, E.; Agaton, C.; Szigyarto, C.A.-K.; Amini, B.; Andersen, E.; Andersson, A.-C.; Angelidou, P.; Asplund, A.; Asplund, C.; et al. A Human Protein Atlas for Normal and Cancer Tissues Based on Antibody Proteomics. Mol. Cell Proteom. 2005, 4, 1920–1932. [Google Scholar] [CrossRef]
- Butler, A.; Hoffman, P.; Smibert, P.; Papalexi, E.; Satija, R. Integrating Single-Cell Transcriptomic Data across Different Conditions, Technologies, and Species. Nat. Biotechnol. 2018, 36, 411–420. [Google Scholar] [CrossRef]
- Xu, C.; Su, Z. Identification of Cell Types from Single-Cell Transcriptomes Using a Novel Clustering Method. Bioinformatics 2015, 31, 1974–1980. [Google Scholar] [CrossRef]
- Mai, T.T.; Hamaï, A.; Hienzsch, A.; Cañeque, T.; Müller, S.; Wicinski, J.; Cabaud, O.; Leroy, C.; David, A.; Acevedo, V.; et al. Salinomycin Kills Cancer Stem Cells by Sequestering Iron in Lysosomes. Nat. Chem. 2017, 9, 1025–1033. [Google Scholar] [CrossRef]
- Ladwa, A.; Elghawy, O.; Schroen, A.; Abernathy, K.; Schlefman, J.; Dillon, P. Complete Response of Triple-Negative Metaplastic Carcinoma of the Breast Using Pembrolizumab. Case Rep. Oncol. 2023, 16, 1129–1135. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, B.; Guan, G.; Kang, R.; Dai, Y.; Tang, D. Cyclophosphamide-Induced GPX4 Degradation Triggers Parthanatos by Activating AIFM1. Biochem. Biophys. Res. Commun. 2022, 606, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Hou, B.; Li, H.; Zhou, H.; Du, B. Cyclophosphamide Induces the Ferroptosis of Tumor Cells Through Heme Oxygenase-1. Front. Pharmacol. 2022, 13, 839464. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.D.; Jung, B.-K.; Lee, D.; Ha, J.; Chang, H.-G.; Lee, J.; Lee, S.; Yun, C.-O.; Kim, Y.-C. Enhanced Immunogenic Cell Death by Apoptosis/Ferroptosis Hybrid Pathway Potentiates PD-L1 Blockade Cancer Immunotherapy. ACS Biomater. Sci. Eng. 2022, 8, 5188–5198. [Google Scholar] [CrossRef] [PubMed]
- Reis-Filho, J.S.; Pusztai, L. Gene Expression Profiling in Breast Cancer: Classification, Prognostication, and Prediction. Lancet 2011, 378, 1812–1823. [Google Scholar] [CrossRef]
- Wang, W.; Green, M.; Choi, J.E.; Gijón, M.; Kennedy, P.D.; Johnson, J.K.; Liao, P.; Lang, X.; Kryczek, I.; Sell, A.; et al. CD8+ T Cells Regulate Tumour Ferroptosis during Cancer Immunotherapy. Nature 2019, 569, 270–274. [Google Scholar] [CrossRef]
- Lee, J.-H.; Jung, S.M.; Yang, K.-M.; Bae, E.; Ahn, S.G.; Park, J.S.; Seo, D.; Kim, M.; Ha, J.; Lee, J.; et al. A20 Promotes Metastasis of Aggressive Basal-like Breast Cancers through Multi-Monoubiquitylation of Snail1. Nat. Cell Biol. 2017, 19, 1260–1273. [Google Scholar] [CrossRef]
- Liu, X.; Jin, X.; Wang, X.; Yan, X.; Wang, C.; Wang, K.; He, X.; Zhai, W. Knockdown of A20 Attenuates Microglial Susceptibility to OGD/R-Induced Ferroptosis and Upregulates Inflammatory Responses. Immunopharmacol. Immunotoxicol. 2023, 45, 539–548. [Google Scholar] [CrossRef]
- Xiao, F.-J.; Zhang, D.; Wu, Y.; Jia, Q.-H.; Zhang, L.; Li, Y.-X.; Yang, Y.-F.; Wang, H.; Wu, C.-T.; Wang, L.-S. miRNA-17-92 Protects Endothelial Cells from Erastin-Induced Ferroptosis through Targeting the A20-ACSL4 Axis. Biochem. Biophys. Res. Commun. 2019, 515, 448–454. [Google Scholar] [CrossRef]
- Song, C.; Kendi, A.T.; Lowe, V.J.; Lee, S. The A20/TNFAIP3-CDC20-CASP1 Axis Promotes Inflammation-Mediated Metastatic Disease in Triple-Negative Breast Cancer. Anticancer Res. 2022, 42, 681–695. [Google Scholar] [CrossRef]
- Kim, S.; Park, S.; Cho, M.S.; Lim, W.; Moon, B.-I.; Sung, S.H. Strong Correlation of Indoleamine 2,3-Dioxygenase 1 Expression with Basal-Like Phenotype and Increased Lymphocytic Infiltration in Triple-Negative Breast Cancer. J. Cancer 2017, 8, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Fiore, A.; Zeitler, L.; Russier, M.; Groß, A.; Hiller, M.-K.; Parker, J.L.; Stier, L.; Köcher, T.; Newstead, S.; Murray, P.J. Kynurenine Importation by SLC7A11 Propagates Anti-Ferroptotic Signaling. Mol. Cell 2022, 82, 920–932.e7. [Google Scholar] [CrossRef] [PubMed]


| Variable | Level | Low (n = 426) | High (n = 656) | Total (n = 1082) | p-Value |
|---|---|---|---|---|---|
| Age at diagnosis | Younger than 65 yo | 283 (66.4) | 462 (70.4) | 745 (68.9) | |
| Older than 65 yo | 143 (33.6) | 194 (29.6) | 337 (31.1) | 0.1871019 | |
| Tumor stage | T1 | 102 (24.1) | 174 (26.5) | 276 (25.6) | |
| T2 | 239 (56.5) | 388 (59.1) | 627 (58.1) | ||
| T3 | 66 (15.6) | 71 (10.8) | 137 (12.7) | ||
| T4 | 16 (3.8) | 23 (3.5) | 39 (3.6) | 0.1341858 | |
| missing | 3 | 0 | 3 | ||
| Node stage | N1 | 141 (33.9) | 214 (33.1) | 355 (33.4) | |
| N0 | 196 (47.1) | 316 (48.9) | 512 (48.2) | ||
| N2 | 46 (11.1) | 73 (11.3) | 119 (11.2) | ||
| N3 | 33 (7.9) | 43 (6.7) | 76 (7.2) | 0.8484744 | |
| missing | 10 | 10 | 20 | ||
| Metastasis stage | M0 | 336 (97.4) | 558 (97.9) | 894 (97.7) | |
| M1 | 9 (2.6) | 12 (2.1) | 21 (2.3) | 0.7909348 | |
| missing | 81 | 86 | 167 | ||
| Pam50 robust | LumB | 154 (36.2) | 169 (25.8) | 323 (29.9) | |
| Her2 | 31 (7.3) | 80 (12.2) | 111 (10.3) | ||
| LumA | 166 (39.0) | 245 (37.3) | 411 (38.0) | ||
| Normal | 14 (3.3) | 28 (4.3) | 42 (3.9) | ||
| Basal | 61 (14.3) | 134 (20.4) | 195 (18.0) | 0.0002416 | |
| Ggi score | mean (sd) | 2.1 (12.6) | 3.4 (13) | 2.9 (12.9) | 0.1112944 |
| G70 score | mean (sd) | −0.23 (0.2) | −0.18 (0.2) | −0.2 (0.2) | 0.0005523 |
| Oncotypedx score | mean (sd) | 61 (35.9) | 65 (36.4) | 63.5 (36.2) | 0.0772518 |
| OS_STATUS | Alive | 356 (83.6) | 575 (87.7) | 931 (86.0) | |
| Dead | 70 (16.4) | 81 (12.3) | 151 (14.0) | 0.0711620 |
| Variable | Level | Low (n = 153) | High (n = 929) | Total (n = 1082) | p-Value |
|---|---|---|---|---|---|
| Age at diagnosis | Younger than 65 yo | 114 (74.5) | 631 (67.9) | 745 (68.9) | |
| Older than 65 yo | 39 (25.5) | 298 (32.1) | 337 (31.1) | 0.124501 | |
| Tumor stage | T1 | 45 (29.4) | 231 (24.9) | 276 (25.6) | |
| T2 | 93 (60.8) | 534 (57.7) | 627 (58.1) | ||
| T3 | 14 (9.2) | 123 (13.3) | 137 (12.7) | ||
| T4 | 1 (0.7) | 38 (4.1) | 39 (3.6) | 0.061994 | |
| missing | 0 | 3 | 3 | ||
| Node stage | N1 | 46 (30.1) | 309 (34.0) | 355 (33.4) | |
| N0 | 91 (59.5) | 421 (46.3) | 512 (48.2) | ||
| N2 | 12 (7.8) | 107 (11.8) | 119 (11.2) | ||
| N3 | 4 (2.6) | 72 (7.9) | 76 (7.2) | 0.006523 | |
| missing | 0 | 20 | 20 | ||
| Metastasis stage | M0 | 140 (99.3) | 754 (97.4) | 894 (97.7) | |
| M1 | 1 (0.7) | 20 (2.6) | 21 (2.3) | 0.288441 | |
| missing | 12 | 155 | 167 | ||
| Pam50 robust | LumB | 26 (17.0) | 297 (32.0) | 323 (29.9) | |
| Her2 | 26 (17.0) | 85 (9.1) | 111 (10.3) | ||
| LumA | 26 (17.0) | 385 (41.4) | 411 (38.0) | ||
| Normal | 4 (2.6) | 38 (4.1) | 42 (3.9) | ||
| Basal | 71 (46.4) | 124 (13.3) | 195 (18.0) | <0.0001 | |
| Ggi score | mean (sd) | 10.5 (10.9) | 1.6 (12.7) | 2.9 (12.9) | <0.0001 |
| G70 score | mean (sd) | 0 (0.2) | −0.2 (0.2) | −0.2 (0.2) | <0.0001 |
| Oncotypedx score | mean (sd) | 84.8 (25.8) | 59.9 (36.5) | 63.5 (36.2) | <0.0001 |
| OS_STATUS | Alive | 141 (92.2) | 790 (85.0) | 931 (86.0) | |
| Dead | 12 (7.8) | 139 (15.0) | 151 (14.0) | 0.025827 |
| Covariates | Hazard Ratios | CI95* Low | CI95* High | p-Value |
|---|---|---|---|---|
| CD274-ferroptosis score [T** high] | 2.012 | 1.063 | 3.810 | 3.18 × 10−2 |
| age categories [T** older than 65 yo] | 2.368 | 1.635 | 3.430 | 5.13 × 10−6 |
| tumor_grade [T** 2] | 1.122 | 0.703 | 1.790 | 6.29 × 10−1 |
| tumor_grade [T** 3] | 1.161 | 0.627 | 2.149 | 6.35 × 10−1 |
| tumor_grade [T** 4] | 1.787 | 0.845 | 3.782 | 1.29 × 10−1 |
| metastasis stage [T** M1] | 3.021 | 1.594 | 5.726 | 6.98 × 10−4 |
| node stage [T** N1] | 1.726 | 1.123 | 2.653 | 1.28 × 10−2 |
| node stage [T** N2] | 2.517 | 1.426 | 4.445 | 1.46 × 10−3 |
| node stage [T** N3] | 2.405 | 1.118 | 5.174 | 2.48 × 10−2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Desterke, C.; Xiang, Y.; Elhage, R.; Duruel, C.; Chang, Y.; Hamaï, A. Ferroptosis Inducers Upregulate PD-L1 in Recurrent Triple-Negative Breast Cancer. Cancers 2024, 16, 155. https://doi.org/10.3390/cancers16010155
Desterke C, Xiang Y, Elhage R, Duruel C, Chang Y, Hamaï A. Ferroptosis Inducers Upregulate PD-L1 in Recurrent Triple-Negative Breast Cancer. Cancers. 2024; 16(1):155. https://doi.org/10.3390/cancers16010155
Chicago/Turabian StyleDesterke, Christophe, Yao Xiang, Rima Elhage, Clémence Duruel, Yunhua Chang, and Ahmed Hamaï. 2024. "Ferroptosis Inducers Upregulate PD-L1 in Recurrent Triple-Negative Breast Cancer" Cancers 16, no. 1: 155. https://doi.org/10.3390/cancers16010155
APA StyleDesterke, C., Xiang, Y., Elhage, R., Duruel, C., Chang, Y., & Hamaï, A. (2024). Ferroptosis Inducers Upregulate PD-L1 in Recurrent Triple-Negative Breast Cancer. Cancers, 16(1), 155. https://doi.org/10.3390/cancers16010155





