PET/CT in Patients with Breast Cancer Treated with Immunotherapy
Abstract
Simple Summary
Abstract
1. Introduction
2. Tumor Microenvironment in the Intersection with Breast Cancer Immunotherapy
3. Clinical Selection of Immunotherapy for Breast Cancer
4. Future Trends in Immunotherapy
5. Correlation between 2-[18F]FDG Uptake and Tumor Heterogeneity
6. 2-[18F]FDG PET/CT Interpretation & Immunotherapy Response Assessment
7. Immuno-PET
| Targeting Molecule | Radiopharmaceutical | Cancer |
|---|---|---|
| HER-2 | [89Zr]trastuzumab [89Zr]pertuzumab | Breast cancer [62,65,66] |
| [64Cu]DOTA-trastuzumab | Breast cancer [67,68] | |
| PD-1 | [89Zr]pembrolizumab [89Zr]nivolumab | NSCLC [60,83] Melanoma [83] |
| PD-L1 | [89Zr]atezolizumab | Metastatic bladder cancer, NSCLC, and TNBC [70] |
| [18F]BMS-986192 | NSCLC [60] | |
| CD8 | [89Zr]Df-IAB22M2C | Melanoma, lung cancer, and hepatocellular carcinoma [61,84] |
| EGFR or VEGF | [89Zr]cetuximab [89Zr]bevacizumab | NSCLC [85,86] Head and neck [86] Breast cancer [63,64] |
| CEA and HSG | [68Ga]IMP288 | Breast cancer [59,69] Colorectal cancer [87] Medullary thyroid carcinoma [88] |
| PSMA | [89Zr]IAB2M | Prostate cancer [89,90] |
| CA-IX | [89Zr]girentuximab | Renal cell carcinoma [91] |
| CA 19-9 | [89Zr]HuMab-5B1 NCT02687230 (ongoing) | Pancreatic ductal adenocarcinoma |
| CTLA-4 | [89Zr]ipilimumab (NCT03313323 ongoing) | Melanoma |
| MUC16 and CD3 | [89Zr]REGN4018 (NCT03564340 ongoing) | Ovarian cancer |
8. Future Molecular Imaging Developments and New Radiopharmaceuticals Pipeline
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BC | breast cancer |
| CAR | chimeric antigen receptor |
| CPS | combined positive score |
| CTLA-4 | cytotoxic T-lymphocyte antigen-4 |
| EBC | early-stage breast cancer |
| EFS | event-free survival |
| EMA | European medicines agency |
| 2-[18F]FDG | fluorodesoxyglucose |
| FDA | food and drug administration |
| HER2 | human epithelial growth factor receptor 2 |
| HR | hazard ration/hormone receptors |
| IATH | intratumoral heterogeneity |
| IETH | intertumoral heterogeneity |
| ICI | immune checkpoint inhibitors |
| irAE | immune-related adverse events |
| ITT | intention-to-treat |
| mAb | monoclonal antibody |
| MHC | major histocompatibility complex |
| MSI | microsatellite instability |
| mTNBC | metastatic triple-negative breast cancer |
| NIRF | near-infrared fluorescence |
| NIS | sodium iodide symporter |
| NLR | neutrophil/lymphocyte ratio |
| ORR | objective response rate |
| OS | overall survival |
| pCR | pathological complete response |
| PD-1 | programmed-death-1 receptor |
| PD-L1 | programmed-death ligand-1 |
| PET/CT | positron emission tomography/computerized tomography |
| PECRIT | PET/CT criteria for early prediction of response to immune checkpoint inhibitor therapy |
| PERCIST | PET response criteria in solid tumors |
| PERCIMT | PET response evaluation criteria for immunotherapy |
| PFS | progression-free survival |
| PMD | progressive metabolic disease |
| PMR | partial metabolic response |
| SMD | stable metabolic disease |
| TILs | tumor-infiltrating lymphocytes |
| TMB | tumor mutational burden |
| TME | tumor microenvironment |
| TNBC | triple negative breast cancer |
| uPMD | unconfirmed progressive metabolic disease |
| VEGF | vascular endothelial growth factor |
References
- NCCN Guidelines Breast Cancer, Version 2.2023. Available online: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (accessed on 11 February 2023).
- Kossai, M.; Radosevic-Robin, N.; Penault-Llorca, F. Refining patient selection for breast cancer immunotherapy: Beyond PD-L1. ESMO Open 2021, 6, 100257. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, A.; Ricci, A.D. Biomarkers for breast cancer immunotherapy: PD-L1, TILs, and beyond. Expert Opin. Investig. Drugs 2022, 31, 549–555. [Google Scholar] [CrossRef]
- Anderson, N.M.; Simon, M.C. The tumor microenvironment. Curr. Biol. 2020, 30, R921–R925. [Google Scholar] [CrossRef]
- Munkácsy, G.; Santarpia, L.; Győrffy, B. Gene Expression Profiling in Early Breast Cancer-Patient Stratification Based on Molecular and Tumor Microenvironment Features. Biomedicines 2022, 10, 248. [Google Scholar] [CrossRef]
- Desmedt, C.; Haibe-Kains, B.; Wirapati, P.; Buyse, M.; Larsimont, D.; Bontempi, G.; Delorenzi, M.; Piccart, M.; Sotiriou, C. Biological processes associated with breast cancer clinical outcome depend on the molecular subtypes. Clin. Cancer Res. 2008, 14, 5158–5165. [Google Scholar] [CrossRef]
- Wagner, J.; Rapsomaniki, M.A.; Chevrier, S.; Anzeneder, T.; Langwieder, C.; Dykgers, A.; Rees, M.; Ramaswamy, A.; Muenst, S.; Soysal, S.D.; et al. A Single-Cell Atlas of the Tumor and Immune Ecosystem of Human Breast Cancer. Cell 2019, 177, 1330–1345.e1318. [Google Scholar] [CrossRef] [PubMed]
- Keren, L.; Bosse, M.; Marquez, D.; Angoshtari, R.; Jain, S.; Varma, S.; Yang, S.R.; Kurian, A.; Van Valen, D.; West, R.; et al. A Structured Tumor-Immune Microenvironment in Triple Negative Breast Cancer Revealed by Multiplexed Ion Beam Imaging. Cell 2018, 174, 1373–1387.e1319. [Google Scholar] [CrossRef] [PubMed]
- Perou, C.M.; Sørlie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular portraits of human breast tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef]
- Lehmann, B.D.; Jovanović, B.; Chen, X.; Estrada, M.V.; Johnson, K.N.; Shyr, Y.; Moses, H.L.; Sanders, M.E.; Pietenpol, J.A. Refinement of Triple-Negative Breast Cancer Molecular Subtypes: Implications for Neoadjuvant Chemotherapy Selection. PLoS ONE 2016, 11, e0157368. [Google Scholar] [CrossRef]
- Gruosso, T.; Gigoux, M.; Manem, V.S.K.; Bertos, N.; Zuo, D.; Perlitch, I.; Saleh, S.M.I.; Zhao, H.; Souleimanova, M.; Johnson, R.M.; et al. Spatially distinct tumor immune microenvironments stratify triple-negative breast cancers. J. Clin. Investig. 2019, 129, 1785–1800. [Google Scholar] [CrossRef]
- Hu, Z.; Li, Z.; Ma, Z.; Curtis, C. Multi-cancer analysis of clonality and the timing of systemic spread in paired primary tumors and metastases. Nat. Genet. 2020, 52, 701–708. [Google Scholar] [CrossRef]
- Szekely, B.; Bossuyt, V.; Li, X.; Wali, V.B.; Patwardhan, G.A.; Frederick, C.; Silber, A.; Park, T.; Harigopal, M.; Pelekanou, V.; et al. Immunological differences between primary and metastatic breast cancer. Ann. Oncol. 2018, 29, 2232–2239. [Google Scholar] [CrossRef] [PubMed]
- Kwok, G.; Yau, T.C.; Chiu, J.W.; Tse, E.; Kwong, Y.L. Pembrolizumab (Keytruda). Hum. Vaccin Immunother. 2016, 12, 2777–2789. [Google Scholar] [CrossRef]
- Cortes, J.; Cescon, D.W.; Rugo, H.S.; Nowecki, Z.; Im, S.A.; Yusof, M.M.; Gallardo, C.; Lipatov, O.; Barrios, C.H.; Holgado, E.; et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): A randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet 2020, 396, 1817–1828. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-pembrolizumab-locally-recurrent-unresectable-or-metastatic-triple (accessed on 7 January 2023).
- Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/keytruda (accessed on 7 January 2023).
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; Di Giacomo, A.M.; De Jesus-Acosta, A.; Delord, J.P.; Geva, R.; Gottfried, M.; Penel, N.; Hansen, A.R.; et al. Efficacy of Pembrolizumab in Patients With Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2020, 38, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Horimoto, Y.; Thinzar Hlaing, M.; Saeki, H.; Kitano, S.; Nakai, K.; Sasaki, R.; Kurisaki-Arakawa, A.; Arakawa, A.; Otsuji, N.; Matsuoka, S.; et al. Microsatellite instability and mismatch repair protein expressions in lymphocyte-predominant breast cancer. Cancer Sci. 2020, 111, 2647–2654. [Google Scholar] [CrossRef]
- Schmid, P.; Adams, S.; Rugo, H.S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Diéras, V.; Hegg, R.; Im, S.A.; Shaw Wright, G.; et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N. Engl. J. Med. 2018, 379, 2108–2121. [Google Scholar] [CrossRef]
- Miles, D.; Gligorov, J.; André, F.; Cameron, D.; Schneeweiss, A.; Barrios, C.; Xu, B.; Wardley, A.; Kaen, D.; Andrade, L.; et al. Primary results from IMpassion131, a double-blind, placebo-controlled, randomised phase III trial of first-line paclitaxel with or without atezolizumab for unresectable locally advanced/metastatic triple-negative breast cancer. Ann. Oncol. 2021, 32, 994–1004. [Google Scholar] [CrossRef]
- Available online: https://ascopost.com/issues/september-25-2021/update-on-us-indication-for-atezolizumab-in-pd-l1-positive-metastatic-triple-negative-breast-cancer/ (accessed on 7 January 2023).
- Stagg, J.; Allard, B. Immunotherapeutic approaches in triple-negative breast cancer: Latest research and clinical prospects. Ther. Adv. Med. Oncol. 2013, 5, 169–181. [Google Scholar] [CrossRef]
- Ghebeh, H.; Mohammed, S.; Al-Omair, A.; Qattan, A.; Lehe, C.; Al-Qudaihi, G.; Elkum, N.; Alshabanah, M.; Bin Amer, S.; Tulbah, A.; et al. The B7-H1 (PD-L1) T lymphocyte-inhibitory molecule is expressed in breast cancer patients with infiltrating ductal carcinoma: Correlation with important high-risk prognostic factors. Neoplasia 2006, 8, 190–198. [Google Scholar] [CrossRef]
- Bailly, C.; Thuru, X.; Quesnel, B. Combined cytotoxic chemotherapy and immunotherapy of cancer: Modern times. NAR Cancer 2020, 2, zcaa002. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Cortes, J.; Pusztai, L.; McArthur, H.; Kümmel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; Harbeck, N.; et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N. Engl. J. Med. 2020, 382, 810–821. [Google Scholar] [CrossRef]
- Schmid, P.; Cortes, J.; Dent, R.; Pusztai, L.; McArthur, H.; Kümmel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; et al. VP7-2021: KEYNOTE-522: Phase III study of neoadjuvant pembrolizumab + chemotherapy vs. placebo + chemotherapy, followed by adjuvant pembrolizumab vs. placebo for early-stage TNBC. Ann. Oncol. 2021, 32, 1198–1200. [Google Scholar] [CrossRef]
- Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pembrolizumab-high-risk-early-stage-triple-negative-breast-cancer (accessed on 7 January 2023).
- Mittendorf, E.A.; Zhang, H.; Barrios, C.H.; Saji, S.; Jung, K.H.; Hegg, R.; Koehler, A.; Sohn, J.; Iwata, H.; Telli, M.L.; et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): A randomised, double-blind, phase 3 trial. Lancet 2020, 396, 1090–1100. [Google Scholar] [CrossRef] [PubMed]
- Loibl, S.; Untch, M.; Burchardi, N.; Huober, J.; Sinn, B.V.; Blohmer, J.U.; Grischke, E.M.; Furlanetto, J.; Tesch, H.; Hanusch, C.; et al. A randomised phase II study investigating durvalumab in addition to an anthracycline taxane-based neoadjuvant therapy in early triple-negative breast cancer: Clinical results and biomarker analysis of GeparNuevo study. Ann. Oncol. 2019, 30, 1279–1288. [Google Scholar] [CrossRef]
- Barroso-Sousa, R.; Li, T.; Reddy, S.; Emens, L.A.; Overmoyer, B.; Lange, P.; Dilullo, M.K.; Attaya, V.; Kimmel, J.; Winer, E.P.; et al. Abstract GS2-10: Nimbus: A phase 2 trial of nivolumab plus ipilimumab for patients with hypermutated her2-negative metastatic breast cancer (MBC). Cancer Res. 2022, 82, GS2-10–GS12-10. [Google Scholar] [CrossRef]
- Nanda, R.; Liu, M.C.; Yau, C.; Shatsky, R.; Pusztai, L.; Wallace, A.; Chien, A.J.; Forero-Torres, A.; Ellis, E.; Han, H.; et al. Effect of Pembrolizumab Plus Neoadjuvant Chemotherapy on Pathologic Complete Response in Women With Early-Stage Breast Cancer: An Analysis of the Ongoing Phase 2 Adaptively Randomized I-SPY2 Trial. JAMA Oncol. 2020, 6, 676–684. [Google Scholar] [CrossRef]
- Gonzalez-Ericsson, P.I.; Wulfkhule, J.D.; Gallagher, R.I.; Sun, X.; Axelrod, M.L.; Sheng, Q.; Luo, N.; Gomez, H.; Sanchez, V.; Sanders, M.; et al. Tumor-Specific Major Histocompatibility-II Expression Predicts Benefit to Anti-PD-1/L1 Therapy in Patients With HER2-Negative Primary Breast Cancer. Clin. Cancer Res. 2021, 27, 5299–5306. [Google Scholar] [CrossRef]
- Park, J.H.; Jonas, S.F.; Bataillon, G.; Criscitiello, C.; Salgado, R.; Loi, S.; Viale, G.; Lee, H.J.; Dieci, M.V.; Kim, S.B.; et al. Prognostic value of tumor-infiltrating lymphocytes in patients with early-stage triple-negative breast cancers (TNBC) who did not receive adjuvant chemotherapy. Ann. Oncol. 2019, 30, 1941–1949. [Google Scholar] [CrossRef]
- Bachelot, T.; Filleron, T.; Bieche, I.; Arnedos, M.; Campone, M.; Dalenc, F.; Coussy, F.; Sablin, M.P.; Debled, M.; Lefeuvre-Plesse, C.; et al. Durvalumab compared to maintenance chemotherapy in metastatic breast cancer: The randomized phase II SAFIR02-BREAST IMMUNO trial. Nat. Med. 2021, 27, 250–255. [Google Scholar] [CrossRef]
- Schmid, P.; Cortes, J.; Dent, R.; Pusztai, L.; McArthur, H.; Kümmel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; et al. Event-free Survival with Pembrolizumab in Early Triple-Negative Breast Cancer. N. Engl. J. Med. 2022, 386, 556–567. [Google Scholar] [CrossRef]
- Loibl, S.; Schneeweiss, A.; Huober, J.; Braun, M.; Rey, J.; Blohmer, J.U.; Furlanetto, J.; Zahm, D.M.; Hanusch, C.; Thomalla, J.; et al. Neoadjuvant durvalumab improves survival in early triple-negative breast cancer independent of pathological complete response. Ann. Oncol. 2022, 33, 1149–1158. [Google Scholar] [CrossRef]
- Tutt, A.N.J.; Garber, J.E.; Kaufman, B.; Viale, G.; Fumagalli, D.; Rastogi, P.; Gelber, R.D.; de Azambuja, E.; Fielding, A.; Balmaña, J.; et al. Adjuvant Olaparib for Patients with BRCA1- or BRCA2-Mutated Breast Cancer. N. Engl. J. Med. 2021, 384, 2394–2405. [Google Scholar] [CrossRef] [PubMed]
- Denduluri, N.; Chavez-MacGregor, M.; Telli, M.L.; Eisen, A.; Graff, S.L.; Hassett, M.J.; Holloway, J.N.; Hurria, A.; King, T.A.; Lyman, G.H.; et al. Selection of Optimal Adjuvant Chemotherapy and Targeted Therapy for Early Breast Cancer: ASCO Clinical Practice Guideline Focused Update. J. Clin. Oncol. 2018, 36, 2433–2443. [Google Scholar] [CrossRef]
- Loibl, S.; O’Shaughnessy, J.; Untch, M.; Sikov, W.M.; Rugo, H.S.; McKee, M.D.; Huober, J.; Golshan, M.; von Minckwitz, G.; Maag, D.; et al. Addition of the PARP inhibitor veliparib plus carboplatin or carboplatin alone to standard neoadjuvant chemotherapy in triple-negative breast cancer (BrighTNess): A randomised, phase 3 trial. Lancet Oncol. 2018, 19, 497–509. [Google Scholar] [CrossRef]
- Xie, Y.; Liu, C.; Zhao, Y.; Gong, C.; Li, Y.; Hu, S.; Song, S.; Hu, X.; Yang, Z.; Wang, B. Heterogeneity derived from 18F-FDG PET/CT predicts immunotherapy outcome for metastatic triple-negative breast cancer patients. Cancer Med. 2022, 11, 1948–1955. [Google Scholar] [CrossRef] [PubMed]
- McDonald, K.A.; Kawaguchi, T.; Qi, Q.; Peng, X.; Asaoka, M.; Young, J.; Opyrchal, M.; Yan, L.; Patnaik, S.; Otsuji, E.; et al. Tumor Heterogeneity Correlates with Less Immune Response and Worse Survival in Breast Cancer Patients. Ann. Surg. Oncol. 2019, 26, 2191–2199. [Google Scholar] [CrossRef]
- Hirakata, T.; Fujii, T.; Kurozumi, S.; Katayama, A.; Honda, C.; Yanai, K.; Tokuda, S.; Nakazawa, Y.; Obayashi, S.; Yajima, R.; et al. FDG uptake reflects breast cancer immunological features: The PD-L1 expression and degree of TILs in primary breast cancer. Breast Cancer Res. Treat. 2020, 181, 331–338. [Google Scholar] [CrossRef]
- Fujii, T.; Yanai, K.; Tokuda, S.; Nakazawa, Y.; Kurozumi, S.; Obayashi, S.; Yajima, R.; Hirakata, T.; Shirabe, K. Relationship Between FDG Uptake and Neutrophil/Lymphocyte Ratio in Patients with Invasive Ductal Breast Cancer. Anticancer Res. 2018, 38, 4927–4931. [Google Scholar] [CrossRef]
- Fujii, T.; Tokuda, S.; Nakazawa, Y.; Kurozumi, S.; Obayashi, S.; Yajima, R.; Shirabe, K. Relationship Between FDG Uptake and the Platelet/lymphocyte Ratio in Patients With Breast Invasive Ductal Cancer. In Vivo 2020, 34, 1365–1369. [Google Scholar] [CrossRef] [PubMed]
- Lopci, E.; Hicks, R.J.; Dimitrakopoulou-Strauss, A.; Dercle, L.; Iravani, A.; Seban, R.D.; Sachpekidis, C.; Humbert, O.; Gheysens, O.; Glaudemans, A.; et al. Joint EANM/SNMMI/ANZSNM practice guidelines/procedure standards on recommended use of [(18)F]FDG PET/CT imaging during immunomodulatory treatments in patients with solid tumors version 1.0. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 2323–2341. [Google Scholar] [CrossRef] [PubMed]
- Gerwing, M.; Herrmann, K.; Helfen, A.; Schliemann, C.; Berdel, W.E.; Eisenblätter, M.; Wildgruber, M. The beginning of the end for conventional RECIST—Novel therapies require novel imaging approaches. Nat. Rev. Clin. Oncol. 2019, 16, 442–458. [Google Scholar] [CrossRef] [PubMed]
- Berz, A.M.; Dromain, C.; Vietti-Violi, N.; Boughdad, S.; Duran, R. Tumor response assessment on imaging following immunotherapy. Front. Oncol. 2022, 12, 982983. [Google Scholar] [CrossRef]
- Klemen, N.D.; Wang, M.; Feingold, P.L.; Cooper, K.; Pavri, S.N.; Han, D.; Detterbeck, F.C.; Boffa, D.J.; Khan, S.A.; Olino, K.; et al. Patterns of failure after immunotherapy with checkpoint inhibitors predict durable progression-free survival after local therapy for metastatic melanoma. J. ImmunoTherapy Cancer 2019, 7, 196. [Google Scholar] [CrossRef]
- Kato, S.; Goodman, A.; Walavalkar, V.; Barkauskas, D.A.; Sharabi, A.; Kurzrock, R. Hyperprogressors after Immunotherapy: Analysis of Genomic Alterations Associated with Accelerated Growth Rate. Clin. Cancer Res. 2017, 23, 4242–4250. [Google Scholar] [CrossRef]
- Saâda-Bouzid, E.; Defaucheux, C.; Karabajakian, A.; Coloma, V.P.; Servois, V.; Paoletti, X.; Even, C.; Fayette, J.; Guigay, J.; Loirat, D.; et al. Hyperprogression during anti-PD-1/PD-L1 therapy in patients with recurrent and/or metastatic head and neck squamous cell carcinoma. Ann. Oncol. 2017, 28, 1605–1611. [Google Scholar] [CrossRef]
- Pons-Tostivint, E.; Latouche, A.; Vaflard, P.; Ricci, F.; Loirat, D.; Hescot, S.; Sablin, M.-P.; Rouzier, R.; Kamal, M.; Morel, C.; et al. Comparative Analysis of Durable Responses on Immune Checkpoint Inhibitors Versus Other Systemic Therapies: A Pooled Analysis of Phase III Trials. JCO Precis. Oncol. 2019, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Goldfarb, L.; Duchemann, B.; Chouahnia, K.; Zelek, L.; Soussan, M. Monitoring anti-PD-1-based immunotherapy in non-small cell lung cancer with FDG PET: Introduction of iPERCIST. EJNMMI Res. 2019, 9, 8. [Google Scholar] [CrossRef]
- Wahl, R.L.; Jacene, H.; Kasamon, Y.; Lodge, M.A. From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. J. Nucl. Med. 2009, 50 (Suppl. S1), 122s–150s. [Google Scholar] [CrossRef]
- Anwar, H.; Sachpekidis, C.; Winkler, J.; Kopp-Schneider, A.; Haberkorn, U.; Hassel, J.C.; Dimitrakopoulou-Strauss, A. Absolute number of new lesionss on (18)F-FDG PET/CT is more predictive of clinical response than SUV changes in metastatic melanoma patients receiving ipilimumab. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 376–383. [Google Scholar] [CrossRef]
- Ito, K.; Teng, R.; Schöder, H.; Humm, J.L.; Ni, A.; Michaud, L.; Nakajima, R.; Yamashita, R.; Wolchok, J.D.; Weber, W.A. (18)F-FDG PET/CT for Monitoring of Ipilimumab Therapy in Patients with Metastatic Melanoma. J. Nucl. Med. 2019, 60, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.Y.; Lipson, E.J.; Im, H.J.; Rowe, S.P.; Gonzalez, E.M.; Blackford, A.; Chirindel, A.; Pardoll, D.M.; Topalian, S.L.; Wahl, R.L. Prediction of Response to Immune Checkpoint Inhibitor Therapy Using Early-Time-Point (18)F-FDG PET/CT Imaging in Patients with Advanced Melanoma. J. Nucl. Med. 2017, 58, 1421–1428. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.B.; Queiroz, M.A.; Barbosa, F.G.; Nunes, R.F.; Zaniboni, E.C.; Ruiz, M.M.; Jardim, D.; Gomes Marin, J.F.; Cerri, G.G.; Buchpiguel, C.A. Reassessing Patterns of Response to Immunotherapy with PET: From Morphology to Metabolism. Radiographics 2021, 41, 120–143. [Google Scholar] [CrossRef]
- Rousseau, C.; Goldenberg, D.M.; Colombié, M.; Sébille, J.C.; Meingan, P.; Ferrer, L.; Baumgartner, P.; Cerato, E.; Masson, D.; Campone, M.; et al. Initial Clinical Results of a Novel Immuno-PET Theranostic Probe in Human Epidermal Growth Factor Receptor 2-Negative Breast Cancer. J. Nucl. Med. 2020, 61, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Niemeijer, A.N.; Leung, D.; Huisman, M.C.; Bahce, I.; Hoekstra, O.S.; van Dongen, G.; Boellaard, R.; Du, S.; Hayes, W.; Smith, R.; et al. Whole body PD-1 and PD-L1 positron emission tomography in patients with non-small-cell lung cancer. Nat. Commun. 2018, 9, 4664. [Google Scholar] [CrossRef] [PubMed]
- Farwell, M.D.; Gamache, R.F.; Babazada, H.; Hellmann, M.D.; Harding, J.J.; Korn, R.; Mascioni, A.; Le, W.; Wilson, I.; Gordon, M.S.; et al. CD8-Targeted PET Imaging of Tumor-Infiltrating T Cells in Patients with Cancer: A Phase I First-in-Humans Study of (89)Zr-Df-IAB22M2C, a Radiolabeled Anti-CD8 Minibody. J. Nucl. Med. 2022, 63, 720–726. [Google Scholar] [CrossRef]
- Dijkers, E.C.; Oude Munnink, T.H.; Kosterink, J.G.; Brouwers, A.H.; Jager, P.L.; de Jong, J.R.; van Dongen, G.A.; Schröder, C.P.; Lub-de Hooge, M.N.; de Vries, E.G. Biodistribution of 89Zr-trastuzumab and PET imaging of HER2-positive lesions in patients with metastatic breast cancer. Clin. Pharmacol. Ther. 2010, 87, 586–592. [Google Scholar] [CrossRef]
- Gaykema, S.B.; Schröder, C.P.; Vitfell-Rasmussen, J.; Chua, S.; Oude Munnink, T.H.; Brouwers, A.H.; Bongaerts, A.H.; Akimov, M.; Fernandez-Ibarra, C.; Lub-de Hooge, M.N.; et al. 89Zr-trastuzumab and 89Zr-bevacizumab PET to evaluate the effect of the HSP90 inhibitor NVP-AUY922 in metastatic breast cancer patients. Clin. Cancer Res. 2014, 20, 3945–3954. [Google Scholar] [CrossRef]
- Gaykema, S.B.; Brouwers, A.H.; Lub-de Hooge, M.N.; Pleijhuis, R.G.; Timmer-Bosscha, H.; Pot, L.; van Dam, G.M.; van der Meulen, S.B.; de Jong, J.R.; Bart, J.; et al. 89Zr-bevacizumab PET imaging in primary breast cancer. J. Nucl. Med. 2013, 54, 1014–1018. [Google Scholar] [CrossRef]
- Gebhart, G.; Lamberts, L.E.; Wimana, Z.; Garcia, C.; Emonts, P.; Ameye, L.; Stroobants, S.; Huizing, M.; Aftimos, P.; Tol, J.; et al. Molecular imaging as a tool to investigate heterogeneity of advanced HER2-positive breast cancer and to predict patient outcome under trastuzumab emtansine (T-DM1): The ZEPHIR trial. Ann. Oncol. 2016, 27, 619–624. [Google Scholar] [CrossRef]
- Ulaner, G.A.; Lyashchenko, S.K.; Riedl, C.; Ruan, S.; Zanzonico, P.B.; Lake, D.; Jhaveri, K.; Zeglis, B.; Lewis, J.S.; O’Donoghue, J.A. First-in-Human Human Epidermal Growth Factor Receptor 2–Targeted Imaging Using 89Zr-Pertuzumab PET/CT: Dosimetry and Clinical Application in Patients with Breast Cancer. J. Nucl. Med. 2018, 59, 900–906. [Google Scholar] [CrossRef] [PubMed]
- Mortimer, J.E.; Bading, J.R.; Colcher, D.M.; Conti, P.S.; Frankel, P.H.; Carroll, M.I.; Tong, S.; Poku, E.; Miles, J.K.; Shively, J.E.; et al. Functional imaging of human epidermal growth factor receptor 2-positive metastatic breast cancer using (64)Cu-DOTA-trastuzumab PET. J. Nucl. Med. 2014, 55, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Kurihara, H.; Yonemori, K.; Tsuda, H.; Suzuki, J.; Kono, Y.; Honda, N.; Kodaira, M.; Yamamoto, H.; Yunokawa, M.; et al. 64Cu-DOTA-trastuzumab PET imaging in patients with HER2-positive breast cancer. J. Nucl. Med. 2013, 54, 1869–1875. [Google Scholar] [CrossRef] [PubMed]
- Pichon, B.; Rousseau, C.; Blanc-Lapierre, A.; Delpon, G.; Ferrer, L.; Libois, V.; Le Turnier, M.; Lenoble, C.; Bodet-Milin, C.; Goldenberg, D.M.; et al. Targeting Stereotactic Body Radiotherapy on Metabolic PET- and Immuno-PET-Positive Vertebral Metastases. Biomedicines 2020, 8, 548. [Google Scholar] [CrossRef]
- Bensch, F.; van der Veen, E.L.; Lub-de Hooge, M.N.; Jorritsma-Smit, A.; Boellaard, R.; Kok, I.C.; Oosting, S.F.; Schröder, C.P.; Hiltermann, T.J.N.; van der Wekken, A.J.; et al. 89Zr-atezolizumab imaging as a non-invasive approach to assess clinical response to PD-L1 blockade in cancer. Nat. Med. 2018, 24, 1852–1858. [Google Scholar] [CrossRef]
- Wijngaarden, J.E.; Huisman, M.C.; Jauw, Y.W.S.; van Dongen, G.; Greuter, H.; Schuit, R.C.; Cleveland, M.; Gootjes, E.C.; Vugts, D.J.; Menke-van der Houven van Oordt, C.W.; et al. Validation of simplified uptake measures against dynamic Patlak K(i) for quantification of lesional (89)Zr-Immuno-PET antibody uptake. Eur. J. Nucl. Med. Mol. Imaging 2023. online ahead of print. [Google Scholar] [CrossRef]
- Wissler, H.L.; Ehlerding, E.B.; Lyu, Z.; Zhao, Y.; Zhang, S.; Eshraghi, A.; Buuh, Z.Y.; McGuth, J.C.; Guan, Y.; Engle, J.W.; et al. Site-Specific Immuno-PET Tracer to Image PD-L1. Mol. Pharm. 2019, 16, 2028–2036. [Google Scholar] [CrossRef]
- Bansal, A.; Pandey, M.K.; Barham, W.; Liu, X.; Harrington, S.M.; Lucien, F.; Dong, H.; Park, S.S.; DeGrado, T.R. Non-invasive immunoPET imaging of PD-L1 using anti-PD-L1-B11 in breast cancer and melanoma tumor model. Nucl. Med. Biol. 2021, 100–101, 4–11. [Google Scholar] [CrossRef]
- Li, M.; Ehlerding, E.B.; Jiang, D.; Barnhart, T.E.; Chen, W.; Cao, T.; Engle, J.W.; Cai, W. In vivo characterization of PD-L1 expression in breast cancer by immuno-PET with (89)Zr-labeled avelumab. Am. J. Transl. Res. 2020, 12, 1862–1872. [Google Scholar]
- Giesen, D.; Broer, L.N.; Lub-de Hooge, M.N.; Popova, I.; Howng, B.; Nguyen, M.; Vasiljeva, O.; de Vries, E.G.E.; Pool, M. Probody Therapeutic Design of (89)Zr-CX-072 Promotes Accumulation in PD-L1-Expressing Tumors Compared to Normal Murine Lymphoid Tissue. Clin. Cancer Res. 2020, 26, 3999–4009. [Google Scholar] [CrossRef]
- Rousseau, C.; Ruellan, A.L.; Bernardeau, K.; Kraeber-Bodéré, F.; Gouard, S.; Loussouarn, D.; Saï-Maurel, C.; Faivre-Chauvet, A.; Wijdenes, J.; Barbet, J.; et al. Syndecan-1 antigen, a promising new target for triple-negative breast cancer immuno-PET and radioimmunotherapy. A preclinical study on MDA-MB-468 xenograft tumors. EJNMMI Res. 2011, 1, 20. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.A.; Kang, L.; Li, C.; Kamkaew, A.; Barrett, K.E.; Aluicio-Sarduy, E.; Yang, Y.; Engle, J.W.; Jiang, D.; Cai, W. ImmunoPET of the differential expression of CD146 in breast cancer. Am. J. Cancer Res. 2021, 11, 1586–1599. [Google Scholar] [PubMed]
- Li, C.; Kang, L.; Fan, K.; Ferreira, C.A.; Becker, K.V.; Huo, N.; Liu, H.; Yang, Y.; Engle, J.W.; Wang, R.; et al. ImmunoPET of CD146 in Orthotopic and Metastatic Breast Cancer Models. Bioconjug. Chem. 2021, 32, 1306–1314. [Google Scholar] [CrossRef]
- Seo, J.W.; Tavaré, R.; Mahakian, L.M.; Silvestrini, M.T.; Tam, S.; Ingham, E.S.; Salazar, F.B.; Borowsky, A.D.; Wu, A.M.; Ferrara, K.W. CD8(+) T-Cell Density Imaging with (64)Cu-Labeled Cys-Diabody Informs Immunotherapy Protocols. Clin. Cancer Res. 2018, 24, 4976–4987. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, L.K.; Fröhlich, C.; Christensen, C.; Melander, M.C.; Poulsen, T.T.; Galler, G.R.; Lantto, J.; Horak, I.D.; Kragh, M.; Nielsen, C.H.; et al. CD4(+) and CD8a(+) PET imaging predicts response to novel PD-1 checkpoint inhibitor: Studies of Sym021 in syngeneic mouse cancer models. Theranostics 2019, 9, 8221–8238. [Google Scholar] [CrossRef] [PubMed]
- Ehlerding, E.B.; Lee, H.J.; Jiang, D.; Ferreira, C.A.; Zahm, C.D.; Huang, P.; Engle, J.W.; McNeel, D.G.; Cai, W. Antibody and fragment-based PET imaging of CTLA-4+ T-cells in humanized mouse models. Am. J. Cancer Res. 2019, 9, 53–63. [Google Scholar]
- Higashikawa, K.; Yagi, K.; Watanabe, K.; Kamino, S.; Ueda, M.; Hiromura, M.; Enomoto, S. 64Cu-DOTA-Anti-CTLA-4 mAb Enabled PET Visualization of CTLA-4 on the T-Cell Infiltrating Tumor Tissues. PLoS ONE 2014, 9, e109866. [Google Scholar] [CrossRef]
- Kok, I.C.; Hooiveld, J.S.; van de Donk, P.P.; Giesen, D.; van der Veen, E.L.; Lub-de Hooge, M.N.; Brouwers, A.H.; Hiltermann, T.J.N.; van der Wekken, A.J.; Hijmering-Kappelle, L.B.M.; et al. (89)Zr-pembrolizumab imaging as a non-invasive approach to assess clinical response to PD-1 blockade in cancer. Ann. Oncol. 2022, 33, 80–88. [Google Scholar] [CrossRef]
- Pandit-Taskar, N.; Postow, M.A.; Hellmann, M.D.; Harding, J.J.; Barker, C.A.; O’Donoghue, J.A.; Ziolkowska, M.; Ruan, S.; Lyashchenko, S.K.; Tsai, F.; et al. First-in-Humans Imaging with (89)Zr-Df-IAB22M2C Anti-CD8 Minibody in Patients with Solid Malignancies: Preliminary Pharmacokinetics, Biodistribution, and Lesion Targeting. J. Nucl. Med. 2020, 61, 512–519. [Google Scholar] [CrossRef]
- Bahce, I.; Huisman, M.C.; Verwer, E.E.; Ooijevaar, R.; Boutkourt, F.; Vugts, D.J.; van Dongen, G.A.; Boellaard, R.; Smit, E.F. Pilot study of (89)Zr-bevacizumab positron emission tomography in patients with advanced non-small cell lung cancer. EJNMMI Res. 2014, 4, 35. [Google Scholar] [CrossRef]
- van Loon, J.; Even, A.J.G.; Aerts, H.; Öllers, M.; Hoebers, F.; van Elmpt, W.; Dubois, L.; Dingemans, A.C.; Lalisang, R.I.; Kempers, P.; et al. PET imaging of zirconium-89 labelled cetuximab: A phase I trial in patients with head and neck and lung cancer. Radiother. Oncol. 2017, 122, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Touchefeu, Y.; Bailly, C.; Frampas, E.; Eugène, T.; Rousseau, C.; Bourgeois, M.; Bossard, C.; Faivre-Chauvet, A.; Rauscher, A.; Masson, D.; et al. Promising clinical performance of pretargeted immuno-PET with anti-CEA bispecific antibody and gallium-68-labelled IMP-288 peptide for imaging colorectal cancer metastases: A pilot study. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Bodet-Milin, C.; Faivre-Chauvet, A.; Carlier, T.; Rauscher, A.; Bourgeois, M.; Cerato, E.; Rohmer, V.; Couturier, O.; Drui, D.; Goldenberg, D.M.; et al. Immuno-PET Using Anticarcinoembryonic Antigen Bispecific Antibody and 68Ga-Labeled Peptide in Metastatic Medullary Thyroid Carcinoma: Clinical Optimization of the Pretargeting Parameters in a First-in-Human Trial. J. Nucl. Med. 2016, 57, 1505–1511. [Google Scholar] [CrossRef]
- Pandit-Taskar, N.; O’Donoghue, J.A.; Ruan, S.; Lyashchenko, S.K.; Carrasquillo, J.A.; Heller, G.; Martinez, D.F.; Cheal, S.M.; Lewis, J.S.; Fleisher, M.; et al. First-in-Human Imaging with 89Zr-Df-IAB2M Anti-PSMA Minibody in Patients with Metastatic Prostate Cancer: Pharmacokinetics, Biodistribution, Dosimetry, and Lesion Uptake. J. Nucl. Med. 2016, 57, 1858–1864. [Google Scholar] [CrossRef]
- Vlachostergios, P.J.; Niaz, M.J.; Thomas, C.; Christos, P.J.; Osborne, J.R.; Margolis, D.J.A.; Khani, F.; Bander, N.H.; Scherr, D.S.; Tagawa, S.T. Pilot study of the diagnostic utility of (89) Zr-df-IAB2M and (68) Ga-PSMA-11 PET imaging and multiparametric MRI in localized prostate cancer. Prostate 2022, 82, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Merkx, R.I.J.; Lobeek, D.; Konijnenberg, M.; Jiménez-Franco, L.D.; Kluge, A.; Oosterwijk, E.; Mulders, P.F.A.; Rijpkema, M. Phase I study to assess safety, biodistribution and radiation dosimetry for (89)Zr-girentuximab in patients with renal cell carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3277–3285. [Google Scholar] [CrossRef]
- Nedrow, J.R.; Josefsson, A.; Park, S.; Bäck, T.; Hobbs, R.F.; Brayton, C.; Bruchertseifer, F.; Morgenstern, A.; Sgouros, G. Pharmacokinetics, microscale distribution, and dosimetry of alpha-emitter-labeled anti-PD-L1 antibodies in an immune competent transgenic breast cancer model. EJNMMI Res. 2017, 7, 57. [Google Scholar] [CrossRef]
- Elboga, U.; Sahin, E.; Kus, T.; Cayirli, Y.B.; Aktas, G.; Uzun, E.; Cinkir, H.Y.; Teker, F.; Sever, O.N.; Aytekin, A.; et al. Superiority of (68)Ga-FAPI PET/CT scan in detecting additional lesions compared to (18)FDG PET/CT scan in breast cancer. Ann. Nucl. Med. 2021, 35, 1321–1331. [Google Scholar] [CrossRef]
- Kömek, H.; Can, C.; Güzel, Y.; Oruç, Z.; Gündoğan, C.; Yildirim, Ö.A.; Kaplan, İ.; Erdur, E.; Yıldırım, M.S.; Çakabay, B. (68)Ga-FAPI-04 PET/CT, a new step in breast cancer imaging: A comparative pilot study with the (18)F-FDG PET/CT. Ann. Nucl. Med. 2021, 35, 744–752. [Google Scholar] [CrossRef]
- Ballal, S.; Yadav, M.P.; Kramer, V.; Moon, E.S.; Roesch, F.; Tripathi, M.; Mallick, S.; ArunRaj, S.T.; Bal, C. A theranostic approach of [(68)Ga]Ga-DOTA.SA.FAPi PET/CT-guided [(177)Lu]Lu-DOTA.SA.FAPi radionuclide therapy in an end-stage breast cancer patient: New frontier in targeted radionuclide therapy. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 942–944. [Google Scholar] [CrossRef]
- Rathke, H.; Fuxius, S.; Giesel, F.L.; Lindner, T.; Debus, J.; Haberkorn, U.; Kratochwil, C. Two Tumors, One Target: Preliminary Experience With 90Y-FAPI Therapy in a Patient With Metastasized Breast and Colorectal Cancer. Clin. Nucl. Med. 2021, 46, 842–844. [Google Scholar] [CrossRef] [PubMed]
- Capaccione, K.M.; Doubrovin, M.; Braumuller, B.; Leibowitz, D.; Bhatt, N.; Momen-Heravi, F.; Molotkov, A.; Kissner, M.; Goldner, K.; Soffing, M.; et al. Evaluating the Combined Anticancer Response of Checkpoint Inhibitor Immunotherapy and FAP-Targeted Molecular Radiotherapy in Murine Models of Melanoma and Lung Cancer. Cancers 2022, 14, 4575. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.K.; Noguera-Ortega, E.; Xiao, Z.; Todd, L.; Scholler, J.; Song, D.; Liousia, M.; Lohith, K.; Xu, K.; Edwards, K.J.; et al. Monitoring Therapeutic Response to Anti-FAP CAR T Cells Using [18F]AlF-FAPI-74. Clin. Cancer Res. 2022, 28, 5330–5342. [Google Scholar] [CrossRef] [PubMed]
- Noortman, W.A.; Vriens, D.; Grootjans, W.; Tao, Q.; de Geus-Oei, L.F.; Van Velden, F.H. Nuclear medicine radiomics in precision medicine: Why we can’t do without artificial intelligence. Q. J. Nucl. Med. Mol. Imaging 2020, 64, 278–290. [Google Scholar] [CrossRef]
- Urso, L.; Manco, L.; Castello, A.; Evangelista, L.; Guidi, G.; Castellani, M.; Florimonte, L.; Cittanti, C.; Turra, A.; Panareo, S. PET-Derived Radiomics and Artificial Intelligence in Breast Cancer: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 3409. [Google Scholar] [CrossRef]
- Napier, T.S.; Hunter, C.L.; Song, P.N.; Larimer, B.M.; Sorace, A.G. Preclinical PET Imaging of Granzyme B Shows Promotion of Immunological Response Following Combination Paclitaxel and Immune Checkpoint Inhibition in Triple Negative Breast Cancer. Pharmaceutics 2022, 14, 440. [Google Scholar] [CrossRef]
- Volpe, A.; Lang, C.; Lim, L.; Man, F.; Kurtys, E.; Ashmore-Harris, C.; Johnson, P.; Skourti, E.; de Rosales, R.T.M.; Fruhwirth, G.O. Spatiotemporal PET Imaging Reveals Differences in CAR-T Tumor Retention in Triple-Negative Breast Cancer Models. Mol. Ther. 2020, 28, 2271–2285. [Google Scholar] [CrossRef]
- Sriraman, S.K.; Davies, C.W.; Gill, H.; Kiefer, J.R.; Yin, J.; Ogasawara, A.; Urrutia, A. Development of an 18.sup.F-labeled anti-human CD8 VHH for same-day immunoPET imaging. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 679. [Google Scholar] [CrossRef]
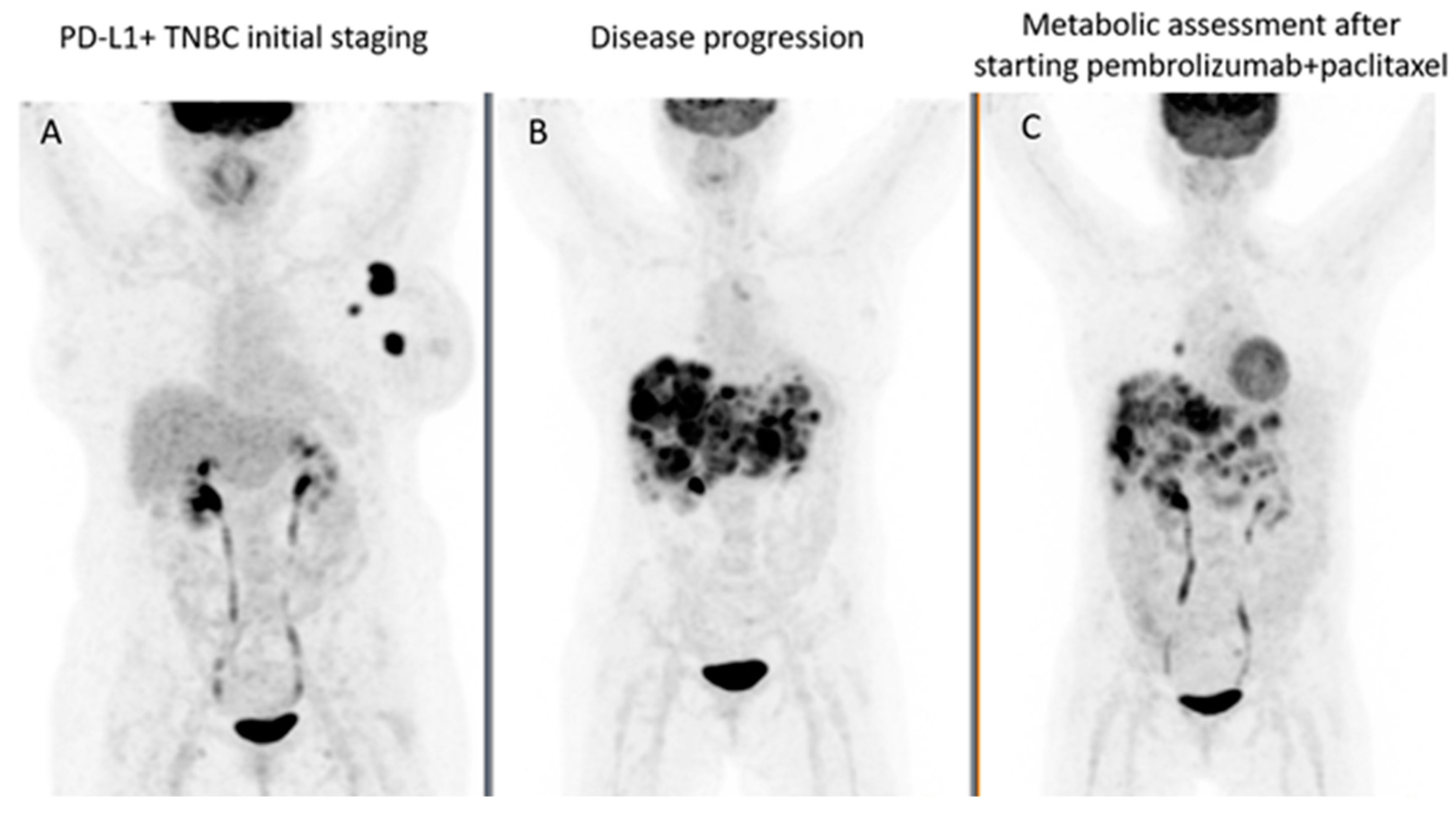
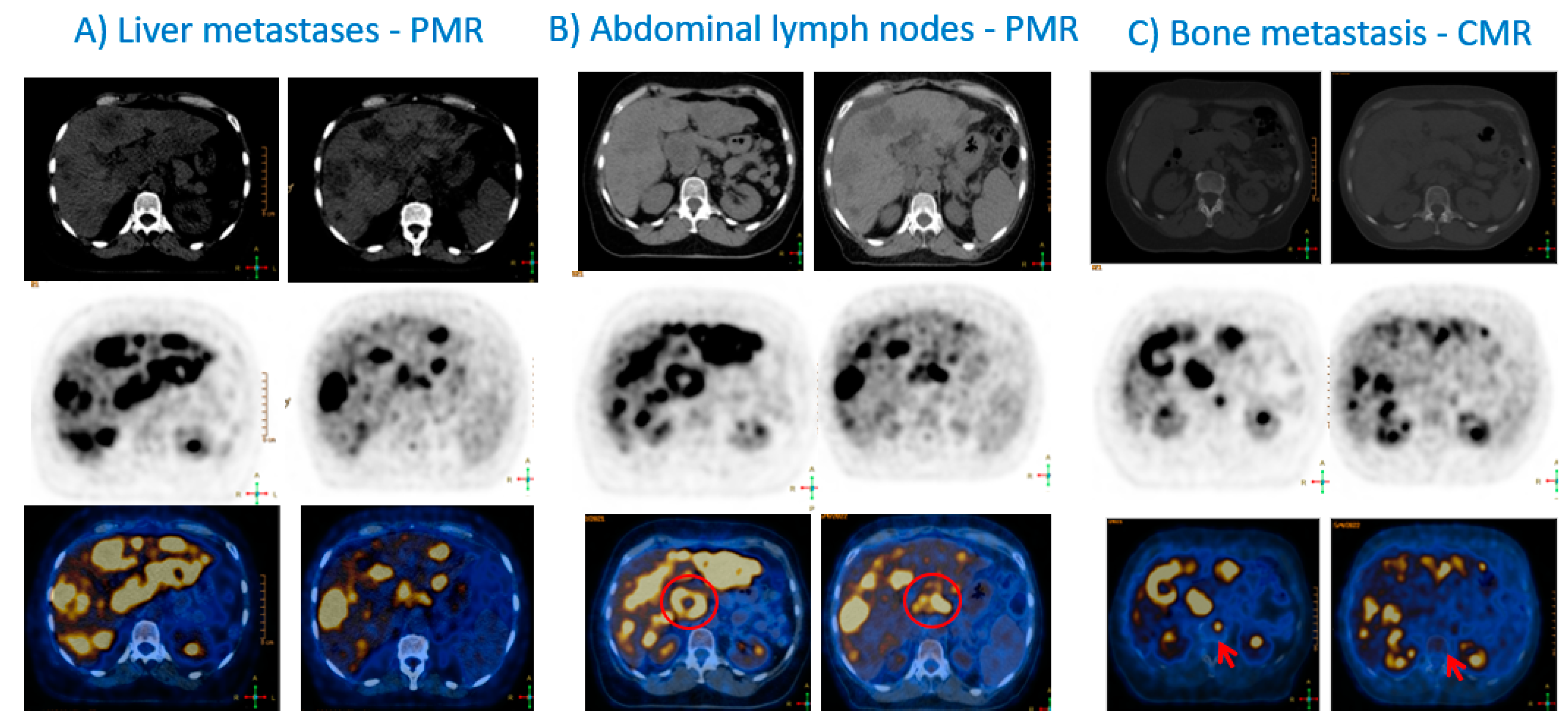
| Trial | Setting | Drugs | Nº of Patients | Primary Outcomes | Main Results | Comments |
|---|---|---|---|---|---|---|
| KEYNOTE-355 [15] Phase III, randomized, placebo-controlled | aTNBC, first line | Pembrolizumab/Placebo + chemotherapy (paclitaxel, nab-paclitaxel, or carbo-gemcitabina) | 847 | Co-primary efficacy endpoints: PFS and OS assessed in the CPS ≥ 10, CPS ≥ f, and ITT populations | PFS (PD-L1 positive: CPS ≥ 10): 9.7 vs. 5.6 months (HR 0.65, 95% CI 0.49–0.86; one-sided p = 0·0012). OS (PD-L1 positive: CPS ≥ 10): 23.0 vs. 16.1 months (HR 0.73; 95%CI 0.55–0.95; p < 0.01). | Based on this trial, FDA and EMA have granted Pembrolizumab approval for first-line aTNBC in PD-L1 positive subpopulation (CPS ≥ 10). |
| KEYNOTE-158 [18] Phase II, open-label one-arm trial | aBC, second or plus lines | Pembrolizumab monotherapy | 233 (5 BC) | Objective response rate per RECIST in confirmed MSI-H/dMMR advanced noncolorectal cancer who experienced failure with prior therapy | Objective response rate: 34.3% (95% CI, 28.3% to 40.8%). Median PFS: 4.1 months (95% CI, 2.4 to 4.9 months). Median OS: 23.5 months (95% CI, 13.5 months to not reached). | FDA first tumor-agnostic approval. |
| Impassion130 [20] Phase III, randomized, placebo-controlled | aTNBC, first line | Atezolizumab/Placebo + nab-paclitaxel | 902 | Co- primary efficacy endpoints: PFS (in ITT and PD-L1–positive subgroups tested with SP142 assay) and OS (tested in ITT—if positive, than tested in the PD-L1–positive subgroup) | PFS (ITT): 7.2 vs. 5.5 months (HR 0.80; 95%CI 0.69–0.92; p < 0.01) PFS (PD-L1 positive: SP142): 7.5 vs. 5.0 months (HR 0.62; 95%CI 0.49–0.78; p < 0.01) OS (ITT): 21.3 vs. 17.6 months (HR 0.84; 95%CI 0.69–1.02; p = 0.08) OS (PD-L1 positive: SP142): 25.0 vs. 15.5 months (HR 0.62; 95%CI 0.45–0.86). Not formally tested due to the hierarchical statistical analysis plan comparison could be conducted as the hierarchical analysis planned for the study had already been considered negative for the co-primary outcome. In the subsequent | EMA granted definitive approval for Atezolizumab based on Impassion130. FDA, however, granted partial approval that was withdrawn after the negative results of the confirmatory trial Impassion131. |
| Impassion131 [21] Phase III, randomized, placebo-controlled | aTNBC, first line | Atezolizumab/Placebo + paclitaxel | 651 | PFS tested hierarchically first in the PD-L1-positive (SP142 assay) population and then in the ITT population. | PFS (PD-L1 positive: SP142): 6.0 vs. 5.7 months (HR 0.82, 95%CI 0.60–1.12; p = 0.20) PFS (ITT): 5.7 vs. 5.6 months (HR 0.86, 95%CI 0.70–1.05; not tested) | After these negative results, the label was dropped in the US |
| KEYNOTE-522 [26] Phase III, randomized, placebo-controlled | eTNBC | Neoadjuvant Pembrolizumab/Placebo + chemotherapy (carbo-paclitaxel followed by AC/EC) followed by adjuvant Pembrolizumab/Placebo | 1174 | pCR and EFS in ITT | pCR: 64.8% vs. 51.2% (∆ 13.6%; 95%CI, 5.4–21.8%; p < 0.00) EFS: median not-reached. HR 0.63, 95%CI 0.43–0.93; p not reported) | After this study, pembrolizumab has been approved for neo + adjuvant use in high-risk eTNBC by the FDA and EMA |
| Impassion031 [29] Phase III, randomized, placebo-controlled (only in the neoadjuvant phase) | eTNBC | Neoadjuvant Atezolizumab/Placebo + chemotherapy (nab-paclitaxel followed by AC) followed by adjuvant Atezolizumab (in the experimental arm) | 455 | pCR in ITT and PD-L1 positive populations | pCR (ITT): 58% vs. 41% (∆ 17%; 95%CI 6–27%; one-sided p = 0·0044) pCR (PD-L1 positive: SP142): 69% vs. 49% (∆ 20%; 95%CI 4–35%; one-sided p = 0·021) | Despite these positive results, atezolizumab was not approved for the neo + adjuvant treatment of eTNBC due to the lack of proof of long- term benefit |
| GeparDouze/NSABP B-59 (NCT02008227) Phase III, randomized, placebo-controlled | eTNBC | Neoadjuvant Atezolizumab/Placebo + chemotherapy (carbo-paclitaxel followed by AC/EC) followed by adjuvant Atezolizumab/Placebo | 1550 | Co-primary: EFS and pCR | Not yet published | If positive, Atezolizumab shall receive approval for eTNBC |
| GeparNuevo [30] Phase II, randomized, placebo-controlled | eTNBC | Durvalumab/Placebo + chemotherapy (nab-paclitaxel followed by EC) | 174 | pCR | pCR: 53.4% vs. 44.2% (∆ 9.2%; p = 0.287). | Durvalumab effect was seen only in the window cohort (pCR 61.0% versus 41.4%) |
| Criteria | PECRIT 2017 [57] | PERCIMT 2018 [55] | iPERCIST 2019 [53] | imPERCIST5 2019 [56] |
|---|---|---|---|---|
| Time for confirming PMD | 3–4 weeks | 3 months | 2 months | 3 months |
| Target lesions | minSUL * = 1.5 × mean SUL liver | Size (metabolically active lesion) > 1.0 or 1.5 cm | minSUL * = 1.5 × mean SUL liver | minSUL * = 1.5 × mean SUL liver |
| New lesions | Progression | PMD | iuPMD | Include in the sum of SULpeak, PMD if SULpeak > 30% |
| Complete metabolic response (CMR) | Disappearance of all target lesions | |||
| Partial metabolic response (PMR) | ↓≥30% from baseline | Disappearance of some metabolically active lesions without new lesions | ↓ SULpeak in target lesions ≥ 30% | ↓ sum of SULpeak in target lesions ≥ 30% and absolute ↓ SUL units ≥ 0.8 |
| Stable metabolic disease (SMD) | Neither of the other options apply | |||
| Progressive metabolic disease (PMD) | ↑≥20% in the nadir of the sum of target lesions (>5 mm) | ≥4 new lesions of <1 cm or ≥3 new lesions of >1 cm or ≥2 new lesions of >1.5 cm | iuPMD *: ↑≥30% in SULpeak or new metabolically active lesions PMD: PET confirmation 4–8 weeks after | ↑>30% in SULpeak, with ↑SUL unit > 0.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vaz, S.C.; Graff, S.L.; Ferreira, A.R.; Debiasi, M.; de Geus-Oei, L.-F. PET/CT in Patients with Breast Cancer Treated with Immunotherapy. Cancers 2023, 15, 2620. https://doi.org/10.3390/cancers15092620
Vaz SC, Graff SL, Ferreira AR, Debiasi M, de Geus-Oei L-F. PET/CT in Patients with Breast Cancer Treated with Immunotherapy. Cancers. 2023; 15(9):2620. https://doi.org/10.3390/cancers15092620
Chicago/Turabian StyleVaz, Sofia C., Stephanie L. Graff, Arlindo R. Ferreira, Márcio Debiasi, and Lioe-Fee de Geus-Oei. 2023. "PET/CT in Patients with Breast Cancer Treated with Immunotherapy" Cancers 15, no. 9: 2620. https://doi.org/10.3390/cancers15092620
APA StyleVaz, S. C., Graff, S. L., Ferreira, A. R., Debiasi, M., & de Geus-Oei, L.-F. (2023). PET/CT in Patients with Breast Cancer Treated with Immunotherapy. Cancers, 15(9), 2620. https://doi.org/10.3390/cancers15092620







