The Effectiveness of the Combination of Arterial Infusion Chemotherapy and Radiotherapy for Biliary Tract Cancer: A Prospective Pilot Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patients
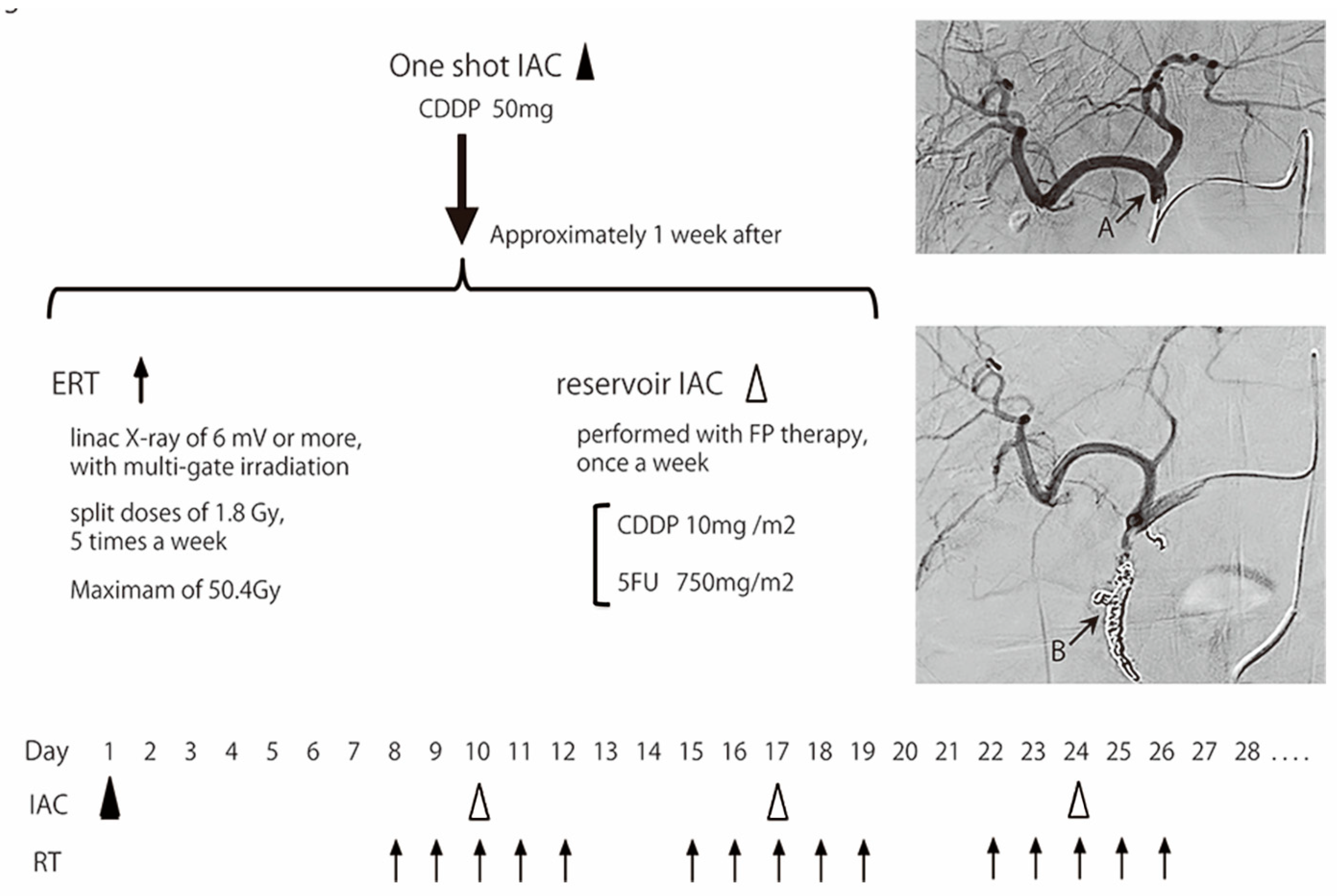
2.3. Treatment
2.4. Assessments
2.5. Statistical Analyses
3. Results
3.1. Tumor Response
3.2. Treatment Compliance
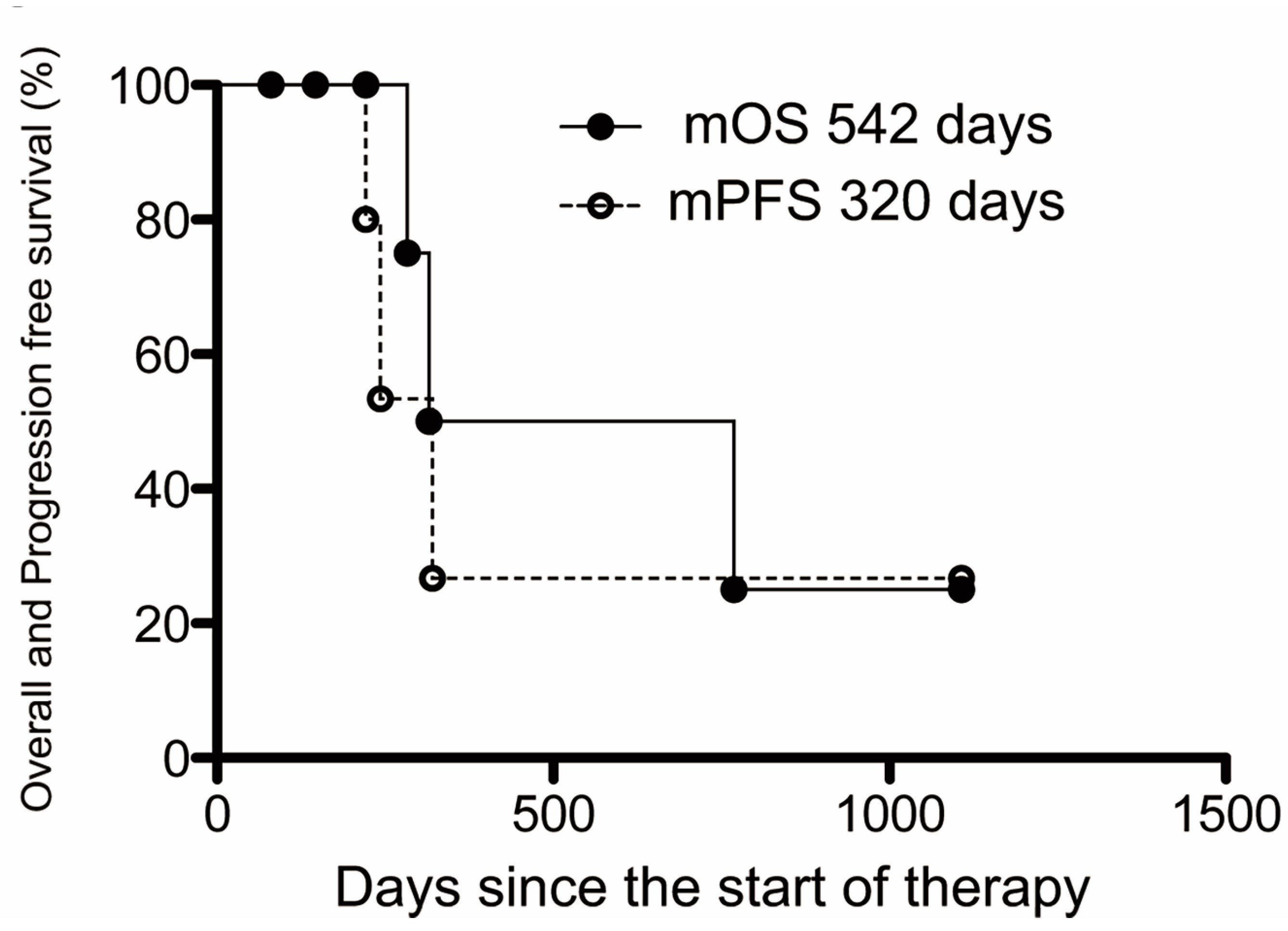
3.3. Survival and Disease Progression
3.4. Adverse Events
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Banales, J.M.; Marin, J.J.G.; Lamarca, A.; Rodrigues, P.M.; Khan, S.A.; Roberts, L.R.; Cardinale, V.; Carpino, G.; Andersen, J.B.; Braconi, C.; et al. Cholangiocarcinoma 2020: The next horizon in mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 557–588. [Google Scholar] [CrossRef] [PubMed]
- Elveni, A.; Laffusa, A.; Scaravaglio, M.; Rossi, R.E.; Longarini, R.; Stagno, A.M.; Cristofen, L.; Ciaccio, A.; Cortinovis, D.L.; Invernizzi, P.; et al. Clinical treatment of cholangiocarcinoma: An updated comprehensive review. Ann Hepatol. 2022, 27, 100737. [Google Scholar] [CrossRef] [PubMed]
- Randi, G.; Malvezzi, M.; Levi, F.; Ferlay, J.; Negri, E.; SFranceschi, S.; Vecchia, C.L. Epidemiology of biliary tract cancers: An update. Ann Oncol. 2009, 20, 146–159. [Google Scholar] [CrossRef]
- Cancer Statistics. Cancer Information Service, National Cancer Center, Japan (Vital Statistics of Japan, Ministry of Health, Labour and Welfare). Available online: https://ganjoho.jp/reg_stat/statistics/data/dl/en.html (accessed on 20 March 2023).
- Ishihara, S.; Horiguchi, A.; Miyakawa, S.; Endo, I.; Miyazaki, M.; Takada, T. Biliary tract cancer registry in Japan from 2008 to 2013. J. Hepatobiliary Pancreat. Sci. 2016, 23, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Davidson, B.R.; Goldin, R.D.; Heaton, N.; Karani, J.; Pereira, S.P.; Rosenberg, W.M.C.; Tait, P.; Taylor-Robinson, S.D.; Thillainayagam, A.V.; et al. Guidelines for the diagnosis and treatment of cholangiocarcinoma: An update. Gut 2012, 61, 1657–1669. [Google Scholar] [CrossRef]
- Simo, K.A.; Halpin, L.E.; McBrier, N.M.; Hessey, J.A.; Baker, E.; Ross, S.; Swan, R.Z.; Iannitti, D.A.; Martinie, J.B. Multimodality treatment of intrahepatic cholangiocarcinoma: A review. J. Surg. Oncol. 2016, 113, 62–83. [Google Scholar] [CrossRef]
- Miyakawa, S.; Ishihara, S.; Horiguchi, A.; Takeda, T.; Nagakawa, M. Biliary tract cancer treatment: 5584 results from the Biliary Tract Cancer Statistics Registry from 1998 to 2004 in Japan. J. Hepatobiliary Pancreat. Surg. 2009, 16, 1–7. [Google Scholar] [CrossRef]
- Valle, J.; Wasan, H.; Palmer, D.H.; Cunningham, D.; Anthoney, A.; Maraveyas, A.; Madhusudan, S.; Iveson, T.; Hughes, S.; Pereira, S.P.; et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N. Engl. J. Med. 2010, 362, 1273–1281. [Google Scholar] [CrossRef]
- Okusaka, T.; Nakachi, K.; Fukutomi, A.; Mizuno, N.; Ohkawa, S.; Funakoshi, A.; Nagino, M.; Kondo, S.; Nagaoka, S.; Funai, J.; et al. Gemcitabine alone or in combination with Cisplatin in patients with biliary tract cancer: A comparative multicentre study in Japan. Br. J. Cancer 2010, 103, 469–474. [Google Scholar] [CrossRef]
- Kanai, M.; Yoshimura, K.; Tsumura, T.; Asada, M.; Suzuki, C.; Niimi, M.; Matsumoto, S.; Nishimura, T.; Nitta, T.; Yasuchika, K.; et al. A multi-institution phase II study of gemcitabine/S-1 combination chemotherapy for patients with advanced biliary tract cancer. Cancer Chemother. Pharmacol. 2011, 67, 1429–1434. [Google Scholar] [CrossRef]
- Morizane, C.; Okusaka, T.; Mizusawa, J.; Takashima, A.; Ueno, M.; Ikeda, M.; Hamamoto, Y.; Ishii, H.; Boku, N.; Furuse, J. Randomized phase II study of gemcitabine plus S-1 versus S-1 in advanced biliary tract cancer: A Japan Clinical Oncology Group trial (JCOG 0805). Cancer Sci. 2013, 104, 1211–1216. [Google Scholar] [CrossRef] [PubMed]
- Mambrini, A.; Guglielmi, A.; Pacetti, P.; Iacono, C.; Torri, T.; Auci, A.; Nicoli, N.; Orlandi, M.; Guadagni, S.; Fiorentini, G.; et al. Capecitabine plus hepatic intra- arterial epirubicin and cisplatin in unresectable biliary cancer: A phase II study. Anticancer Res. 2007, 27, 3009–3013. [Google Scholar] [PubMed]
- Hyder, O.; Marsh, J.W.; Salem, R.; Petre, E.N.; Kalva, S.; Liapi, E.; Cosgrove, D.; Neal, D.; Kamel, I.; Zhu, A.X.; et al. Intra-arterial Therapy for Advanced Intrahepatic Cholangiocarcinoma: AMulti-institutional Analysis intra-arterial therapy (IAT) for, I.C.C. Ann. Surg. Oncol. 2013, 20, 3779–3786. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Feng WZheng, Y.; Bao, Y.; Feng, M. Intra-arterial chemotherapy improved survival of stage 2–3 gallbladder cancer after curative resection. Onco. Targets Ther. 2018, 11, 2975–2979. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Zeng, Z.C.; Tang, Z.Y.; Fan, J.; Zhou, J.; Zeng, M.S.; Zhang, J.Y.; Chen, Y.X.; Tan, Y.S. Benefit of radiotherapy for 90 patients with resected intrahepatic cholangiocarcinoma and concurrent lymph node metastases. J. Cancer Res. Clin. Oncol. 2010, 136, 1323–1331. [Google Scholar] [CrossRef]
- Moureau-Zabotto, L.; Turrini, O.; Resbeut, M.; Raoul, J.L.; Giovannini, M.; Poizat, F.; Piana, G.; Delpero, J.R.; Bertucci, F. Impact of radiotherapy in the management of locally advanced extrahepatic cholangiocarcinoma. BMC Cancer 2013, 13, 568. [Google Scholar] [CrossRef]
- Shinohara, E.T.; Mitra, N.; Guo, M.; Metz, J.M. Radiation therapy is associated with improved survival in the adjuvant and definitive treatment of intrahepatic cholangiocarcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 1495–1501. [Google Scholar] [CrossRef]
- Goto, T.; Saito, H.; Sasajima, J.; Kawamoto, T.; Fujinaga, A.; Utsumi, T.; Yanagawa, N.; Hiramatsu, K.; Takamura, A.; Sato, H.; et al. High response rate and prolonged survival of unresectable biliary tract cancer treated with a new combination therapy consisting of intraarterial chemotherapy plus radiotherapy. Front Oncol. 2020, 10, 597813. [Google Scholar] [CrossRef]
- Arai, Y.; Kido, C.; Ariyoshi, Y. Pharmacokinetics in Arterial Infusion Chemotherapy. Gan Kagaku Ryoho. 1993, 20, 1755–1761. (In Japanese) [Google Scholar]
- Fukuda, S.; Tanaka, M.; Okuda, Y. Current status of biliary and pancreatic cancer chemotherapy in Japan. Tan Sui. 2000, 21, 841–845. (In Japanese) [Google Scholar]
- Graham, J.S.; Boyd, K.; Coxon, F.Y.; Wall, L.R.; Eatock, M.M.; Maughan, T.S.; Highley, M.; Soulis, E.; Harden, S.; Bützberger-Zimmerli, P.; et al. A phase II study of capecitabine and oxaliplatin combination chemotherapy in patients with inoperable adenocarcinoma of the gall bladder or biliary tract. BMC Res. Notes 2016, 9, 161. [Google Scholar] [CrossRef] [PubMed]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.W.K.; Askan, G.; Daniel, T.D.; Lowery, M.; Klimstra, D.S.; Abou-Alfa, G.K.; Shia, J. Biliary carcinomas: Pathology and the role of DNA mismatch repair deficiency. Chin. Clin. Oncol. 2016, 5, 62. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.-Y.; He, A.R.; Qin, S.; Chen, L.-T.; Okusaka, T.; Vogel, A.; Kim, J.W.; Suksombooncharoen, T.; Lee, M.A.; Kitano, M.; et al. A phase 3 randomized, double-blind, placebo-controlled study of durvalumab in combination with gemcitabine plus cisplatin (GemCis) in patients (pts) with advanced biliary tract cancer (BTC): TOPAZ-1. JCO 2022, 40, 378. [Google Scholar] [CrossRef]
- Oh, D.-Y.; Ruth, H.A.; Qin, S.; Chen, L.-T.; Okusaka, T.; Vogel, A.; Kim, J.W.; Suksombooncharoen, T.; Lee, M.A.; Kitano, M.; et al. Durvalumab plus Gemcitabine and Cisplatin in Advanced Biliary Tract Cancer. NEJM Evid. 2022, 1, EVIDoa2200015. [Google Scholar] [CrossRef]
- Massard, C.; Michiels, S.; Ferte, C.; Le Deley, M.-C.; Lacroix, L.; Hollebecque, A.; Verlingue, L.; Ileana, E.; Rosellini, S.; Ammari, S.; et al. High-Throughput Genomics and Clinical Outcome in Hard-to-Treat Advanced Cancers: Results of the MOSCATO 01 Trial. Cancer Discov. 2017, 7, 586–595. [Google Scholar] [CrossRef]
- Verlingue, L.; Malka, D.; Allorant, A.; Massard, C.; Ferte, C.; Lacroix, L.; Rouleau, E.; Auger, N.; Ngo, M.; Nicotra, C.; et al. Precision medicine for patients with advanced biliary tract cancers: An effective strategy within the prospective MOSCATO-01 trial. Eur. J. Cancer 2017, 87, 122–130. [Google Scholar] [CrossRef]
- Regimbeau, J.M.; Fuks, D.; Bachellier, P.; Treut YPLe Pruvot, F.R.; Navarro, F.; Chiche, L.; Farges, O. Prognostic value of jaundice in patients with gallbladder cancer by the AFC-GBC-2009 study group. Eur. J. Surg. Oncol. 2011, 37, 505–512. [Google Scholar] [CrossRef]
- Fukutomi, A.; Furuse, J.; Okusaka, T.; Miyazaki, M.; Taketsuna, M.; Koshiji, M.; Nimura, Y. Effect of biliary drainage on chemotherapy in patients with biliary tract cancer: An exploratory analysis of the BT22 study. HPB 2012, 14, 221–227. [Google Scholar] [CrossRef]
| Total, N | Biliary Tract Cancer N = 7 |
|---|---|
| Age, years | |
| Median | 71 |
| Range | 55–76 |
| Sex, n (%) | |
| Female | 4 (57.1) |
| Male | 3 (42.9) |
| Performance status, n (%) | |
| 0 | 5 (71.4) |
| 1 | 2 (28.6) |
| Primary site, n (%) | |
| Gallbladder | 3 (42.9) |
| Perihilar extrahepatic bile duct | 2 (28.6) |
| Distal extrahepatic bile duct | 2 (28.6) |
| Type of tumor, n (%) | |
| Adenocarcinoma * | 7 (100) |
| Stage (UICC 8), n (%) | |
| Stage 2b | 1 (14.3) |
| Stage 3c | 1 (14.3) |
| Stage 4 | H 1 (14.3) |
| Stage 4b | H 2, N 2 (57.1) |
| Jaundice (at diagnosis), n (%) | |
| No | 2 (28.6) |
| Yes | 5 (71.4) |
| Jaundice (at the start of treatment), n (%) | |
| No | 7 (100) |
| Yes | 0 (0) |
| Child–Pugh scores | |
| A | 6 (85.7) |
| B | 1 (14.3) |
| CEA value, ng/mL (Normal; <5) | |
| Median | 2.2 |
| Range | 1.1–8.7 |
| CA19-9 value, U/mL (Normal; <37) | |
| Median | 51 |
| Range | 13–4584 |
| Primary tumor diameter, mm | |
| Median | 34.2 |
| Range | 29.8–52.8 |
| Total tumor diameter, mm | |
| Median | 62.2 |
| Range | 31.0–96.3 |
| Total, N | Biliary Tract Cancer N = 7 |
|---|---|
| CR:PR:SD:PD | RECIST 0:4:3:0, Clinically 1:4:2:0 |
| Response rate | RECIST 57.1% (4/7), Clinically 71.4% (5/7) |
| Disease control rate | 100% (7/7) |
| Case | Pre-Treatment Diameter (mm) | Post-Treatment Diameter (mm) | Reduction Rate (%) | Overall Survival (Day) | Progression-Free Survival (Day) |
|---|---|---|---|---|---|
| Case 1 | 78.4 | 47.4 | 39.5 | 283 | 172 |
| Case 2 | 45.3 | 34.2 > 0 | 24.5 > 100 | 1107 | 179 |
| Case 3 | 96.3 | 44.7 | 53.6 | 769 | 202 |
| Case 4 | 31.0 | 26.1 | 15.8 | 315 | 201 |
| Case 5 | 29.3 | 19.8 | 32.4 | 221 | 207 |
| Case 6 | 69.4 | 43.3 | 37.6 | 146 | 146 |
| Case 7 | 62.2 | 53.2 | 14.5 | 80 | 80 |
| Total, n | Biliary Tract Cancer N = 7 | |
|---|---|---|
| All Grades (%) | Grade 3, 4 (%) | |
| Hematologic | ||
| Leukopenia | 6 (85.7) | 5 (71.4) |
| Neutropenia | 6 (85.7) | 5 (71.4) |
| Anemia | 6 (85.7) | 2 (28.6) |
| Thrombocytopenia | 7 (100) | 4 (57.1) |
| Liver dysfunction | 2 (28.6) | 1 (14.3) |
| Pancreatic enzyme elevation | 2 (28.6) | 2 (28.6) |
| Renal dysfunction | 1 (14.3) | 0 (0.0) |
| Hypoalbuminemia | 2 (28.6) | 0 (0.0) |
| Non-hematologic | ||
| Anorexia | 3 (42.9) | 1 (14.3) |
| Abdominal pain | 5 (71.4) | 1 (14.3) |
| Nausea | 4 (57.1) | 0 (0.0) |
| Diarrhea | 0 (0.0) | 0 (0.0) |
| Gastroduodenal ulcer | 3 (42.9) | 1 (14.3) |
| Cholangitis | 2 (28.6) | 2 (28.6) |
| Fatigue | 2 (28.6) | 0 (0.0) |
| Rash | 1 (14.3) | 0 (0.0) |
| Liver abscess | 1 (14.3) | 1 (14.3) |
| Catheter deviation | 1 (14.3) | 1 (14.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goto, T.; Sato, H.; Fujibayashi, S.; Okada, T.; Hayashi, A.; Kawabata, H.; Yuzawa, S.; Ishitoya, S.; Yamashina, M.; Fujiya, M. The Effectiveness of the Combination of Arterial Infusion Chemotherapy and Radiotherapy for Biliary Tract Cancer: A Prospective Pilot Study. Cancers 2023, 15, 2616. https://doi.org/10.3390/cancers15092616
Goto T, Sato H, Fujibayashi S, Okada T, Hayashi A, Kawabata H, Yuzawa S, Ishitoya S, Yamashina M, Fujiya M. The Effectiveness of the Combination of Arterial Infusion Chemotherapy and Radiotherapy for Biliary Tract Cancer: A Prospective Pilot Study. Cancers. 2023; 15(9):2616. https://doi.org/10.3390/cancers15092616
Chicago/Turabian StyleGoto, Takuma, Hiroki Sato, Shugo Fujibayashi, Tetsuhiro Okada, Akihiro Hayashi, Hidemasa Kawabata, Sayaka Yuzawa, Syunta Ishitoya, Masaaki Yamashina, and Mikihiro Fujiya. 2023. "The Effectiveness of the Combination of Arterial Infusion Chemotherapy and Radiotherapy for Biliary Tract Cancer: A Prospective Pilot Study" Cancers 15, no. 9: 2616. https://doi.org/10.3390/cancers15092616
APA StyleGoto, T., Sato, H., Fujibayashi, S., Okada, T., Hayashi, A., Kawabata, H., Yuzawa, S., Ishitoya, S., Yamashina, M., & Fujiya, M. (2023). The Effectiveness of the Combination of Arterial Infusion Chemotherapy and Radiotherapy for Biliary Tract Cancer: A Prospective Pilot Study. Cancers, 15(9), 2616. https://doi.org/10.3390/cancers15092616









