Lymphovascular Space Invasion in Early-Stage Endometrial Cancer (LySEC): Patterns of Recurrence and Predictors. A Multicentre Retrospective Cohort Study of the Spain Gynecologic Oncology Group
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Setting, and Oversight
2.2. Cohort Selection and Study Variables
2.3. Outcomes
2.4. Statistical Analysis
3. Results
3.1. Study Population
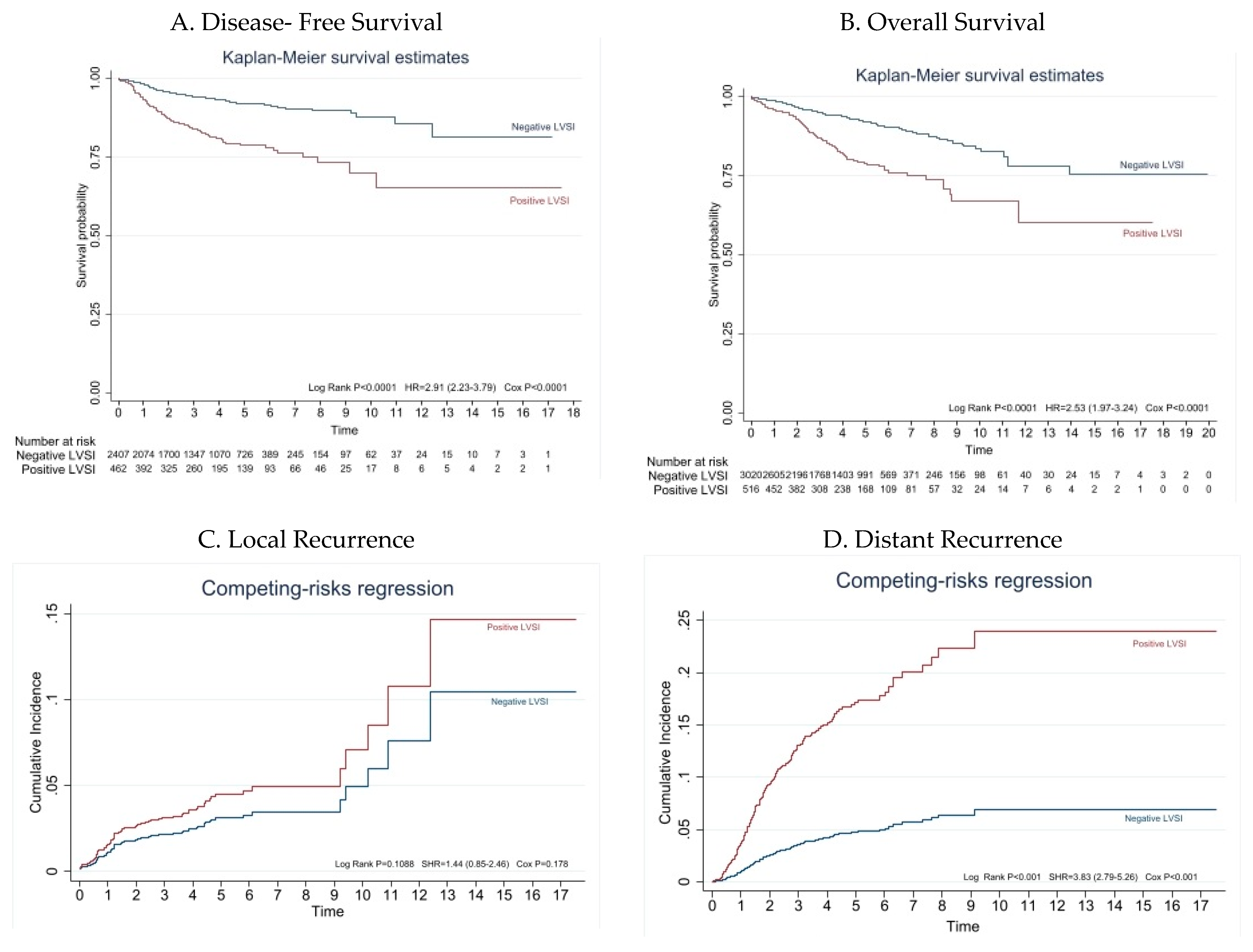
3.2. Survival Analysis According to LVSI Status
3.3. Patterns of Recurrence
3.4. Predictors of LVSI
4. Discussion
4.1. Main Findings
4.2. Results in the Context of Published Literature
4.3. Strengths and Limitations
4.4. Implications for Future Research and Practice
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tortorella, L.; Restaino, S.; Zannoni, G.F.; Vizzielli, G.; Chiantera, V.; Cappuccio, S.; Gioè, A.; La Fera, E.; Dinoi, G.; Angelico, G.; et al. Substantial lymph-vascular space invasion (LVSI) as predictor of distant relapse and poor prognosis in low-risk early-stage endometrial cancer. J. Gynecol. Oncol. 2021, 32, e11. [Google Scholar] [CrossRef]
- Harris, K.L.; Maurer, K.A.; Jarboe, E.; Werner, T.L.; Gaffney, D. LVSI positive and NX in early endometrial cancer: Surgical restaging (and no further treatment if N0), or adjuvant ERT? Gynecol. Oncol. 2020, 156, 243–250. [Google Scholar] [CrossRef]
- Boothe, D.; Wolfson, A.; Christensen, M.; Francis, S.; Werner, T.L.; Gaffney, D.K. Lymphovascular invasion in endometrial cancer: Prognostic value and implications on adjuvant radiation therapy use. Am. J. Clin. Oncol. 2019, 42, 549–554. [Google Scholar] [CrossRef]
- Bosse, T.; Peters, E.E.; Creutzberg, C.L.; Jürgenliemk-Schulz, I.M.; Jobsen, J.J.; Mens, J.W.M.; Lutgens, L.C.; van der Steen-Banasik, E.M.; Smit, V.T.; Nout, R.A. Substantial lymph-vascular space invasion (LVSI) is a significant risk factor for recurrence in endometrial cancer—A pooled analysis of PORTEC 1 and 2 trials. Eur. J. Cancer 2015, 51, 1742–1750. [Google Scholar] [CrossRef] [PubMed]
- Concin, N.; Matias-Guiu, X.; Vergote, I.; Cibula, D.; Mirza, M.R.; Marnitz, S.; Ledermann, J.; Bosse, T.; Chargari, C.; Fagotti, A.; et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int. J. Gynecol. Cancer 2021, 31, 12–39. [Google Scholar] [CrossRef]
- Abu-Rustum, N.; Yashar, C.; Arend, R.; Barber, E.; Bradley, K.; Brooks, R.; Campos, S.M.; Chino, J.; Chon, H.S.; Chu, C.; et al. Uterine Neoplasms, Version 1.2023, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2023, 21, 181–209. [Google Scholar] [CrossRef] [PubMed]
- Murali, R.; A Soslow, R.; Weigelt, B. Classification of endometrial carcinoma: More than two types. Lancet Oncol. 2014, 15, e268–e278. [Google Scholar] [CrossRef]
- Karabagli, P.; Ugras, S.; Yilmaz, B.S.; Celik, C. The evaluation of reliability and contribution of frozen section pathology to staging endometrioid adenocarcinomas. Arch. Gynecol. Obstet. 2015, 292, 391–397. [Google Scholar] [CrossRef]
- Kumar, S.; Bandyopadhyay, S.; Semaan, A.; Shah, J.P.; Mahdi, H.; Morris, R.; Munkarah, A.; Ali-Fehmi, R. The Role of Frozen Section in Surgical Staging of Low Risk Endometrial Cancer. PLoS ONE 2011, 6, e21912. [Google Scholar] [CrossRef]
- Rtoft, G.; Lausten-Thomsen, L.; Høgdall, C.; Hansen, E.S.; Dueholm, M. Lymph-vascular space invasion (LVSI) as a strong and independent predictor for non-locoregional recurrences in endometrial cancer: A danish gynecological cancer group study. J. Gynecol. Oncol. 2019, 30, e84. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, K.; Garcia-Sayre, J.; Medeiros, F.; Casabar, J.K.; Machida, H.; Moeini, A.; Roman, L.D. Impact of depth and extent of lymphovascular space invasion on lymph node metastasis and recurrence patterns in endometrial cancer. J. Surg. Oncol. 2015, 112, 669–676. [Google Scholar] [CrossRef]
- Weinberg, L.E.; Kunos, C.A.; Zanotti, K.M. Lymphovascular Space Invasion (LVSI) Is an Isolated Poor Prognostic Factor for Recurrence and Survival Among Women With Intermediate- to High-Risk Early-Stage Endometrioid Endometrial Cancer. Int. J. Gynecol. Cancer 2013, 23, 1438–1445. [Google Scholar] [CrossRef]
- Oliver-Perez, M.R.; Magriña, J.; Villalain-Gonzalez, C.; Jimenez-Lopez, J.S.; Lopez-Gonzalez, G.; Barcena, C.; Martinez-Biosques, C.; Gil-Ibañez, B.; Tejerizo-Garcia, A. Lymphovascular space invasion in endometrial carcinoma: Tumor size and location matter. Surg. Oncol. 2021, 37, 101541. [Google Scholar] [CrossRef]
- Laufer, J.; Scasso, S.; Papadia, A.; Sosa, C.; Cirillo, F.; Raspagliesi, F. Association between tumor diameter and lymphovascular space invasion among women with early-stage endometrial cancer. Int. J. Gynecol. Obstet. 2013, 123, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Meydanli, M.M.; Aslan, K.; Öz, M.; Muftuoglu, K.H.; Yalcin, I.; Engin-Ustun, Y. Is it possible to develop a prediction model for lymphovascular space invasion in endometrioid endometrial cancer? Int. J. Gynecol. Pathol. 2020, 39, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Capozzi, V.A.; Sozzi, G.; Uccella, S.; Ceni, V.; Cianciolo, A.; Gambino, G.; Armano, G.; Pugliese, M.; Scambia, G.; Chiantera, V.; et al. Novel preoperative predictive score to evaluate lymphovascular space involvement in endometrial cancer: An aid to the sentinel lymph node algorithm. Int. J. Gynecol. Cancer 2020, 30, 806–812. [Google Scholar] [CrossRef]
- Radley-Gardner, O.; Beale, H.; Zimmermann, R. (Eds.) Fundamental Texts On European Private Law; Hart Publishing: London, UK, 2016. [Google Scholar]
- FIGO. Staging for carcinoma of the vulva, cervix, and corpus uteri. Int. J. Gynecol. Obstet. 2014, 125, 97–98. [Google Scholar] [CrossRef] [PubMed]
- FIGO. Announcements, stages-1988 Revision. Gynecol. Oncol. 1989, 35, 125. [Google Scholar]
- Pollom, E.L.; Conklin, C.M.J.; von Eyben, R.; Folkins, A.K.; Kidd, E.A. Nomogram to Predict Risk of Lymph Node Metastases in Patients With Endometrioid Endometrial Cancer. Int. J. Gynecol. Pathol. 2016, 35, 395–401. [Google Scholar] [CrossRef]
- Jorge, S.; Hou, J.Y.; Tergas, A.I.; Burke, W.M.; Huang, Y.; Hu, J.C.; Ananth, C.V.; Neugut, A.I.; Hershman, D.L.; Wright, J.D. Magnitude of risk for nodal metastasis associated with lymphvascular space invasion for endometrial cancer. Gynecol. Oncol. 2016, 140, 387–393. [Google Scholar] [CrossRef]
- Beavis, A.L.; Yen, T.T.; Stone, R.L.; Wethington, S.L.; Carr, C.; Son, J.; Chambers, L.; Michener, C.M.; Ricci, S.; Burkett, W.C.; et al. Adjuvant therapy for early stage, endometrial cancer with lymphovascular space invasion: Is there a role for chemotherapy? Gynecol. Oncol. 2020, 156, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Wortman, B.G.; Creutzberg, C.L.; Putter, H.; Jürgenliemk-Schulz, I.M.; Jobsen, J.J.; Lutgens, L.C.H.W.; van der Steen-Banasik, E.M.; Mens, J.W.M.; Slot, A.; Stenfert Kroese, M.C.; et al. Ten-year results of the PORTEC-2 trial for high-intermediate risk endometrial carcinoma: Improving patient selection for adjuvant therapy. Br. J. Cancer 2018, 119, 1067–1074. [Google Scholar] [CrossRef]
- Leon-Castillo, A.; Horeweg, N.; Peters, E.E.; Rutten, T.; ter Haar, N.; Smit, V.T.; Kroon, C.D.; Boennelycke, M.; Hogdall, E.; Hogdall, C.; et al. Prognostic relevance of the molecular classification in high-grade endometrial cancer for patients staged by lymphadenectomy and without adjuvant treatment. Gynecol. Oncol. 2022, 164, 577–586. [Google Scholar] [CrossRef]
- Stelloo, E.; Nout, R.A.; Osse, E.M.; Jürgenliemk-Schulz, I.J.; Jobsen, J.J.; Lutgens, L.C.; van der Steen-Banasik, E.M.; Nijman, H.W.; Putter, H.; Bosse, T.; et al. Improved risk assessment by integrating molecular and clinicopathological factors in early-stage endometrial cancer-combined analysis of the PORTEC cohorts. Clin. Cancer Res. 2016, 22, 4215–4224. [Google Scholar] [CrossRef] [PubMed]
- de Boer, S.M.; Powell, M.E.; Mileshkin, L.; Katsaros, D.; Bessette, P.; Haie-Meder, C.; Ottevanger, P.B.; Ledermann, J.A.; Khaw, P.; Colombo, A.; et al. Faculty Opinions recommendation of Adjuvant chemoradiotherapy versus radiotherapy alone for women with high-risk endometrial cancer (PORTEC-3): Final results of an international, open-label, multicentre, randomised, phase 3 trial. Lancet Oncol. 2019, 19, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Ytre-Hauge, S.; Husby, J.A.; Magnussen, I.J.; Werner, H.M.; Salvesen, O.; Bjørge, L.; Trovik, J.; Stefansson, I.M.; Salvesen, H.B.; Haldorsen, I.S. Preoperative Tumor Size at MRI Predicts Deep Myometrial Invasion, Lymph Node Metastases, and Patient Outcome in Endometrial Carcinomas. Int. J. Gynecol. Cancer 2015, 25, 459–466. [Google Scholar] [CrossRef]
- Bourgioti, C.; Chatoupis, K.; Tzavara, C.; Antoniou, A.; Rodolakis, A.; Moulopoulos, L. Predictive ability of maximal tumor diameter on MRI for high-risk endometrial cancer. Abdom. Imaging 2016, 41, 2484–2495. [Google Scholar] [CrossRef]
| Characteristic | Total (n = 3546) | Negative LVSI (n = 3028) | Positive LVSI (n = 518) | p-Value |
|---|---|---|---|---|
| Age (yr) | ||||
| <70 | 2362 (66.7) | 2043 (67.5) | 319 (61.6) | |
| ≥70 | 1181 (33.3) | 982 (32.5) | 199 (38.4) | 0.008 |
| NR | 3 | 3 | 0 | |
| BMI | ||||
| Median (IQR) | 31.2 (26.7–35.9) | 31.2 (26.7–36.1) | 30.5 (26.1–35.0) | 0.042 |
| NR | 634 | 559 | 75 | |
| Surgical approach | ||||
| MIS | 2699 (76.2) | 2287 (75.6) | 412 (79.5) | 0.054 |
| Laparotomy | 844 (23.8) | 738 (24.4) | 106 (20.5) | |
| NR | 3 | 3 | ||
| LN assessment a | 1465 (41.3) | 1156 (38.2) | 309 (59.6) | <0.001 |
| Tumour diameter (cm) b | ||||
| <2 | 693 (26.4) | 645 (28.8) | 48 (12.3) | |
| ≥2 | 1934 (73.6) | 1592 (71.2) | 342 (87.7) | <0.001 |
| NR | 919 | 791 | 128 | |
| Grading c | ||||
| Low grade (G1, G2) | 3175 (89.7) | 2786 (92.2) | 389 (75.1) | |
| High grade (G3) | 364 (10.3) | 235 (7.8) | 129 (24.9) | <0.001 |
| NR | 7 | 7 | 0 | |
| MI ≥ 50% | 973 (27.4) | 693 (22.9) | 280 (54.1) | <0.001 |
| FIGO stage d | ||||
| I | 3308 (93.3) | 2865 (94.6) | 443 (85.5) | <0.001 |
| II | 238 (6.7) | 163 (5.4) | 75 (14.5) | |
| Adjuvant treatment | ||||
| None | 2041 (57.6) | 1955 (64.6) | 86 (16.6) | |
| BT alone | 708 (20.0) | 544 (18.0) | 164 (31.7) | |
| EBRT+/−BT | 738 (20.8) | 495 (16.3) | 243 (46.9) | <0.001 |
| CT alone or with RT | 59 (1.6) | 34 (1.1) | 25 (4.8) | |
| Recurrence | ||||
| No | 3268 (92.2) | 2842 (93.9) | 426 (82.2) | <0.001 |
| Yes | 278 (7.8) | 186 (6.1) | 92 (17.8) | |
| Recurrence e | ||||
| No | 3268 (92.2) | 2842 (93.9) | 426 (82.2) | <0.001 |
| Local and vaginal vault | 92 (2.5) | 72 (2.3) | 20 (3.8) | |
| Distant metastases | 186 (5.3) | 114 (3.8) | 72 (14.0) |
| Characteristics | DFS | OS | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||
| HR (95%CI) | p-Value | HR (95% CI) | p-Value | HR (95%CI) | p-Value | HR (95% CI) | p-Value | |
| LVSI present | 2.9 (2.2–3.8) | <0.001 | 1.9 (1.3–2.5) | <0.001 f | 2.5 (1.9–3.2) | <0.001 | 2.1 (1.5–2.9) | <0.001 g |
| Age ≥ 70 y. | 1.6(1.3–2.1) | <0.001 | 1.6 (1.2–2.1) | <0.002 | 3.80 (3.0–4.8) | <0.001 | 3.6 (2.7–4.7) | <0.001 |
| Tumour diameter ≥ 2 cm a | 2.4(1.6–3.6) | <0.001 | 1. 7 (1.1–2.6) | 0.015 | 2.1 (1.5–3.1) | <0.001 | 1.63 (1.1–2.4) | 0.012 |
| High grade b | 3.4 (2.5–4.5) | <0.001 | 2.6 (1.8–3.5) | <0.001 | 2.4 (1.8–3.1) | <0.001 | 2.5 (1.8–3.5) | <0.001 |
| MI ≥ 50% | 2.1 (1.6–2.7) | <0.001 | 1.3 (0.9–1.8) | 0.066 | 1.7 (1.3–2.1) | <0.001 | 1.5 (1.0–2.0) | 0.026 |
| Figo Stage II c | 2.8 (1.9–3.9) | <0.001 | 1.8 (1.2–2.7) | 0.007 | 1.7 (1.2–2.4) | 0.007 | 1.4 (0.9–2.3) | 0.124 |
| LN assessment d | 1.1 (0.9–1.5) | 0.309 | - | - | 0.7 (0.5–0.9) | 0.0009 | 0.5 (0.3–0.6) | <0.001 |
| Adjuvant treatment e | 2.1 (1.6–2.7) | <0.001 | - | - | 1.2 (0.9–1.5) | 0.105 | 0.6 (0.4–0.8) | 0.002 |
| Distant Recurrence h | Local Recurrence h | |||||||
| Univariate | Multivariate | Univariate | Multivariate | |||||
| HR (95%CI) | p-Value | HR (95% CI) | p-Value | HR (95%CI) | p-Value | HR (95% CI) | p-Value | |
| LVSI present | 3.9 (2.8–5.3) | <0.001 | 2.4 (1.7–3.4) | <0.001 i | 1.5 (0.9–2.6) | 0.112 | - | - |
| Age ≥ 70 y. | 1.6 (1.1–2.2) | 0.005 | 1.4 (1.0–1.9) | 0.037 | 1.7 (1.0–2.7) | 0.019 | 1.8 (1.1–2.9) | 0.022 |
| Tumour diameter ≥ 2 cm a | 2.4 (1.5–4.1) | 0.001 | - | - | 2.3 (1.1–4.6) | 0.022 | 2.3 (1.1–4.8) | 0.020 |
| High grade b | 4.1 (2.9–5.7) | <0.001 | 2.6 (1.8–3.7) | <0.001 | 2.2 (1.2–3.9) | 0.006 | 3.1 (1.6–5.9) | 0.001 |
| MI ≥ 50% | 2.7 (1.9–3.7) | <0.001 | 1.5 (1.0–2.1) | 0.034 | 1.3 (0.8–2.1) | 0.26 | - | - |
| LN assessment d | 1.5 (1.1–2.1) | 0.007 | - | - | 0.6 (0.4–0.9) | 0.029 | - | - |
| Adjuvant treatment e | 3.5(2.5–5.1) | <0.001 | 1.6 (1.0–2.6) | 0.031 | 0.9 (0.6–1.4) | 0.519 | - | - |
| Location | Total (n = 278) | LVSI Negative (n = 186) | LVSI Positive (n = 92) | p-Value |
|---|---|---|---|---|
| Local and Vaginal vault | 92 (33.1) | 72 (38.7) | 20 (21.7) | 0.012 |
| Peritoneal carcinomatosis | 44 (15.8) | 31 (16.8) | 13 (14.2) | |
| Metastatic lymph nodes | 44 (15.8) | 24 (12.9) | 20 (21.7) | |
| Visceral metastases | 98 (35.3) | 59 (31.7) | 39 (42.4) | |
| Local and vaginal vault a | 92 (33.1) | 72 (38.7) | 20 (21.8) | 0.01 |
| Distant metastases b | 186 (66.9) | 114 (61.3) | 72 (78.2) |
| Characteristics | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| 0 R (95%CI) | p-Value | 0 R (95% CI) | p-Value | |
| Age ≥70 years | 1.29 (1.07–1.57) | 0.008 | 0.83 (0.64–1.08) | 0.17 |
| BMI | 0.98 (0.96–0.99) | 0.027 | 0.98 (0.96–1.00) | 0.054 |
| Tumor diameter ≥ 2 cm a | 2.88 (2.10–3.95) | <0.001 | 2.03 (1.45–2.85) | <0.001 |
| Myometrial invasion ≥ 50% | 3.96 (3.26–4.80) | <0.001 | 3.04 (2.36–3.92) | <0.001 |
| High grade (G3) b | 3.9 (3.09–4.99) | <0.001 | 2.54 (1.84–3.5) | <0.001 |
| FIGO II c | 2.97 (2.22–3.98) | <0.001 | 2.01 (1.34–3.02) | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliver-Perez, M.R.; Padilla-Iserte, P.; Arencibia-Sanchez, O.; Martin-Arriscado, C.; Muruzabal, J.C.; Diaz-Feijóo, B.; Cabrera, S.; Coronado, P.; Martín-Salamanca, M.B.; Pantoja-Garrido, M.; et al. Lymphovascular Space Invasion in Early-Stage Endometrial Cancer (LySEC): Patterns of Recurrence and Predictors. A Multicentre Retrospective Cohort Study of the Spain Gynecologic Oncology Group. Cancers 2023, 15, 2612. https://doi.org/10.3390/cancers15092612
Oliver-Perez MR, Padilla-Iserte P, Arencibia-Sanchez O, Martin-Arriscado C, Muruzabal JC, Diaz-Feijóo B, Cabrera S, Coronado P, Martín-Salamanca MB, Pantoja-Garrido M, et al. Lymphovascular Space Invasion in Early-Stage Endometrial Cancer (LySEC): Patterns of Recurrence and Predictors. A Multicentre Retrospective Cohort Study of the Spain Gynecologic Oncology Group. Cancers. 2023; 15(9):2612. https://doi.org/10.3390/cancers15092612
Chicago/Turabian StyleOliver-Perez, M. Reyes, Pablo Padilla-Iserte, Octavio Arencibia-Sanchez, Cristina Martin-Arriscado, Juan Carlos Muruzabal, Berta Diaz-Feijóo, Silvia Cabrera, Pluvio Coronado, M. Belen Martín-Salamanca, Manuel Pantoja-Garrido, and et al. 2023. "Lymphovascular Space Invasion in Early-Stage Endometrial Cancer (LySEC): Patterns of Recurrence and Predictors. A Multicentre Retrospective Cohort Study of the Spain Gynecologic Oncology Group" Cancers 15, no. 9: 2612. https://doi.org/10.3390/cancers15092612
APA StyleOliver-Perez, M. R., Padilla-Iserte, P., Arencibia-Sanchez, O., Martin-Arriscado, C., Muruzabal, J. C., Diaz-Feijóo, B., Cabrera, S., Coronado, P., Martín-Salamanca, M. B., Pantoja-Garrido, M., Marcos-Sanmartin, J., Cabezas-López, E., Lorenzo, C., Beric, D., Rodriguez-Hernandez, J. R., Roldan-Rivas, F., Gilabert-Estelles, J., Sanchez, L., Laseca-Modrego, M., ... on behalf of the Spain-GOG Group. (2023). Lymphovascular Space Invasion in Early-Stage Endometrial Cancer (LySEC): Patterns of Recurrence and Predictors. A Multicentre Retrospective Cohort Study of the Spain Gynecologic Oncology Group. Cancers, 15(9), 2612. https://doi.org/10.3390/cancers15092612






