Interstitial Photodynamic Therapy of Glioblastomas: A Long-Term Follow-up Analysis of Survival and Volumetric MRI Data
Abstract
Simple Summary
Abstract
1. Introduction
2. Patients and Methods
2.1. Patients
2.2. Methods
2.2.1. Survival Analysis
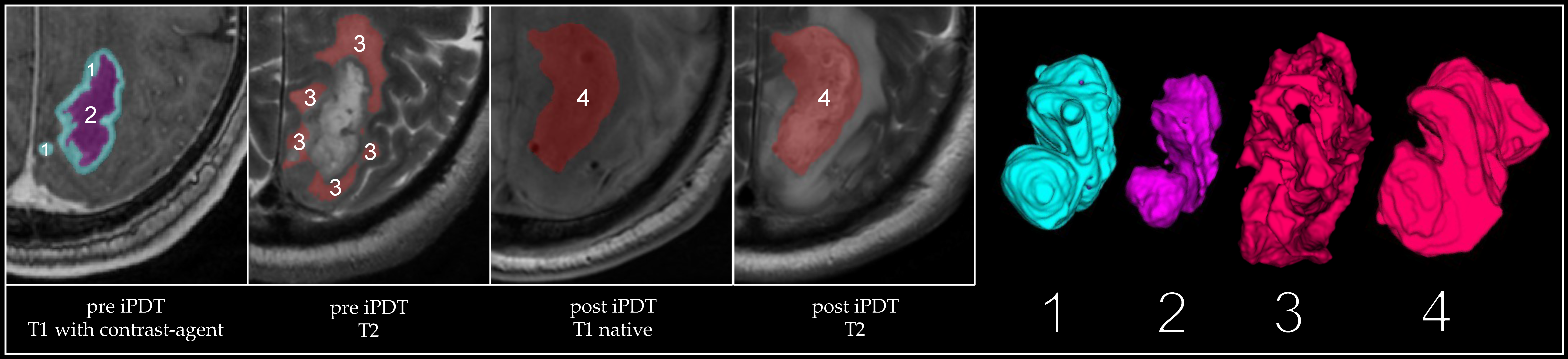
2.2.2. MRI Analysis
2.2.3. Statistics
3. Results
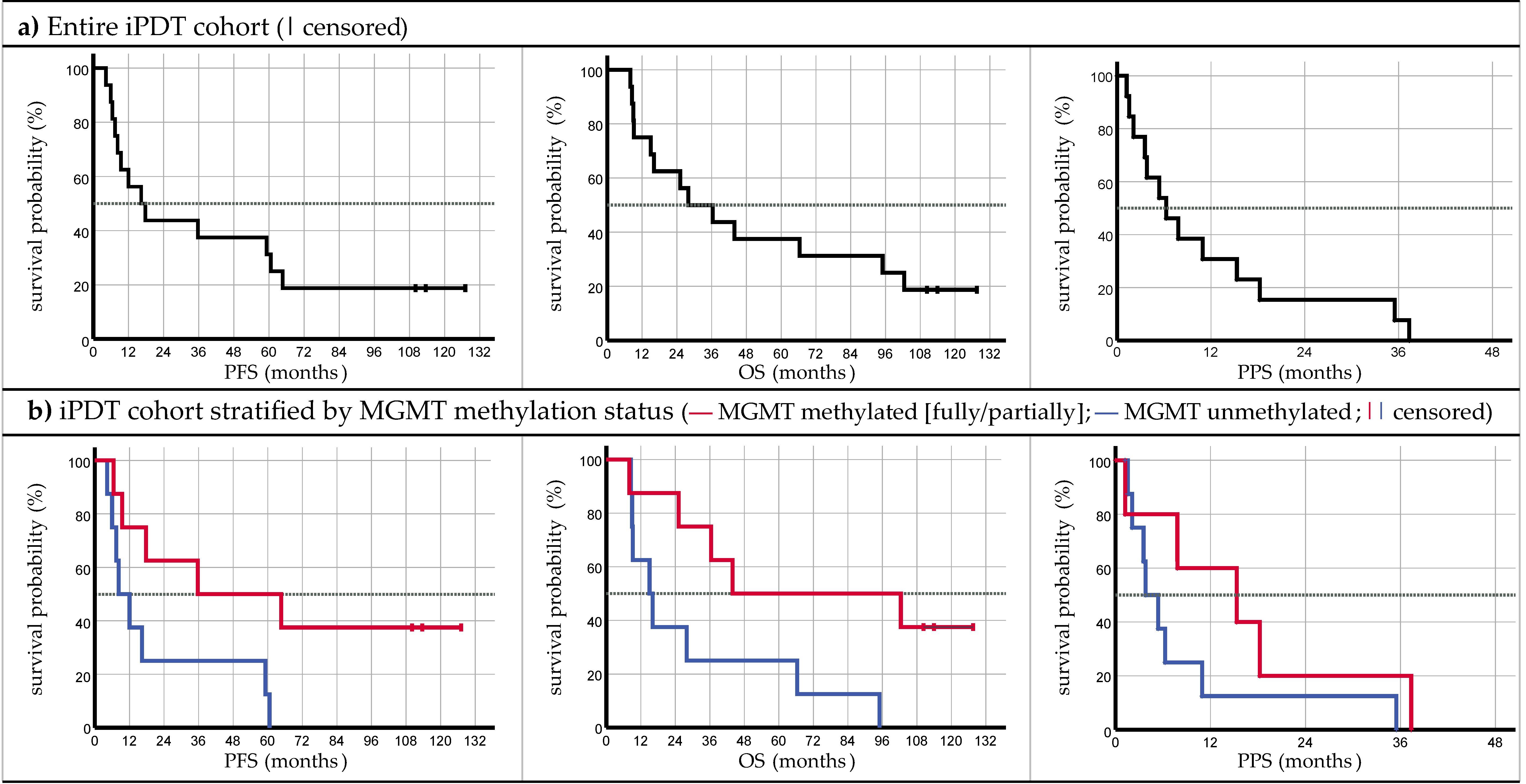
3.1. Survival
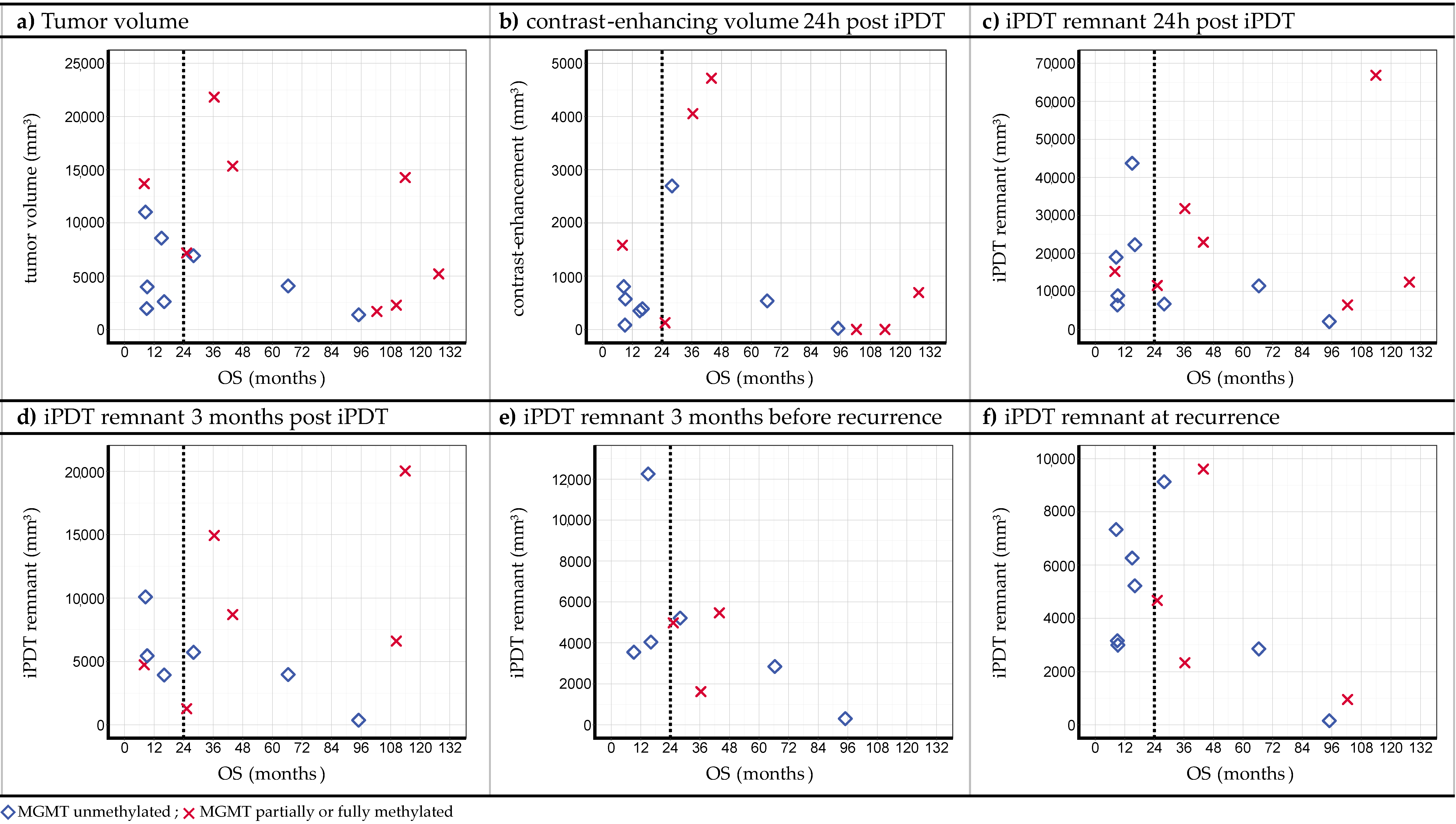
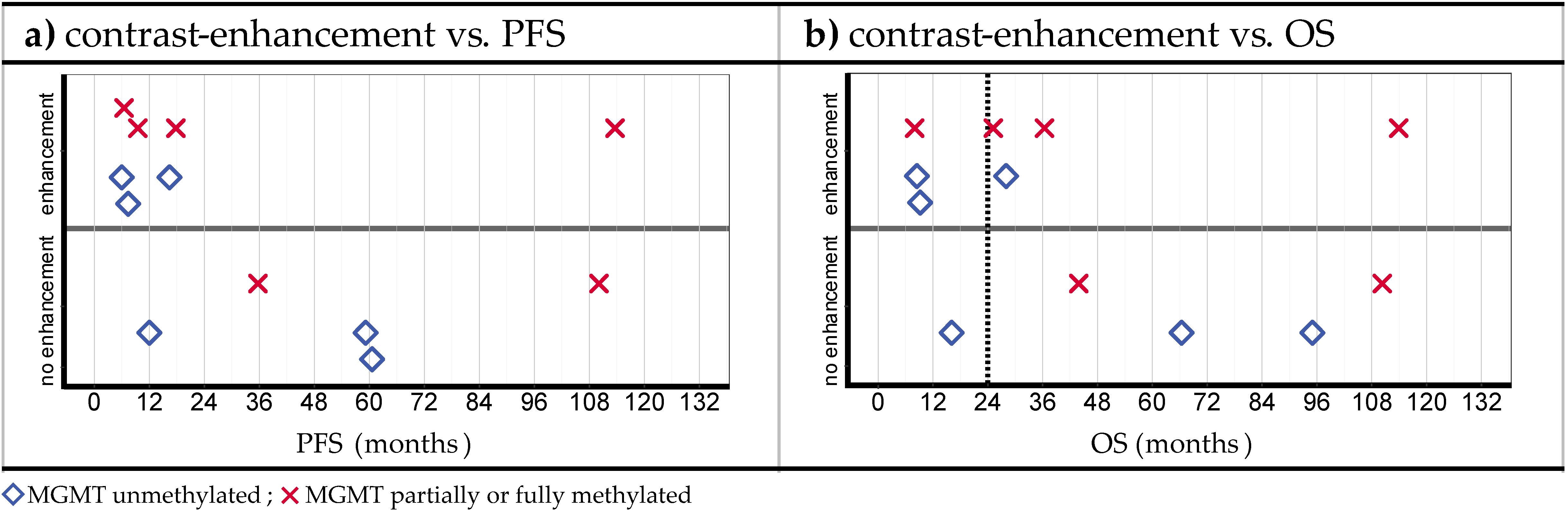
3.2. Volumetric Assessments
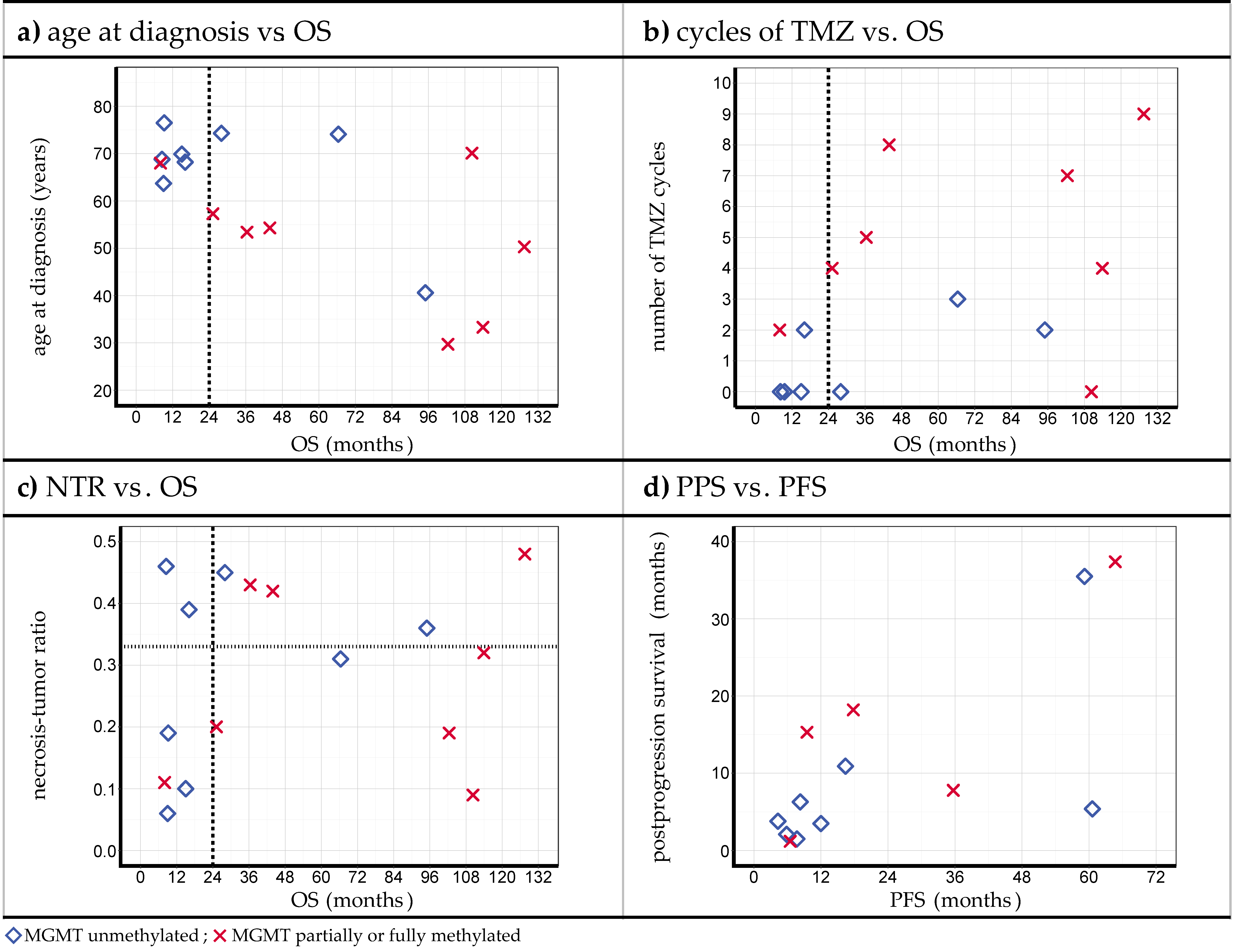
3.3. Relations between Observations
4. Discussion
4.1. Survival Comparison
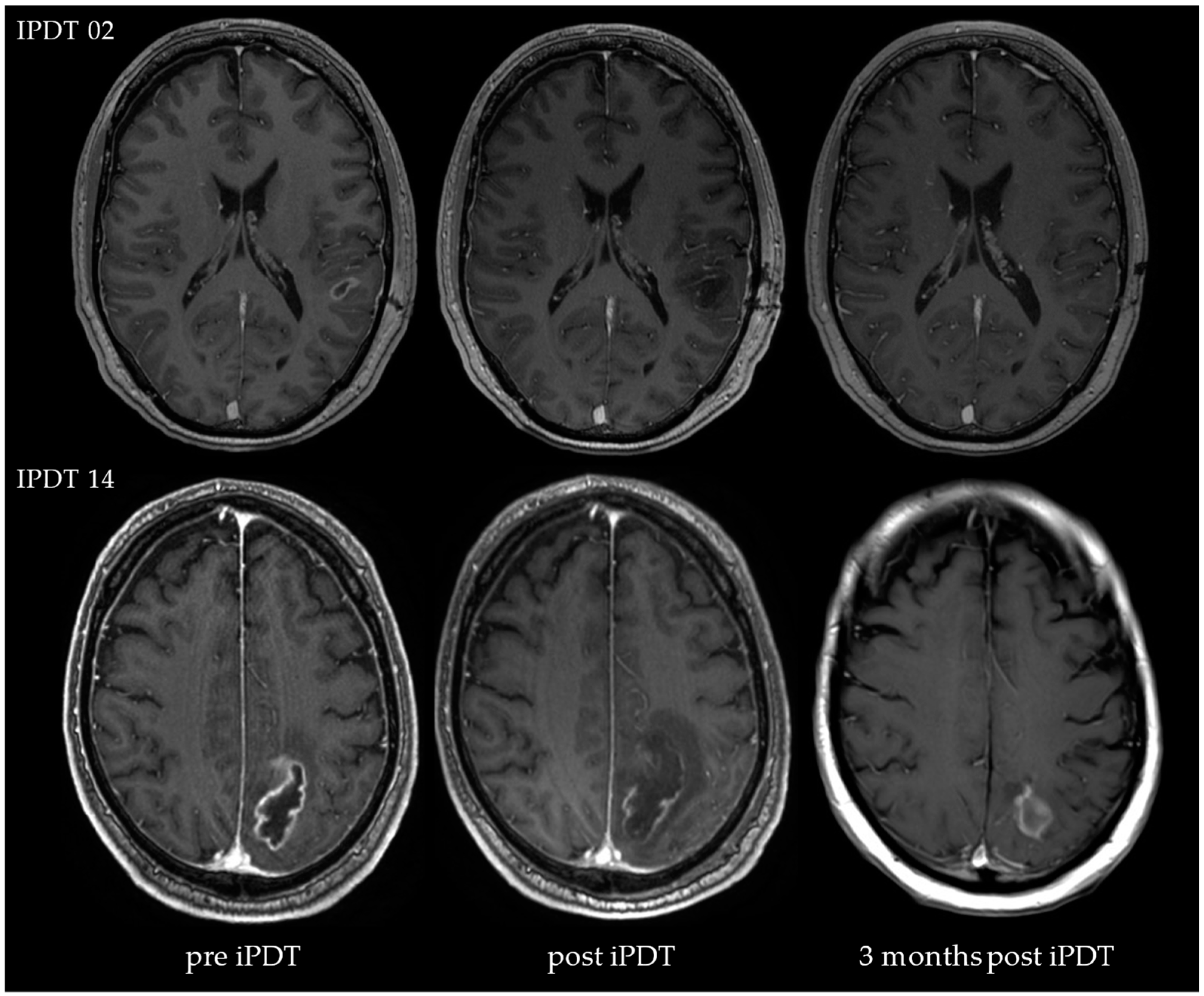
4.2. Posttreatment CE
4.3. iPDT Remnant
4.4. Recurrence Patterns
4.5. Examination of Possibly Survival-Related Factors
4.6. Quality Assessment of the Performed Treatment
4.7. Discussion of Materials and Methods
4.8. Limitations
4.9. Recapitulation and Outlook
- The presented data indicate that the first results obtained through the iPDT-treatment of patients with de novo glioblastomas are promising, e.g., in terms of OS. The possibility of treating nonresectable tumors should especially be highly appreciated, as it would greatly improve the prognosis of the affected patients. For recurrent glioblastomas, iPDT was already tested on a larger number of patients, showing the feasibility and safety of the treatment [16].
- It appears that the good performance of iPDT regarding survival is not only due to a good local tumor-debulking effect but is also based on immunogenic effects. This can be concluded from the observed unusual recurrence pattern characterized by a lower rate of local recurrence than that seen in a plenitude of other glioblastoma patient cohorts [78,79,80,81,82].
- The confirmation of the importance of the MGMT methylation status [17] can be seen as an important step in the improvement of the patient selection of iPDT for de novo glioblastomas.
- The assessment of MRI data showed peculiarities compared to the SOC treatment. In the case of residual or increasing CE after iPDT, advanced imaging techniques (e.g., DWI, MR perfusion and spectroscopy, PET) should be considered to enable better differentiation between vital tumor reactions and tissue reactions/pseudoprogression.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Ostrom, Q.T.; Price, M.; Neff, C.; Cioffi, G.; Waite, K.A.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2015–2019. Neuro-oncology 2022, 24 (Suppl. 5), v1–v95. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; Van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Friedmann-Morvinski, D. Glioblastoma heterogeneity and cancer cell plasticity. Crit. Rev. Oncog. 2014, 19, 327–336. [Google Scholar] [CrossRef]
- Orzan, F.; De Bacco, F.; Crisafulli, G.; Pellegatta, S.; Mussolin, B.; Siravegna, G.; D’Ambrosio, A.; Comoglio, P.M.; Finocchiaro, G.; Boccaccio, C. Genetic Evolution of Glioblastoma Stem-Like Cells from Primary to Recurrent Tumor. Stem Cells 2017, 35, 2218–2228. [Google Scholar] [CrossRef]
- Lara-Velazquez, M.; Al-Kharboosh, R.; Jeanneret, S.; Vazquez-Ramos, C.; Mahato, D.; Tavanaiepour, D.; Rahmathulla, G.; Quinones-Hinojosa, A. Advances in Brain Tumor Surgery for Glioblastoma in Adults. Brain Sci. 2017, 7, 166. [Google Scholar] [CrossRef]
- Venkataramani, V.; Yang, Y.; Schubert, M.C.; Reyhan, E.; Tetzlaff, S.K.; Wißmann, N.; Botz, M.; Soyka, S.J.; Beretta, C.A.; Pramatarov, R.L.; et al. Glioblastoma hijacks neuronal mechanisms for brain invasion. Cell 2022, 185, 2899–2917.e2831. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.P.; Adamson, D.C. Current FDA-Approved Therapies for High-Grade Malignant Gliomas. Biomedicines 2021, 9, 324. [Google Scholar] [CrossRef]
- Sun, R.; Cuthbert, H.; Watts, C. Fluorescence-Guided Surgery in the Surgical Treatment of Gliomas: Past, Present and Future. Cancers 2021, 13, 3508. [Google Scholar] [CrossRef]
- Wilson, B.C.; Eu, D. Optical spectroscopy and imaging in surgical management of cancer patients. Transl. Biophotonics 2022, 4, e202100009. [Google Scholar] [CrossRef]
- Thon, N.; Thorsteinsdottir, J.; Eigenbrod, S.; Schüller, U.; Lutz, J.; Kreth, S.; Belka, C.; Tonn, J.C.; Niyazi, M.; Kreth, F.W. Outcome in unresectable glioblastoma: MGMT promoter methylation makes the difference. J. Neurol. 2017, 264, 350–358. [Google Scholar] [CrossRef]
- Azoulay, M.; Chang, S.D.; Gibbs, I.C.; Hancock, S.L.; Pollom, E.L.; Harsh, G.R.; Adler, J.R.; Harraher, C.; Li, G.; Hayden Gephart, M.; et al. A phase I/II trial of 5-fraction stereotactic radiosurgery with 5-mm margins with concurrent temozolomide in newly diagnosed glioblastoma: Primary outcomes. Neuro-oncology 2020, 22, 1182–1189. [Google Scholar] [CrossRef]
- Oermann, E.; Collins, B.T.; Erickson, K.T.; Yu, X.; Lei, S.; Suy, S.; Hanscom, H.N.; Kim, J.; Park, H.U.; Eldabh, A.; et al. CyberKnife enhanced conventionally fractionated chemoradiation for high grade glioma in close proximity to critical structures. J. Hematol. Oncol. 2010, 3, 22. [Google Scholar] [CrossRef]
- Barbarite, E.; Sick, J.T.; Berchmans, E.; Bregy, A.; Shah, A.H.; Elsayyad, N.; Komotar, R.J. The role of brachytherapy in the treatment of glioblastoma multiforme. Neurosurg. Rev. 2017, 40, 195–211. [Google Scholar] [CrossRef] [PubMed]
- Stummer, W.; Beck, T.; Beyer, W.; Mehrkens, J.H.; Obermeier, A.; Etminan, N.; Stepp, H.; Tonn, J.C.; Baumgartner, R.; Herms, J.; et al. Long-sustaining response in a patient with non-resectable, distant recurrence of glioblastoma multiforme treated by interstitial photodynamic therapy using 5-ALA: Case report. J. Neurooncol. 2008, 87, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Beck, T.J.; Kreth, F.W.; Beyer, W.; Mehrkens, J.H.; Obermeier, A.; Stepp, H.; Stummer, W.; Baumgartner, R. Interstitial photodynamic therapy of nonresectable malignant glioma recurrences using 5-aminolevulinic acid induced protoporphyrin IX. Lasers Surg. Med. 2007, 39, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Lietke, S.; Schmutzer, M.; Schwartz, C.; Weller, J.; Siller, S.; Aumiller, M.; Heckl, C.; Forbrig, R.; Niyazi, M.; Egensperger, R.; et al. Interstitial Photodynamic Therapy Using 5-ALA for Malignant Glioma Recurrences. Cancers 2021, 13, 1767. [Google Scholar] [CrossRef] [PubMed]
- Quach, S.; Schwartz, C.; Aumiller, M.; Foglar, M.; Schmutzer, M.; Katzendobler, S.; Forbrig, R.; Bochmann, K.; Egensperger, R.; Sroka, R.; et al. Interstitial photodynamic therapy for newly diagnosed glioblastoma. J. Neuro-Oncol. 2023. ahead of print. [Google Scholar] [CrossRef]
- Traylor, J.I.; Pernik, M.N.; Sternisha, A.C.; McBrayer, S.K.; Abdullah, K.G. Molecular and Metabolic Mechanisms Underlying Selective 5-Aminolevulinic Acid-Induced Fluorescence in Gliomas. Cancers 2021, 13, 580. [Google Scholar] [CrossRef]
- Malik, Z. Fundamentals of 5-aminolevulinic acid photodynamic therapy and diagnosis: An overview. Transl. Biophotonics 2020, 2, e201900022. [Google Scholar] [CrossRef]
- Kiening, M.; Lange, N. A Recap of Heme Metabolism towards Understanding Protoporphyrin IX Selectivity in Cancer Cells. Int. J. Mol. Sci. 2022, 23, 7974. [Google Scholar] [CrossRef]
- Aumiller, M.; Heckl, C.; Quach, S.; Stepp, H.; Ertl-Wagner, B.; Sroka, R.; Thon, N.; Rühm, A. Interrelation between Spectral Online Monitoring and Postoperative T1-Weighted MRI in Interstitial Photodynamic Therapy of Malignant Gliomas. Cancers 2021, 14, 120. [Google Scholar] [CrossRef] [PubMed]
- Stepp, H.; Stummer, W. 5-ALA in the management of malignant glioma. Lasers Surg. Med. 2018, 50, 399–419. [Google Scholar] [CrossRef] [PubMed]
- Leroy, H.A.; Guérin, L.; Lecomte, F.; Baert, G.; Vignion, A.S.; Mordon, S.; Reyns, N. Is interstitial photodynamic therapy for brain tumors ready for clinical practice? A systematic review. Photodiagn. Photodyn. Ther. 2021, 36, 102492. [Google Scholar] [CrossRef] [PubMed]
- Nkune, N.W.; Simelane, N.W.N.; Montaseri, H.; Abrahamse, H. Photodynamic Therapy-Mediated Immune Responses in Three-Dimensional Tumor Models. Int. J. Mol. Sci. 2021, 22, 2618. [Google Scholar] [CrossRef]
- Donohoe, C.; Senge, M.O.; Arnaut, L.G.; Gomes-da-Silva, L.C. Cell death in photodynamic therapy: From oxidative stress to anti-tumor immunity. Biochim. Biophys. Acta Rev. Cancer 2019, 1872, 188308. [Google Scholar] [CrossRef]
- Hamblin, M.R.; Abrahamse, H. Factors Affecting Photodynamic Therapy and Anti-Tumor Immune Response. Anticancer Agents Med. Chem. 2021, 21, 123–136. [Google Scholar] [CrossRef]
- Yu, X.; Zheng, H.; Chan, M.T.V.; Wu, W.K.K. Immune consequences induced by photodynamic therapy in non-melanoma skin cancers: A review. Environ. Sci. Pollut. Res. Int. 2018, 25, 20569–20574. [Google Scholar] [CrossRef]
- Gollnick, S.O.; Brackett, C.M. Enhancement of anti-tumor immunity by photodynamic therapy. Immunol. Res. 2010, 46, 216–226. [Google Scholar] [CrossRef]
- Castano, A.P.; Mroz, P.; Hamblin, M.R. Photodynamic therapy and anti-tumour immunity. Nat. Rev. Cancer 2006, 6, 535–545. [Google Scholar] [CrossRef]
- Li, F.; Cheng, Y.; Lu, J.; Hu, R.; Wan, Q.; Feng, H. Photodynamic therapy boosts anti-glioma immunity in mice: A dependence on the activities of T cells and complement C3. J. Cell. Biochem. 2011, 112, 3035–3043. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Reifenberger, G.; Von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef]
- Smoll, N.R.; Schaller, K.; Gautschi, O.P. Long-term survival of patients with glioblastoma multiforme (GBM). J. Clin. Neurosci. 2013, 20, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Krex, D.; Klink, B.; Hartmann, C.; Von Deimling, A.; Pietsch, T.; Simon, M.; Sabel, M.; Steinbach, J.P.; Heese, O.; Reifenberger, G.; et al. Long-term survival with glioblastoma multiforme. Brain 2007, 130, 2596–2606. [Google Scholar] [CrossRef] [PubMed]
- Jovčevska, I. Genetic secrets of long-term glioblastoma survivors. Bosn. J. Basic. Med. Sci. 2019, 19, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Gately, L.; McLachlan, S.A.; Philip, J.; Ruben, J.; Dowling, A. Long-term survivors of glioblastoma: A closer look. J. Neurooncol. 2018, 136, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.R.; Ma, D.J.; Buckner, J.C.; Hammack, J.E. Conditional probability of long-term survival in glioblastoma: A population-based analysis. Cancer 2012, 118, 5608–5613. [Google Scholar] [CrossRef] [PubMed]
- Anselmo, P.; Maranzano, E.; Selimi, A.; Lupattelli, M.; Palumbo, I.; Bini, V.; Casale, M.; Trippa, F.; Bufi, A.; Arcidiacono, F.; et al. Clinical characterization of glioblastoma patients living longer than 2 years: A retrospective analysis of two Italian institutions. Asia Pac. J. Clin. Oncol. 2021, 17, 273–279. [Google Scholar] [CrossRef]
- Gately, L.; McLachlan, S.A.; Dowling, A.; Philip, J. Life beyond a diagnosis of glioblastoma: A systematic review of the literature. J. Cancer Surviv. 2017, 11, 447–452. [Google Scholar] [CrossRef]
- Poon, M.T.C.; Sudlow, C.L.M.; Figueroa, J.D.; Brennan, P.M. Longer-term (≥2 years) survival in patients with glioblastoma in population-based studies pre- and post-2005: A systematic review and meta-analysis. Sci. Rep. 2020, 10, 11622. [Google Scholar] [CrossRef]
- Avants, B.; Tustison, N.; Song, G. Advanced normalization tools (ANTS). Insight J. 2008, 2, 1–35. [Google Scholar]
- Yushkevich, P.A.; Piven, J.; Hazlett, H.C.; Smith, R.G.; Ho, S.; Gee, J.C.; Gerig, G. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage 2006, 31, 1116–1128. [Google Scholar] [CrossRef] [PubMed]
- Henker, C.; Hiepel, M.C.; Kriesen, T.; Scherer, M.; Glass, Ä.; Herold-Mende, C.; Bendszus, M.; Langner, S.; Weber, M.A.; Schneider, B.; et al. Volumetric assessment of glioblastoma and its predictive value for survival. Acta Neurochir. 2019, 161, 1723–1732. [Google Scholar] [CrossRef] [PubMed]
- IBM Corp. IBM SPSS Statistics for Windows, Version 29; IBM Corp: Armonk, NY, USA, 2022. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core: Vienna, Austria, 2022. [Google Scholar]
- Wickham, H. Data analysis. In ggplot2; Springer: Berlin/Heidelberg, Germany, 2016; pp. 189–201. [Google Scholar]
- Mitchell, M.; Muftakhidinov, B.; Winchen, T. Engauge Digitizer Software. 2019. Available online: https://markummitchell.github.io/engauge-digitizer (accessed on 5 February 2023).
- Schwartz, C.; Rühm, A.; Tonn, J.-C.; Kreth, S.; Kreth, F.-W. Surg-25interstitial Photodynamic Therapy of De-Novo Glioblastoma Multiforme Who IV. Neuro-Oncology 2015, 17, v219. [Google Scholar] [CrossRef]
- Epstein, N.E. A review of the risks and benefits of differing prophylaxis regimens for the treatment of deep venous thrombosis and pulmonary embolism in neurosurgery. Surg. Neurol. 2005, 64, 295–301; discussion 302. [Google Scholar] [CrossRef]
- Bartek, J., Jr.; Alattar, A.A.; Dhawan, S.; Ma, J.; Koga, T.; Nakaji, P.; Dusenbery, K.E.; Chen, C.C. Receipt of brachytherapy is an independent predictor of survival in glioblastoma in the Surveillance, Epidemiology, and End Results database. J. Neurooncol. 2019, 145, 75–83. [Google Scholar] [CrossRef]
- Norred, S.E.; Johnson, J.A. Magnetic resonance-guided laser induced thermal therapy for glioblastoma multiforme: A review. Biomed. Res. Int. 2014, 2014, 761312. [Google Scholar] [CrossRef]
- Traylor, J.I.; Patel, R.; Muir, M.; De Almeida Bastos, D.C.; Ravikumar, V.; Kamiya-Matsuoka, C.; Rao, G.; Thomas, J.G.; Kew, Y.; Prabhu, S.S. Laser Interstitial Thermal Therapy for Glioblastoma: A Single-Center Experience. World Neurosurg. 2021, 149, e244–e252. [Google Scholar] [CrossRef]
- Binabaj, M.M.; Bahrami, A.; ShahidSales, S.; Joodi, M.; Joudi Mashhad, M.; Hassanian, S.M.; Anvari, K.; Avan, A. The prognostic value of MGMT promoter methylation in glioblastoma: A meta-analysis of clinical trials. J. Cell. Physiol. 2018, 233, 378–386. [Google Scholar] [CrossRef]
- Weller, M.; Tabatabai, G.; Kästner, B.; Felsberg, J.; Steinbach, J.P.; Wick, A.; Schnell, O.; Hau, P.; Herrlinger, U.; Sabel, M.C.; et al. MGMT Promoter Methylation Is a Strong Prognostic Biomarker for Benefit from Dose-Intensified Temozolomide Rechallenge in Progressive Glioblastoma: The DIRECTOR Trial. Clin. Cancer Res. 2015, 21, 2057–2064. [Google Scholar] [CrossRef]
- Fuster-Garcia, E.; Lorente Estellés, D.; Álvarez-Torres, M.D.M.; Juan-Albarracín, J.; Chelebian, E.; Rovira, A.; Acosta, C.A.; Pineda, J.; Oleaga, L.; Mollá-Olmos, E.; et al. MGMT methylation may benefit overall survival in patients with moderately vascularized glioblastomas. Eur. Radiol. 2021, 31, 1738–1747. [Google Scholar] [CrossRef]
- Hegi, M.E.; Diserens, A.C.; Gorlia, T.; Hamou, M.F.; De Tribolet, N.; Weller, M.; Kros, J.M.; Hainfellner, J.A.; Mason, W.; Mariani, L.; et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N. Engl. J. Med. 2005, 352, 997–1003. [Google Scholar] [CrossRef]
- Gerson, S.L. MGMT: Its role in cancer aetiology and cancer therapeutics. Nat. Rev. Cancer 2004, 4, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Dean, M.; Fojo, T.; Bates, S. Tumour stem cells and drug resistance. Nat. Rev. Cancer 2005, 5, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Yuan, X.; Zeng, Z.; Tunici, P.; Ng, H.; Abdulkadir, I.R.; Lu, L.; Irvin, D.; Black, K.L.; Yu, J.S. Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol. Cancer 2006, 5, 67. [Google Scholar] [CrossRef]
- Salmaggi, A.; Boiardi, A.; Gelati, M.; Russo, A.; Calatozzolo, C.; Ciusani, E.; Sciacca, F.L.; Ottolina, A.; Parati, E.A.; La Porta, C.; et al. Glioblastoma-derived tumorospheres identify a population of tumor stem-like cells with angiogenic potential and enhanced multidrug resistance phenotype. Glia 2006, 54, 850–860. [Google Scholar] [CrossRef]
- Chinot, O.L.; Macdonald, D.R.; Abrey, L.E.; Zahlmann, G.; Kerloëguen, Y.; Cloughesy, T.F. Response assessment criteria for glioblastoma: Practical adaptation and implementation in clinical trials of antiangiogenic therapy. Curr. Neurol. Neurosci. Rep. 2013, 13, 347. [Google Scholar] [CrossRef]
- Wen, P.Y.; Macdonald, D.R.; Reardon, D.A.; Cloughesy, T.F.; Sorensen, A.G.; Galanis, E.; Degroot, J.; Wick, W.; Gilbert, M.R.; Lassman, A.B.; et al. Updated response assessment criteria for high-grade gliomas: Response assessment in neuro-oncology working group. J. Clin. Oncol. 2010, 28, 1963–1972. [Google Scholar] [CrossRef]
- Albert, F.K.; Forsting, M.; Sartor, K.; Adams, H.P.; Kunze, S. Early postoperative magnetic resonance imaging after resection of malignant glioma: Objective evaluation of residual tumor and its influence on regrowth and prognosis. Neurosurgery 1994, 34, 45–60; discussion 60–61. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Liang, H.; Cheng, P.; Yang, H.; Zhao, P. Gross Total vs. Subtotal Resection on Survival Outcomes in Elderly Patients With High-Grade Glioma: A Systematic Review and Meta-Analysis. Front. Oncol. 2020, 10, 151. [Google Scholar] [CrossRef]
- Sanai, N.; Polley, M.Y.; McDermott, M.W.; Parsa, A.T.; Berger, M.S. An extent of resection threshold for newly diagnosed glioblastomas. J. Neurosurg. 2011, 115, 3–8. [Google Scholar] [CrossRef]
- Stummer, W.; Reulen, H.J.; Meinel, T.; Pichlmeier, U.; Schumacher, W.; Tonn, J.C.; Rohde, V.; Oppel, F.; Turowski, B.; Woiciechowsky, C.; et al. Extent of resection and survival in glioblastoma multiforme: Identification of and adjustment for bias. Neurosurgery 2008, 62, 564–576; discussion 564–576. [Google Scholar] [CrossRef]
- Kreth, F.W.; Thon, N.; Simon, M.; Westphal, M.; Schackert, G.; Nikkhah, G.; Hentschel, B.; Reifenberger, G.; Pietsch, T.; Weller, M.; et al. Gross total but not incomplete resection of glioblastoma prolongs survival in the era of radiochemotherapy. Ann. Oncol. 2013, 24, 3117–3123. [Google Scholar] [CrossRef]
- Lacroix, M.; Abi-Said, D.; Fourney, D.R.; Gokaslan, Z.L.; Shi, W.; DeMonte, F.; Lang, F.F.; McCutcheon, I.E.; Hassenbusch, S.J.; Holland, E.; et al. A multivariate analysis of 416 patients with glioblastoma multiforme: Prognosis, extent of resection, and survival. J. Neurosurg. 2001, 95, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Lu, Z.R. Gadolinium-based contrast agents for magnetic resonance cancer imaging. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2013, 5, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Kiviniemi, A.; Gardberg, M.; Ek, P.; Frantzén, J.; Bobacka, J.; Minn, H. Gadolinium retention in gliomas and adjacent normal brain tissue: Association with tumor contrast enhancement and linear/macrocyclic agents. Neuroradiology 2019, 61, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Hirschberg, H.; Uzal, F.A.; Chighvinadze, D.; Zhang, M.J.; Peng, Q.; Madsen, S.J. Disruption of the blood-brain barrier following ALA-mediated photodynamic therapy. Lasers Surg. Med. 2008, 40, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Brandsma, D.; Van den Bent, M.J. Pseudoprogression and pseudoresponse in the treatment of gliomas. Curr. Opin. Neurol. 2009, 22, 633–638. [Google Scholar] [CrossRef]
- Kumar, A.J.; Leeds, N.E.; Fuller, G.N.; Van Tassel, P.; Maor, M.H.; Sawaya, R.E.; Levin, V.A. Malignant gliomas: MR imaging spectrum of radiation therapy- and chemotherapy-induced necrosis of the brain after treatment. Radiology 2000, 217, 377–384. [Google Scholar] [CrossRef]
- Brandsma, D.; Stalpers, L.; Taal, W.; Sminia, P.; Van den Bent, M.J. Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol. 2008, 9, 453–461. [Google Scholar] [CrossRef]
- Dolmans, D.E.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef]
- Schwake, M.; Nemes, A.; Dondrop, J.; Schroeteler, J.; Schipmann, S.; Senner, V.; Stummer, W.; Ewelt, C. In-Vitro Use of 5-ALA for Photodynamic Therapy in Pediatric Brain Tumors. Neurosurgery 2018, 83, 1328–1337. [Google Scholar] [CrossRef]
- Karmakar, S.; Banik, N.L.; Patel, S.J.; Ray, S.K. 5-Aminolevulinic acid-based photodynamic therapy suppressed survival factors and activated proteases for apoptosis in human glioblastoma U87MG cells. Neurosci. Lett. 2007, 415, 242–247. [Google Scholar] [CrossRef] [PubMed]
- De Bonis, P.; Anile, C.; Pompucci, A.; Fiorentino, A.; Balducci, M.; Chiesa, S.; Lauriola, L.; Maira, G.; Mangiola, A. The influence of surgery on recurrence pattern of glioblastoma. Clin. Neurol. Neurosurg. 2013, 115, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.C.; Veeravagu, A.; Hsu, A.R.; Tse, V.C. Recurrent glioblastoma multiforme: A review of natural history and management options. Neurosurg. Focus 2006, 20, E5. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, L.E.; Fisher, B.J.; Macdonald, D.R.; LeBer, D.V.; Halperin, E.C.; Schold, S.C., Jr.; Cairncross, J.G. Supratentorial malignant glioma: Patterns of recurrence and implications for external beam local treatment. Int. J. Radiat. Oncol. Biol. Phys. 1992, 24, 55–57. [Google Scholar] [CrossRef] [PubMed]
- Hess, C.F.; Schaaf, J.C.; Kortmann, R.D.; Schabet, M.; Bamberg, M. Malignant glioma: Patterns of failure following individually tailored limited volume irradiation. Radiother. Oncol. 1994, 30, 146–149. [Google Scholar] [CrossRef]
- Lee, S.W.; Fraass, B.A.; Marsh, L.H.; Herbort, K.; Gebarski, S.S.; Martel, M.K.; Radany, E.H.; Lichter, A.S.; Sandler, H.M. Patterns of failure following high-dose 3-D conformal radiotherapy for high-grade astrocytomas: A quantitative dosimetric study. Int. J. Radiat. Oncol. Biol. Phys. 1999, 43, 79–88. [Google Scholar] [CrossRef]
- Lohmann, P.; Stavrinou, P.; Lipke, K.; Bauer, E.K.; Ceccon, G.; Werner, J.M.; Neumaier, B.; Fink, G.R.; Shah, N.J.; Langen, K.J.; et al. FET PET reveals considerable spatial differences in tumour burden compared to conventional MRI in newly diagnosed glioblastoma. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 591–602. [Google Scholar] [CrossRef]
- Schucht, P.; Knittel, S.; Slotboom, J.; Seidel, K.; Murek, M.; Jilch, A.; Raabe, A.; Beck, J. 5-ALA complete resections go beyond MR contrast enhancement: Shift corrected volumetric analysis of the extent of resection in surgery for glioblastoma. Acta Neurochir. 2014, 156, 305–312; discussion 312. [Google Scholar] [CrossRef]
- Laws, E.R.; Parney, I.F.; Huang, W.; Anderson, F.; Morris, A.M.; Asher, A.; Lillehei, K.O.; Bernstein, M.; Brem, H.; Sloan, A.; et al. Survival following surgery and prognostic factors for recently diagnosed malignant glioma: Data from the Glioma Outcomes Project. J. Neurosurg. 2003, 99, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Li, S.W.; Qiu, X.G.; Chen, B.S.; Zhang, W.; Ren, H.; Wang, Z.C.; Jiang, T. Prognostic factors influencing clinical outcomes of glioblastoma multiforme. Chin. Med. J. Engl. 2009, 122, 1245–1249. [Google Scholar] [PubMed]
- Kaneko, S. Photodynamic therapy for human malignant gliomas. J. Jpn. Soc. Laser Surg. Med. 2011, 32, 131–138. [Google Scholar] [CrossRef]
- Van Veen, R.L.P.; Robinson, D.J.; Sterenborg, H.; Aans, J.B.; Tan, I.B.; Vrieze, O.H.; Hoebers, F.; Witjes, M.J.H.; Levendag, P.C. Treatment planning for Interstitial Photodynamic Therapy for head and neck cancer. Head Neck Oncol. 2010, 2, O45. [Google Scholar] [CrossRef]
- Leroy, H.A.; Baert, G.; Guerin, L.; Delhem, N.; Mordon, S.; Reyns, N.; Vignion-Dewalle, A.S. Interstitial Photodynamic Therapy for Glioblastomas: A Standardized Procedure for Clinical Use. Cancers 2021, 13, 5754. [Google Scholar] [CrossRef] [PubMed]
- Dupont, C.; Betrouni, N.; Mordon, S.; Reyns, N.; Vermandel, M. 5-ALA Photodynamic Therapy in Neurosurgery, Towards the Design of a Treatment Planning System: A Proof of Concept. Innov. Res. BioMed. Eng. 2016, 38, 34–41. [Google Scholar] [CrossRef]
- Shafirstein, G.; Bellnier, D.; Oakley, E.; Hamilton, S.; Potasek, M.; Beeson, K.; Parilov, E. Interstitial Photodynamic Therapy—A Focused Review. Cancers 2017, 9, 12. [Google Scholar] [CrossRef]
- Yassine, A.A.; Lilge, L.; Betz, V. Optimizing interstitial photodynamic therapy with custom cylindrical diffusers. J. Biophotonics 2019, 12, e201800153. [Google Scholar] [CrossRef]
- Yassine, A.A.; Lilge, L.; Betz, V. Optimizing Interstitial Photodynamic Therapy Planning with Reinforcement Learning-Based Diffuser Placement. IEEE Trans. Biomed. Eng. 2021, 68, 1668–1679. [Google Scholar] [CrossRef]
- Yassine, A.A.; Kingsford, W.; Xu, Y.; Cassidy, J.; Lilge, L.; Betz, V. Automatic interstitial photodynamic therapy planning via convex optimization. Biomed. Opt. Express 2018, 9, 898–920. [Google Scholar] [CrossRef]
- Molinaro, A.M.; Hervey-Jumper, S.; Morshed, R.A.; Young, J.; Han, S.J.; Chunduru, P.; Zhang, Y.; Phillips, J.J.; Shai, A.; Lafontaine, M.; et al. Association of Maximal Extent of Resection of Contrast-Enhanced and Non-Contrast-Enhanced Tumor with Survival Within Molecular Subgroups of Patients With Newly Diagnosed Glioblastoma. JAMA Oncol. 2020, 6, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Rühm, A.; Stepp, H.; Beyer, W.; Hennig, G.; Pongratz, T.; Sroka, R.; Schnell, O.; Tonn, J.-C.; Kreth, F.-W. 5-ALA based photodynamic management of glioblastoma. SPIE 2014, 8928, 69–75. [Google Scholar]
- Leao, D.J.; Craig, P.G.; Godoy, L.F.; Leite, C.C.; Policeni, B. Response Assessment in Neuro-Oncology Criteria for Gliomas: Practical Approach Using Conventional and Advanced Techniques. AJNR Am. J. Neuroradiol. 2020, 41, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Bar, E.E.; Lin, A.; Mahairaki, V.; Matsui, W.; Eberhart, C.G. Hypoxia increases the expression of stem-cell markers and promotes clonogenicity in glioblastoma neurospheres. Am. J. Pathol. 2010, 177, 1491–1502. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.S.; Han, J.; Shi, H.; Koo, S.; Singh, H.; Kim, H.-J.; Sessler, J.L.; Lee, J.Y.; Kim, J.-H.; Kim, J.S. Overcoming the Limits of Hypoxia in Photodynamic Therapy: A Carbonic Anhydrase IX-Targeted Approach. J. Am. Chem. Soc. 2017, 139, 7595–7602. [Google Scholar] [CrossRef]
- Chelakkot, V.S.; Liu, K.; Yoshioka, E.; Saha, S.; Xu, D.; Licursi, M.; Dorward, A.; Hirasawa, K. MEK reduces cancer-specific PpIX accumulation through the RSK-ABCB1 and HIF-1α-FECH axes. Sci. Rep. 2020, 10, 22124. [Google Scholar] [CrossRef]
- Müller, P.; Abdel Gaber, S.A.; Zimmermann, W.; Wittig, R.; Stepp, H. ABCG2 influence on the efficiency of photodynamic therapy in glioblastoma cells. J. Photochem. Photobiol. B 2020, 210, 111963. [Google Scholar] [CrossRef]
- Abdel Gaber, S.A.; Müller, P.; Zimmermann, W.; Hüttenberger, D.; Wittig, R.; Abdel Kader, M.H.; Stepp, H. ABCG2-mediated suppression of chlorin e6 accumulation and photodynamic therapy efficiency in glioblastoma cell lines can be reversed by KO143. J. Photochem. Photobiol. B 2018, 178, 182–191. [Google Scholar] [CrossRef]
- Schimanski, A.; Ebbert, L.; Sabel, M.C.; Finocchiaro, G.; Lamszus, K.; Ewelt, C.; Etminan, N.; Fischer, J.C.; Sorg, R.V. Human glioblastoma stem-like cells accumulate protoporphyrin IX when subjected to exogenous 5-aminolaevulinic acid, rendering them sensitive to photodynamic treatment. J. Photochem. Photobiol. B 2016, 163, 203–210. [Google Scholar] [CrossRef]
- Fahey, J.M.; Emmer, J.V.; Korytowski, W.; Hogg, N.; Girotti, A.W. Antagonistic Effects of Endogenous Nitric Oxide in a Glioblastoma Photodynamic Therapy Model. Photochem. Photobiol. 2016, 92, 842–853. [Google Scholar] [CrossRef]
- Madsen, S.J.; Sun, C.H.; Tromberg, B.J.; Hirschberg, H. Repetitive 5-aminolevulinic acid-mediated photodynamic therapy on human glioma spheroids. J. Neurooncol. 2003, 62, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Casas, A.; Perotti, C.; Ortel, B.; Di Venosa, G.; Saccoliti, M.; Batlle, A.; Hasan, T. Tumor cell lines resistant to ALA-mediated photodynamic therapy and possible tools to target surviving cells. Int. J. Oncol. 2006, 29, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Miretti, M.; González Graglia, M.A.; Suárez, A.I.; Prucca, C.G. Photodynamic therapy for glioblastoma: A light at the end of the tunnel. J. Photochem. Photobiol. 2023, 13, 100161. [Google Scholar] [CrossRef]
- Fujita, Y.; Nagashima, H.; Tanaka, K.; Hashiguchi, M.; Itoh, T.; Sasayama, T. Hyperintense signal on diffusion-weighted imaging for monitoring the acute response and local recurrence after photodynamic therapy in malignant gliomas. J. Neurooncol. 2021, 155, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, K.; Sheehan, D.; Sulaiman, M.; Padilla, F.; Moore, D.; Sheehan, J.; Xu, Z. Investigation of the tumoricidal effects of sonodynamic therapy in malignant glioblastoma brain tumors. J. Neurooncol. 2020, 148, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Liu, Y.; Cao, Y.; Liu, Z. Engineering Macrophage Exosome Disguised Biodegradable Nanoplatform for Enhanced Sonodynamic Therapy of Glioblastoma. Adv. Mater. 2022, 34, e2110364. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, J.; Liu, W.; Zheng, X.; Zhang, W.; Lee, C.S.; Wang, P. A novel hypocrellin-based assembly for sonodynamic therapy against glioblastoma. J. Mater. Chem. B 2021, 10, 57–63. [Google Scholar] [CrossRef]
- Guo, Q.L.; Dai, X.L.; Yin, M.Y.; Cheng, H.W.; Qian, H.S.; Wang, H.; Zhu, D.M.; Wang, X.W. Nanosensitizers for sonodynamic therapy for glioblastoma multiforme: Current progress and future perspectives. Mil. Med. Res. 2022, 9, 26. [Google Scholar] [CrossRef]
- Suehiro, S.; Ohnishi, T.; Yamashita, D.; Kohno, S.; Inoue, A.; Nishikawa, M.; Ohue, S.; Tanaka, J.; Kunieda, T. Enhancement of antitumor activity by using 5-ALA-mediated sonodynamic therapy to induce apoptosis in malignant gliomas: Significance of high-intensity focused ultrasound on 5-ALA-SDT in a mouse glioma model. J. Neurosurg. 2018, 129, 1416–1428. [Google Scholar] [CrossRef]
- Omuro, A. Immune-checkpoint inhibitors for glioblastoma: What have we learned? Arq. Neuropsiquiatr. 2022, 80, 266–269. [Google Scholar] [CrossRef]
- Medikonda, R.; Dunn, G.; Rahman, M.; Fecci, P.; Lim, M. A review of glioblastoma immunotherapy. J. Neurooncol. 2021, 151, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Yang, J.; Ji, J.; Wang, P.; Zhang, L.; Yan, G.; Wu, Y.; Chen, Q.; Liu, J.; Zhang, G.; et al. PD-L1 blockade potentiates the antitumor effects of ALA-PDT and optimizes the tumor microenvironment in cutaneous squamous cell carcinoma. Oncoimmunology 2022, 11, 2061396. [Google Scholar] [CrossRef] [PubMed]
- Werner, M.; Lyu, C.; Stadlbauer, B.; Schrader, I.; Buchner, A.; Stepp, H.; Sroka, R.; Pohla, H. The role of Shikonin in improving 5-aminolevulinic acid-based photodynamic therapy and chemotherapy on glioblastoma stem cells. Photodiagn. Photodyn. Ther. 2022, 39, 102987. [Google Scholar] [CrossRef] [PubMed]
| IPDT No. (⟳ PFS, † OS) | Sex (m/f) | Age at PDT (Years) | PFS (Months) | OS (Months) | PPS (Months) | Status |
|---|---|---|---|---|---|---|
| IPDT 01 (⟳ 65, † 102) | m | 29.7 | 64.7 | 102.4 | 37.4 | † |
| IPDT 02 (⟳ 59, † 95) | m | 40.6 | 59.2 | 95.0 | 35.5 | † |
| IPDT 03 (128) | f | 50.3 | 127.1 * | 127.5 * | - | A |
| IPDT 04 (⟳ 8, † 15) | m | 69.9 | 8.3 | 15.0 | 6.3 | † |
| IPDT 05 (⟳ 12, † 16) | m | 68.2 | 12.0 | 16.1 | 3.5 | † |
| IPDT 06 (⟳ 4, † 9) | m | 63.7 | 4.3 | 9.0 | 3.8 | † |
| IPDT 07 (110) | m | 70.1 | 110.1 * | 110.3 * | - | A |
| IPDT 08 (⟳ 61, † 66) | f | 74.1 | 60.6 | 66.4 | 5.4 | † |
| IPDT 09 (114) | m | 33.3 | 113.6 * | 113.9 * | - | A |
| IPDT 10 (⟳ 16, † 28) | f | 74.3 | 16.4 | 28.0 | 10.9 | † |
| IPDT 11 (⟳ 6, † 9) | m | 68.8 | 6.0 | 8.5 | 2.1 | † |
| IPDT 12 (⟳ 7, † 8) | m | 68.0 | 6.5 | 8.0 | 1.2 | †\REC |
| IPDT 13 (⟳ 10, † 25) | f | 57.3 | 9.5 | 25.2 | 15.3 | † |
| IPDT 14 (⟳ 36, † 44) | m | 54.3 | 35.7 | 43.9 | 7.8 | † |
| IPDT 15 (⟳ 7, † 9) | m | 76.5 | 7.4 | 9.2 | 1.5 | † |
| IPDT 16 (⟳ 18, † 36) | m | 53.4 | 17.8 | 36.4 | 18.2 | † |
| Median | 65.8 | 16.4 a | 28.0 a | 6.3 a | ||
| Average | 59.5 | 43.1 a | 52.9 a | 11.5 a | ||
| Max | 76.5 | 127.1 | 127.5 | 37.4 | ||
| Min | 29.7 | 4.3 | 8.0 | 1.2 |
| IPDT No. (⟳ PFS, † OS) | MGMT Methylation b | IDH1 | IDH2 | Ki67 | Tumor location | Side | TMZ during Radiation | TMZ Cycles after Radiation |
|---|---|---|---|---|---|---|---|---|
| IPDT 01 (⟳ 65, † 102) | yes | yes | no | n/a | frontal supraventricular | right | yes | 7 |
| IPDT 02 (⟳ 59, † 95) | no | no | no | 10% | temporoparietal | left | partially | 2 |
| IPDT 03 (128) | yes | no | no | 10% | temporo-occipital | left | yes | 9 |
| IPDT 04 (⟳ 8, † 15) | no | no | no | 30% | temporal | left | yes | 0 |
| IPDT 05 (⟳ 12, † 16) | no | no | no | 10–15% | frontal | left | yes | 2 |
| IPDT 06 (⟳ 4, † 9) | no | no | no | 20% | temporal | right | yes | 0 |
| IPDT 07 (110) | yes | no | no | 25% | temporal | left | yes | 0 |
| IPDT 08 (⟳ 61, † 66) | no | no | no | 25% | median frontal gyrus | left | yes | 3 |
| IPDT 09 (114) | partially | yes | no | 85% | temporal | left | yes | 4 |
| IPDT 10 (⟳ 16, † 28) | no | no | no | 30% | central gyrus and subcentral lobe | left | yes | 0 |
| IPDT 11 (⟳ 6, † 9) | no | no | no | 21% | superficial parietal gyrus | left | no | 0 |
| IPDT 12 (⟳ 7, † 8) | yes | no | no | 7% | parieto-occipital | left | yes | 2 |
| IPDT 13 (⟳ 10, † 25) | partially | no | no | 15% | temporoparietal | left | partially | 4 |
| IPDT 14 (⟳ 36, † 44) | yes | no | no | 15% | parieto-occipital | left | partially | 8 |
| IPDT 15 (⟳ 7, † 9) | no | no | no | 10% | temporal/parietal | left | n/a | n/a |
| IPDT 16 (⟳ 18, † 36) | yes | no | no | 28% | parietal | left | yes | 5 |
| iPDT All Cases | iPDT MGMT Unmethylated | iPDT MGMT Methylated | Stupp [2] c | Chemoradiation [10] d | CyberKnife® [12] d | Stereotactic Radiosurgery [11] c | ||
|---|---|---|---|---|---|---|---|---|
| Subjects (n) | 16 | 8 | 8 | 287 | 56 | 12 | 30 | |
| PFS (months) | Median [95% CI] | 16.4 [5.1; 27.6] | 8.3 [1.9; 14.7] | 35.7 [0.0; 100.7] | 6.9 [5.8; 8.2] | 8.0 [5.6; 10.4] | 16.0 [10.4; 21.6] | 8.2 [4.6; 10.5] |
| OS (months) | Median [95% CI] | 28.0 [6.0; 50.0] | 15.0 [5.3; 24.7] | 43.9 [0.0; 135.4] | 14.6 [13.2; 16.8] | 12.0 [9.6; 14.4] | 18.0 [10.9; 25.1] | 14.8 [10.9; 19.9] |
| PPS (months) | Median [95% CI] | 6.3 [1.6; 11.0] | 3.8 [1.3; 6.4] | 15.3 [0.0; 31.4] | n/a | 4.0 [3.0; 5.0] | 3.0 [2.0; 4.0] | n/a |
| iPDT MGMT Methylated vs. Unmethylated | iPDT vs. Stupp [2] | iPDT vs. Chemoradiation [10] | iPDT vs. CyberKnife® [12] | iPDT vs. Stereotactic Radiosurgery [11] | |
|---|---|---|---|---|---|
| PFS | 0.030 | <0.001 | 0.005 | 0.341 | n/a |
| OS | 0.031 | 0.017 | 0.022 | 0.280 | 0.036 |
| PPS | 0.192 | n/a | 0.516 | 0.237 | n/a |
| Volume | Tumor Pre iPDT (mm³) | CE Pre iPDT (mm³) | Necrosis Pre iPDT (mm³) | CE 1 Day Post iPDT (mm³) | iPDT Remnant 1 Day Post iPDT (mm³) | iPDT Remnant 3 Months Post iPDT (mm³) | iPDT Remnant 3 Months Pre Recurrence (mm³) | iPDT Remnant at Recurrence (mm³) | Recurrent Tumor at Recurrence (mm³) |
|---|---|---|---|---|---|---|---|---|---|
| Median | 6063 | 3533 | 1350 | 535 | 12,426 | 5585 | 4036 | 3914 | 5893 |
| Average | 7622 | 5180 | 2442 | 1108 | 19,166 | 7151 | 4474 | 4556 | 6645 |
| Max | 21,825 | 12,362 | 9463 | 4720 | 66,857 | 20,027 | 12,254 | 9607 | 17,177 |
| Min | 1362 | 878 | 118 | 0 | 2062 | 370 | 305 | 157 | 6 |
| SD | 5849 | 3761 | 2579 | 1463 | 16,571 | 5395 | 3188 | 2917 | 6115 |
| iPDT | Chemoradiation [56] | |||
|---|---|---|---|---|
| MGMT Unmethylated | MGMT Methylated | MGMT Unmethylated e | MGMT Methylated e | |
| PFS median (months) | 8.3 | 35.7 | 5.3 | 10.3 |
| OS median (months) | 15.0 | 43.9 | 12.7 | 21.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foglar, M.; Aumiller, M.; Bochmann, K.; Buchner, A.; El Fahim, M.; Quach, S.; Sroka, R.; Stepp, H.; Thon, N.; Forbrig, R.; et al. Interstitial Photodynamic Therapy of Glioblastomas: A Long-Term Follow-up Analysis of Survival and Volumetric MRI Data. Cancers 2023, 15, 2603. https://doi.org/10.3390/cancers15092603
Foglar M, Aumiller M, Bochmann K, Buchner A, El Fahim M, Quach S, Sroka R, Stepp H, Thon N, Forbrig R, et al. Interstitial Photodynamic Therapy of Glioblastomas: A Long-Term Follow-up Analysis of Survival and Volumetric MRI Data. Cancers. 2023; 15(9):2603. https://doi.org/10.3390/cancers15092603
Chicago/Turabian StyleFoglar, Marco, Maximilian Aumiller, Katja Bochmann, Alexander Buchner, Mohamed El Fahim, Stefanie Quach, Ronald Sroka, Herbert Stepp, Niklas Thon, Robert Forbrig, and et al. 2023. "Interstitial Photodynamic Therapy of Glioblastomas: A Long-Term Follow-up Analysis of Survival and Volumetric MRI Data" Cancers 15, no. 9: 2603. https://doi.org/10.3390/cancers15092603
APA StyleFoglar, M., Aumiller, M., Bochmann, K., Buchner, A., El Fahim, M., Quach, S., Sroka, R., Stepp, H., Thon, N., Forbrig, R., & Rühm, A. (2023). Interstitial Photodynamic Therapy of Glioblastomas: A Long-Term Follow-up Analysis of Survival and Volumetric MRI Data. Cancers, 15(9), 2603. https://doi.org/10.3390/cancers15092603







