A Multicenter, Open-Label, Phase I/II Study of FN-1501 in Patients with Advanced Solid Tumors
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
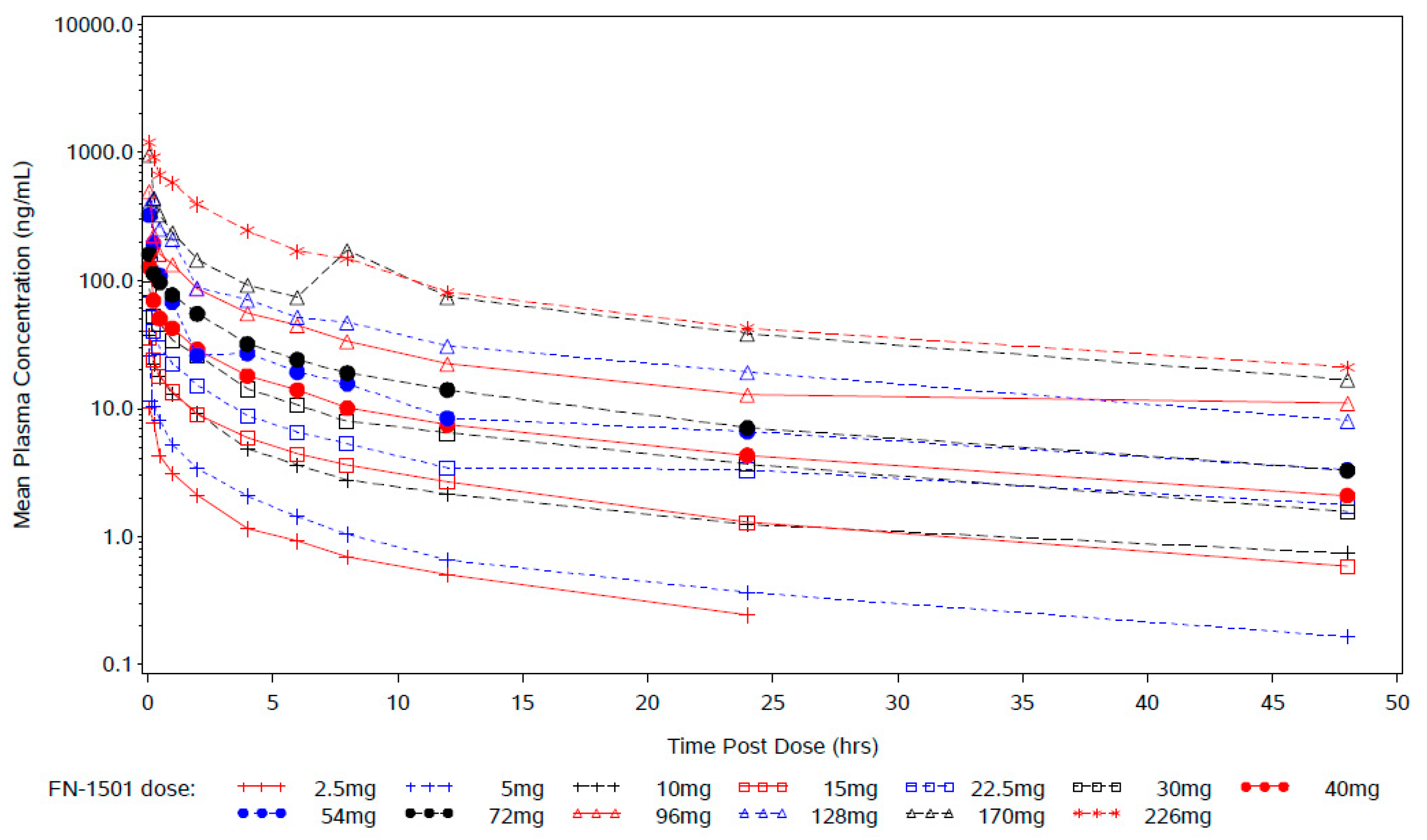
2.2. Pharmacokinetics
2.3. Statistical Methods
3. Results
3.1. Patient Disposition
3.2. Demography and Baseline Characteristics
3.3. Safety and Tolerability
3.4. Pharmacokinetic Profile of FN-1501
3.5. Efficacy Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Du, Z.; Lovly, C.M. Mechanisms of receptor tyrosine kinase activation in cancer. Mol. Cancer 2018, 17, 58. [Google Scholar] [CrossRef]
- Lemmon, M.A.; Schlessinger, J. Cell signaling by receptor tyrosine kinases. Cell 2010, 141, 1117–1134. [Google Scholar] [CrossRef] [PubMed]
- McDonell, L.M.; Kernohan, K.D.; Boycott, K.M.; Sawyer, S.L. Receptor tyrosine kinase mutations in developmental syndromes and cancer: Two sides of the same coin. Hum. Mol. Genet. 2015, 24, R60–R66. [Google Scholar] [CrossRef] [PubMed]
- Li, E.; Hristova, K. Role of receptor tyrosine kinase transmembrane domains in cell signaling and human pathologies. Biochemistry 2006, 45, 6241–6251. [Google Scholar] [CrossRef] [PubMed]
- Grafone, T.; Palmisano, M.; Nicci, C.; Storti, S. An overview on the role of FLT3-tyrosine kinase receptor in acute myeloid leukemia: Biology and treatment. Oncol. Rev. 2012, 6, e8. [Google Scholar] [CrossRef] [PubMed]
- Parcells, B.W.; Ikeda, A.K.; Simms-Waldrip, T.; Moore, T.B.; Sakamoto, K.M. FMS-like tyrosine kinase 3 in normal hematopoiesis and acute myeloid leukemia. Stem Cells 2006, 24, 1174–1184. [Google Scholar] [CrossRef]
- Nitika; Wei, J.; Hui, A.-M. Role of Biomarkers in FLT3 AML. Cancers 2022, 14, 1164. [Google Scholar] [CrossRef]
- Zheng, R.; Bailey, E.; Nguyen, B.; Yang, X.; Piloto, O.; Levis, M.; Small, D. Further activation of FLT3 mutants by FLT3 ligand. Oncogene 2011, 30, 4004–4014. [Google Scholar] [CrossRef]
- Kiyoi, H.; Kawashima, N.; Ishikawa, Y. FLT3 mutations in acute myeloid leukemia: Therapeutic paradigm beyond inhibitor development. Cancer Sci. 2020, 111, 312–322. [Google Scholar] [CrossRef]
- Kennedy, V.E.; Smith, C.C. FLT3 Mutations in Acute Myeloid Leukemia: Key Concepts and Emerging Controversies. Front. Oncol. 2020, 10, 612880. [Google Scholar] [CrossRef]
- Lim, S.H.; Kim, S.Y.; Kim, K.; Jang, H.; Ahn, S.; Kim, K.M.; Kim, N.K.; Park, W.; Lee, S.J.; Kim, S.T.; et al. The implication of FLT3 amplification for FLT targeted therapeutics in solid tumors. Oncotarget 2017, 8, 3237–3245. [Google Scholar] [CrossRef] [PubMed]
- AACR Project Genie Consortium; AACR Project GENIE Consortium; André, F.; Arnedos, M.; Baras, A.S.; Baselga, J.; Bedard, P.L.; Berger, M.F.; Bierkens, M.; Calvo, F.; et al. AACR Project GENIE: Powering Precision Medicine through an International Consortium. Cancer Discov. 2017, 7, 818–831. [Google Scholar] [CrossRef]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Short, N.J.; Kantarjian, H.; Ravandi, F.; Daver, N. Emerging treatment paradigms with FLT3 inhibitors in acute myeloid leukemia. Ther. Adv. Hematol. 2019, 10, 2040620719827310. [Google Scholar] [CrossRef]
- Muller, J.P.; Schmidt-Arras, D. Novel Approaches to Target Mutant FLT3 Leukaemia. Cancers 2020, 12, 2806. [Google Scholar] [CrossRef]
- Gebru, M.T.; Wang, H.-G. Therapeutic targeting of FLT3 and associated drug resistance in acute myeloid leukemia. J. Hematol. Oncol. 2020, 13, 155. [Google Scholar] [CrossRef]
- Capelli, D.; Menotti, D.; Fiorentini, A.; Saraceni, F.; Olivieri, A. Overcoming Resistance: FLT3 Inhibitors Past, Present, Future and the Challenge of Cure. Cancers 2022, 14, 4315. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.-S.; Liu, S.-B.; Xue, S.-L. Developments and challenges of FLT3 inhibitors in acute myeloid leukemia. Front. Oncol. 2022, 12, 996438. [Google Scholar] [CrossRef]
- Michaelis, J.; Grabbert, M.; Sigle, A.; Yilmaz, M.; Schlager, D.; Gratzke, C.; Miernik, A.; Schoeb, D.S. Tyrosine Kinase Inhibitors in the Treatment of Metastasised Renal Cell Carcinoma-Future or the Past? Cancers 2022, 14, 3777. [Google Scholar] [CrossRef]
- Yang, Y.; Li, S.; Wang, Y.; Zhao, Y.; Li, Q. Protein tyrosine kinase inhibitor resistance in malignant tumors: Molecular mechanisms and future perspective. Signal Transduct. Target. Ther. 2022, 7, 329. [Google Scholar] [CrossRef]
- Rollig, C.; Serve, H.; Noppeney, R.; Hanoun, M.; Krug, U.; Baldus, C.D.; Brandts, C.H.; Kunzmann, V.; Einsele, H.; Krämer, A.; et al. Sorafenib or placebo in patients with newly diagnosed acute myeloid leukaemia: Long-term follow-up of the randomized controlled SORAML trial. Leukemia 2021, 35, 2517–2525. [Google Scholar] [CrossRef] [PubMed]
- Röllig, C.; Serve, H.; Hüttmann, A.; Noppeney, R.; Müller-Tidow, C.; Krug, U.; Baldus, C.D.; Brandts, C.H.; Kunzmann, V.; Einsele, H.; et al. Addition of sorafenib versus placebo to standard therapy in patients aged 60 years or younger with newly diagnosed acute myeloid leukaemia (SORAML): A multicentre, phase 2, randomised controlled trial. Lancet Oncol. 2015, 16, 1691–1699. [Google Scholar] [CrossRef]
- Al-Rajabi, R.M.d.T.; Richardson, G.E.; Uprety, D.; Williamson, S.K.; Hamid, A.; Baranda, J.C.; Mamdani, H.; Lee, Y.; Li, C.; Nitika; et al. A multicenter, open-label, phase I/II study of FN-1501 in patients with advanced solid tumors and acute myeloid leukemia. J. Clin. Oncol. 2022, 40 (Suppl. 16), e15083. [Google Scholar] [CrossRef]
- Wander, S.A.; Levis, M.J.; Fathi, A.T. The evolving role of FLT3 inhibitors in acute myeloid leukemia: Quizartinib and beyond. Ther. Adv. Hematol. 2014, 5, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Antar, A.I.; Otrock, Z.K.; Jabbour, E.; Mohty, M.; Bazarbachi, A. FLT3 inhibitors in acute myeloid leukemia: Ten frequently asked questions. Leukemia 2020, 34, 682–696. [Google Scholar] [CrossRef] [PubMed]
- Knight, T.E.; Edwards, H.; Meshinchi, S.; Taub, J.W.; Ge, Y. “FLipping” the Story: FLT3-Mutated Acute Myeloid Leukemia and the Evolving Role of FLT3 Inhibitors. Cancers 2022, 14, 3398. [Google Scholar] [CrossRef]
- Cerchione, C.; Peleteiro Raíndo, A.; Mosquera Orgueira, A.; Mosquera Torre, A.; Bao Pérez, L.; Marconi, G.; Isidori, A.; Perez Encinas, M.M.; Martinelli, G. Safety of FLT3 inhibitors in patients with acute myeloid leukemia. Expert Rev. Hematol. 2021, 14, 851–865. [Google Scholar] [CrossRef] [PubMed]
- Levis, M.; Perl, A.E. Gilteritinib: Potent targeting of FLT3 mutations in AML. Blood Adv. 2020, 4, 1178–1191. [Google Scholar] [CrossRef]
- Tarver, T.C.; Hill, J.E.; Rahmat, L.; Perl, A.E.; Bahceci, E.; Mori, K.; Smith, C.C. Gilteritinib is a clinically active FLT3 inhibitor with broad activity against FLT3 kinase domain mutations. Blood Adv. 2020, 4, 514–524. [Google Scholar] [CrossRef]

| Dose Level | Dose | Number of Patients | Dose-Limiting Toxicity |
|---|---|---|---|
| 1 | 2.5 mg/day | 4 | 0 |
| 2 | 5 mg/day | 3 | 0 |
| 3 | 10 mg/day | 5 | 0 |
| 4 | 15 mg/day | 3 | 0 |
| 5 | 22.5 mg/day | 4 | 0 |
| 6 | 30 mg/day | 3 | 0 |
| 7 | 40 mg/day | 4 | 0 |
| 8 | 54 mg/day | 3 | 0 |
| 9 | 72 mg/day | 3 | 0 |
| 10 | 96 mg/day | 5 | 0 |
| 11 | 128 mg/day | 3 | 0 |
| 12 | 170 mg/day | 4 | 0 |
| 13 | 226 mg/day | 4 | 2 * |
| Characteristic | Number | Percentage |
|---|---|---|
| All | 48 | 100 |
| Gender | ||
| Male | 21 | 44 |
| Female | 27 | 56 |
| Ethnicity | ||
| Caucasian | 41 | 85 |
| African American | 5 | 10 |
| Asian | 2 | 4 |
| Median age, years (range) | 65 (30–92) | |
| Primary tumor | ||
| Ovarian | 9 | 19 |
| Colorectal | 7 | 15 |
| Endometrial | 3 | 6 |
| Gastric | 2 | 4 |
| Head/neck | 1 | 2 |
| Uterine | 1 | 2 |
| AML | 1 | 2 |
| Other | 10 | 21 |
| Median number of prior therapies (range) | 5 (1–12) |
| FN-1501 Dose (mg/day) IV TIW × 2 Weeks in 21 Day Cycle | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2.5 (N = 4) | 5 (N = 3) | 10 (N = 5) | 15 (N = 3) | 22.5 (N = 4) | 30 (N = 3) | 40 (N = 4) | 54 (N = 3) | 72 (N = 3) | 96 (N = 5) | 128 (N = 3) | 170 (N = 4) | 226 (N = 4) | Total (N = 48) | |
| Total number of TEAEs | 20 | 23 | 25 | 41 | 26 | 16 | 21 | 12 | 15 | 33 | 43 | 60 | 46 | 381 |
| Total patients with at least 1 TEAE | 4 | 3 | 4 | 3 | 4 | 3 | 4 | 3 | 2 | 5 | 2 | 4 | 4 | 45 |
| Fatigue | 1 | 1 | 2 | 0 | 2 | 1 | 1 | 2 | 0 | 0 | 1 | 4 | 0 | 15 |
| Nausea | 1 | 1 | 1 | 2 | 1 | 1 | 0 | 0 | 1 | 2 | 1 | 1 | 1 | 15 |
| Diarrhea | 0 | 1 | 0 | 2 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 2 | 3 | 12 |
| Vomiting | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 2 | 0 | 1 | 2 | 10 |
| Abdominal pain | 1 | 1 | 0 | 1 | 2 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 9 |
| Constipation | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 2 | 0 | 7 |
| Dizziness | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 7 |
| Infusion-related reaction | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 2 | 1 | 7 |
| Back pain | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 2 | 6 |
| Decreased appetite | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 6 |
| Dyspnea | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 6 |
| Oedema peripheral | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 5 |
| Pyrexia | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 5 |
| Dose Level (mg) | AUC∞ (h × ng/mL) | AUClast (h × ng/mL) | t1/2 (h) | MRT∞ (h) | Cmax (ng/mL) | tmax (h) | CL (L/h) | Vss (L) | Vz (L) |
|---|---|---|---|---|---|---|---|---|---|
| 2.5 | 32.4 ± 13.1 | 29.4 ± 11.9 | 12.8 ± 3.5 | 11.7 ± 4.59 | 12.0 ± 1.42 | 1.2 ± 0.10 | 85.1 ± 26.8 | 913 ± 206 | 1510 ± 421 |
| 5 | 56.5 ± 18.2 | 52.1 ± 18.2 | 18.6 ± 2.34 | 13.7 ± 3.74 | 25.5 ± 17.3 | 1.1 ± 0.02 | 93.9 ± 25.2 | 1320 ± 619 | 2570 ± 932 |
| 10 | 129 ± 41.3 | 119 ± 32.6 | 13.7 ± 2.68 | 12.5 ± 2.84 | 36.9 ± 17.5 | 1.2 ± 0.15 | 84.7 ± 32.4 | 1110 ± 664 | 1720 ± 925 |
| 15 | 153 ± 8.45 | 138 ± 10.8 | 16.6 ± 3 | 16.1 ± 2.64 | 35.6 ± 2.15 | 1.2 ± 0.14 | 98.5 ± 5.29 | 1600 ± 331 | 2370 ± 536 |
| 22.5 | 239 ± 55.4 | 251 ± 87.8 | 18.3 ± 0.49 | 16.4 ± 1.01 | 65.8 ± 24.5 | 1.2 ± 0.19 | 96.9 ± 22.5 | 1600 ± 466 | 2570 ± 663 |
| 30 | 341 ± 47.1 | 297 ± 55.8 | 12.4 ± 7.43 | 13.4 ± 8.49 | 87.8 ± 37.5 | 1.1 ± 0.11 | 89.1 ± 13.3 | 1130 ± 619 | 1500 ± 786 |
| 40 | 499 ± 122 | 437 ± 77.2 | 18.7 ± 5.68 | 18.3 ± 6.19 | 128 ± 41.9 | 1.1 ± 0.044 | 83.6 ± 18.6 | 1450 ± 185 | 2160 ± 195 |
| 54 | 868 ± 181 | 773 ± 180 | 19.6 ± 6.44 | 16.9 ± 7.34 | 323 ± 55.6 | 1.1 ± 0.05 | 63.9 ± 12.4 | 1080 ± 516 | 1820 ± 740 |
| 72 | 823 ± 292 | 742 ± 275 | 17.2 ± 1.58 | 16.8 ± 1.12 | 161 ± 67.7 | 1.3 ± 0.24 | 94.2 ± 28.8 | 1580 ± 489 | 2370 ± 833 |
| 96 | 1650 ± 229 | 1420 ± 236 | 16.1 ± 6.28 | 15.4 ± 6.58 | 494 ± 289 | 1.1 ± 0.13 | 59.1 ± 8.20 | 921 ± 425 | 1410 ± 682 |
| 128 | 1980 ± 1120 | 1730 ± 850 | 17.8 ± 7.00 | 18.1 ± 5.53 | 533 ± 344 | 1.1 ± 0.11 | 79.9 ± 4.21 | 1320 ± 473 | 1790 ± 366 |
| 170 | 3760 ± 1980 | 3340 ± 1500 | 15.0 ± 3.64 | 15.8 ± 7.89 | 955 ± 330 | 1.3 ± 0.26 | 56.5 ± 31.5 | 757 ± 218 | 1120 ± 419 |
| 226 | 6070 ± 1670 | 5620 ± 1390 | 14.2 ± 2.2 | 15.2 ± 3.92 | 1180 ± 232 | 1.2 ± 0.02 | 39.4 ± 0.17 | 569 ± 30.8 | 787 ± 139 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Richardson, G.E.; Al-Rajabi, R.; Uprety, D.; Hamid, A.; Williamson, S.K.; Baranda, J.; Mamdani, H.; Lee, Y.-L.; Nitika; Li, L.; et al. A Multicenter, Open-Label, Phase I/II Study of FN-1501 in Patients with Advanced Solid Tumors. Cancers 2023, 15, 2553. https://doi.org/10.3390/cancers15092553
Richardson GE, Al-Rajabi R, Uprety D, Hamid A, Williamson SK, Baranda J, Mamdani H, Lee Y-L, Nitika, Li L, et al. A Multicenter, Open-Label, Phase I/II Study of FN-1501 in Patients with Advanced Solid Tumors. Cancers. 2023; 15(9):2553. https://doi.org/10.3390/cancers15092553
Chicago/Turabian StyleRichardson, Gary Edward, Raed Al-Rajabi, Dipesh Uprety, Anis Hamid, Stephen K. Williamson, Joaquina Baranda, Hirva Mamdani, Ya-Li Lee, Nitika, Li Li, and et al. 2023. "A Multicenter, Open-Label, Phase I/II Study of FN-1501 in Patients with Advanced Solid Tumors" Cancers 15, no. 9: 2553. https://doi.org/10.3390/cancers15092553
APA StyleRichardson, G. E., Al-Rajabi, R., Uprety, D., Hamid, A., Williamson, S. K., Baranda, J., Mamdani, H., Lee, Y.-L., Nitika, Li, L., Wang, X., & Dong, X. (2023). A Multicenter, Open-Label, Phase I/II Study of FN-1501 in Patients with Advanced Solid Tumors. Cancers, 15(9), 2553. https://doi.org/10.3390/cancers15092553





