Bone Remodeling Markers in Children with Acute Lymphoblastic Leukemia after Intensive Chemotherapy: The Screenshot of a Biochemical Signature
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Risk Stratification and Treatment
2.3. Clinical Data
2.4. Bone Metabolism Markers
2.5. Bone Remodeling Cytokines Assessment
2.6. Statistical Analys
3. Results
3.1. Patient Population
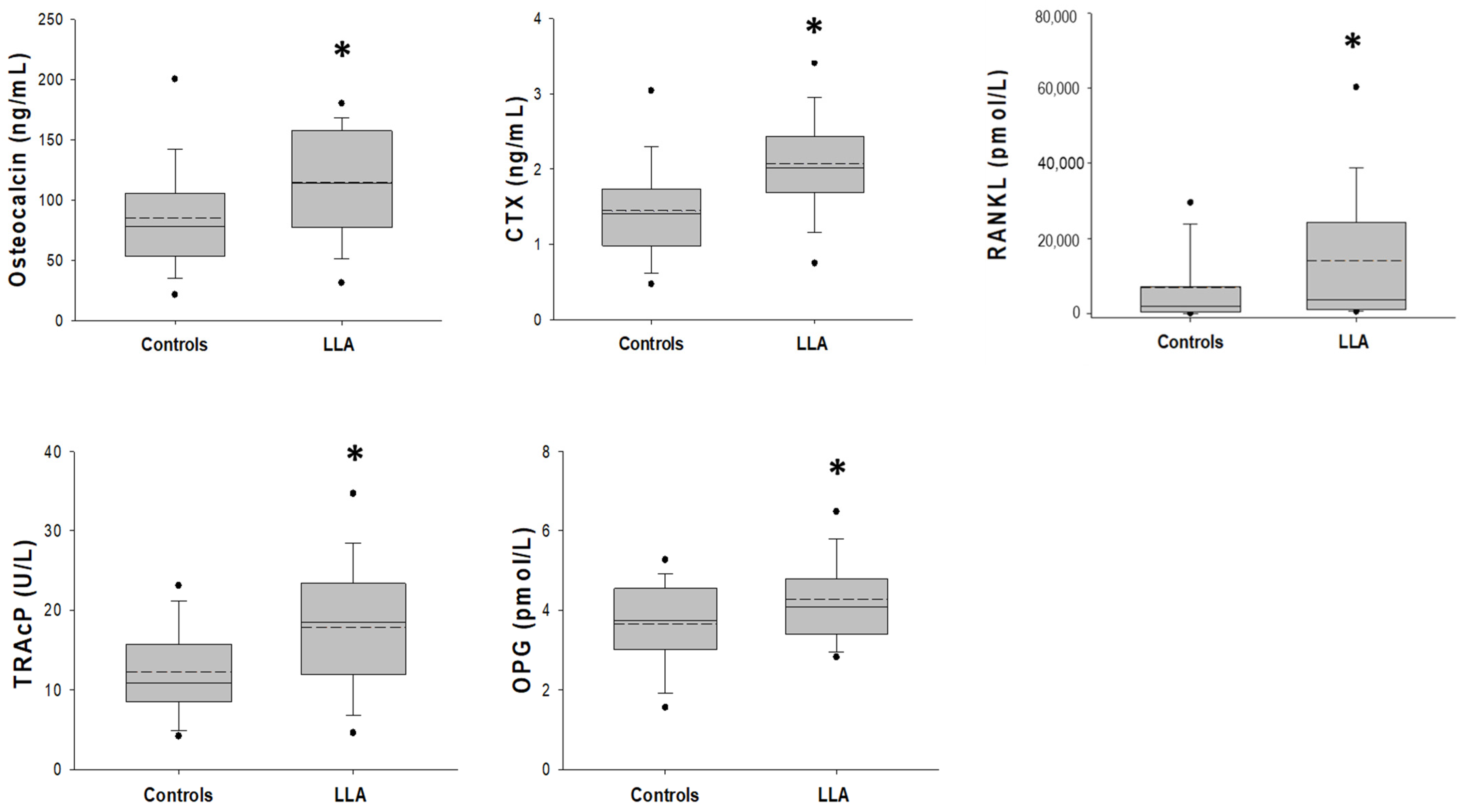
3.2. Bone Metabolism and Bone Remodeling Markers
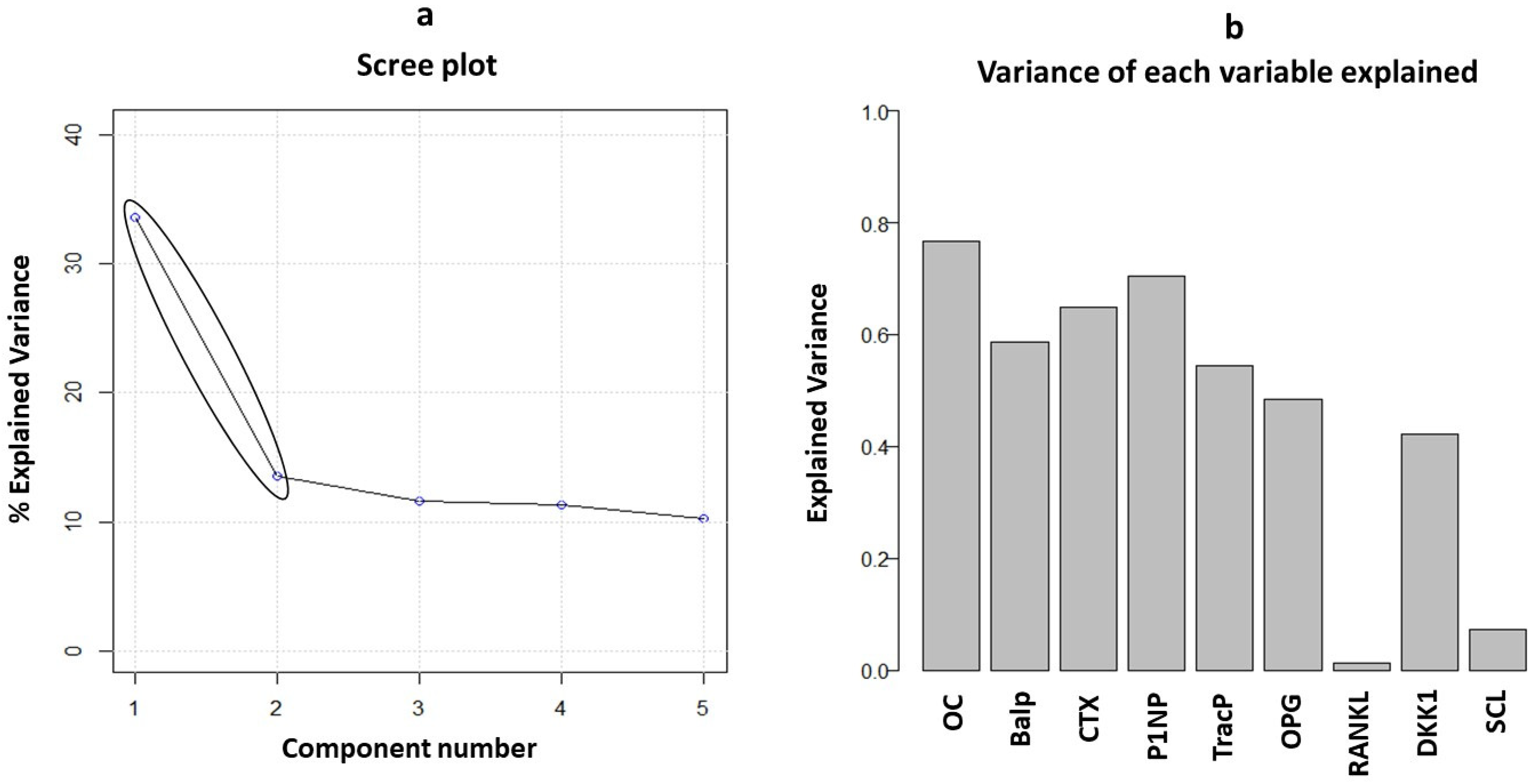
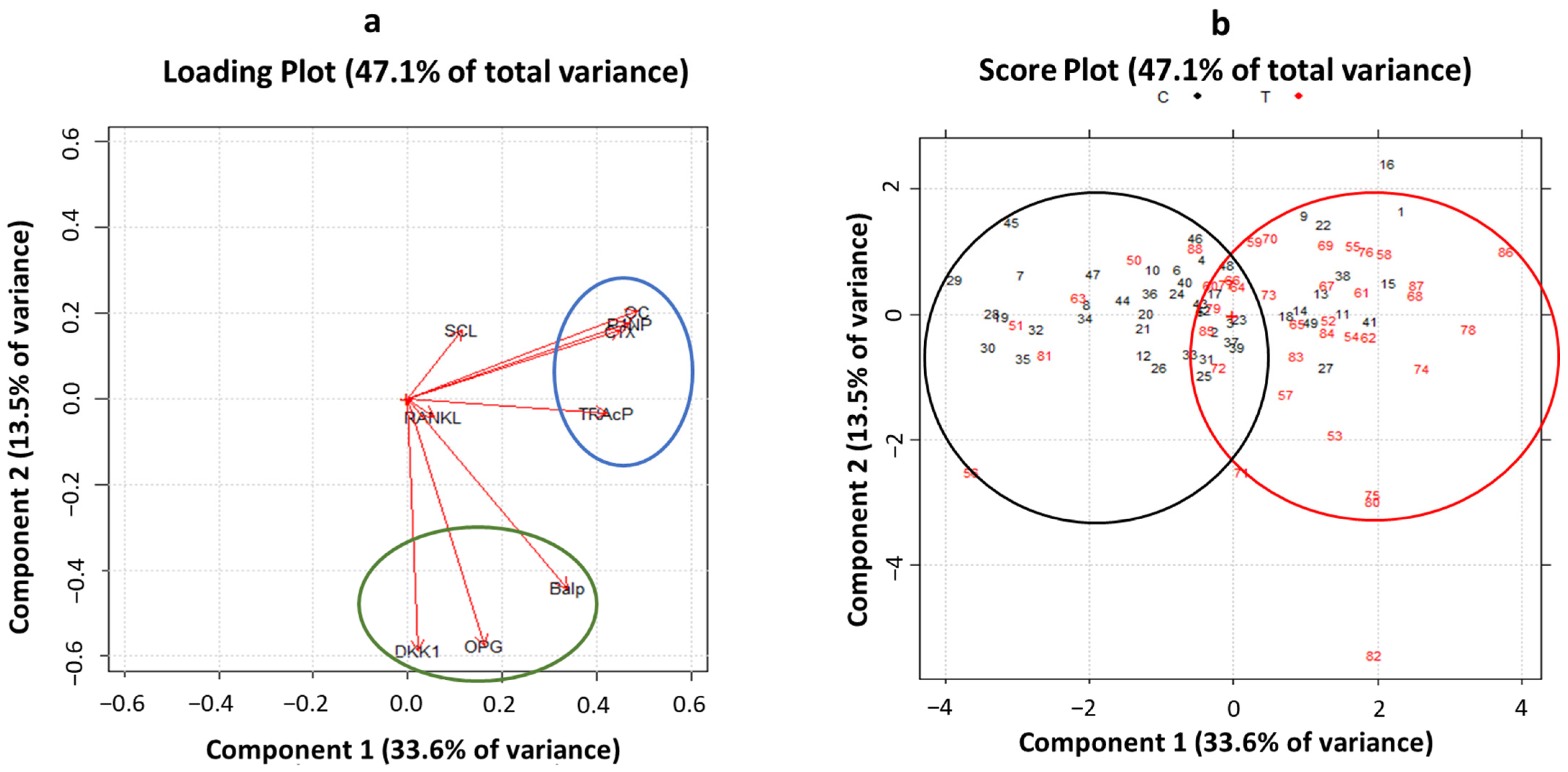
3.3. Principal Component Analysis of Biomarkers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ward, E.; DeSantis, C.; Robbins, A.; Kohler, B.; Jemal, A. Childhood and adolescent cancer statistics, 2014. CA Cancer J. Clin. 2014, 64, 83–103. [Google Scholar] [CrossRef]
- Inaba, H.; Greaves, M.; Mullighan, C.G. Acute lymphoblastic leukaemia. Lancet 2013, 381, 1943–1955. [Google Scholar] [CrossRef] [PubMed]
- Delvecchio, M.; Muggeo, P.; Monteduro, M.; Lassandro, G.; Novielli, C.; Valente, F.; Salinaro, E.; Zito, A.; Ciccone, M.M.; Miniello, V.L.; et al. Non-alcoholic fatty liver disease is associated with early left ventricular dysfunction in childhood acute lymphoblastic leukaemia survivors. Eur. J. Endocrinol. 2017, 176, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Giordano, P.; Muggeo, P.; Delvecchio, M.; Carbonara, S.; Romano, A.; Altomare, M.; Ricci, G.; Valente, F.; Zito, A.; Scicchitano, P.; et al. Endothelial dysfunction and cardiovascular risk factors in childhood acute lymphoblastic leukemia survivors. Int. J. Cardiol. 2017, 228, 621–627. [Google Scholar] [CrossRef]
- Muggeo, P.; Muggeo, V.M.R.; Giordano, P.; Delvecchio, M.; Altomare, M.; Novielli, C.; Ciccone, M.M.; D’Amato, G.; Faienza, M.F.; Santoro, N. Cardiovascular dysfunction and vitamin D status in childhood acute lymphoblastic leukemia survivors. World J. Pediatr. 2019, 15, 465–470. [Google Scholar] [CrossRef]
- Inaba, H.; Cao, X.; Han, A.Q.; Panetta, J.C.; Ness, K.K.; Metzger, M.L.; Rubnitz, J.E.; Ribeiro, R.C.; Sandlund, J.T.; Jeha, S.; et al. Bone mineral density in children with acute lymphoblastic leukemia. Cancer 2018, 124, 1025–1035. [Google Scholar] [CrossRef]
- Frenkel, B.; White, W.; Tuckermann, J. Glucocorticoid-Induced Osteoporosis. Adv. Exp. Med. Biol. 2015, 872, 179–215. [Google Scholar]
- Faienza, M.F.; Luce, V.; Lonero, A.; Ventura, A.; Colaianni, G.; Colucci, S.; Cavallo, L.; Grano, M.; Brunetti, G. Treatment of osteoporosis in children with glucocorticoid-treated diseases. Expert. Rev. Endocrinol. Metab. 2014, 9, 525–534. [Google Scholar] [CrossRef]
- Urquiaga, M.; Saag, K.G. Risk for osteoporosis and fracture with glucocorticoids. Best. Pract. Res. Clin. Rheumatol. 2022, 36, 101793. [Google Scholar] [CrossRef]
- Winkel, M.L.; Pieters, R.; Hop, W.C.; Roos, J.C.; Bokkerink, J.P.; Leeuw, J.A.; Bruin, M.C.; Kollen, W.J.; Veerman, A.J.; de Groot-Kruseman, H.A.; et al. Bone mineral density at diagnosis determines fracture rate in children with acute lymphoblastic leukemia treated according to the DCOG-ALL9 protocol. Bone 2014, 59, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Cummings, E.A.; Ma, J.; Fernandez, C.V.; Halton, J.; Alos, N.; Miettunen, P.M.; Jaremko, J.L.; Ho, J.; Shenouda, N.; Matzinger, M.A.; et al. Incident vertebral fractures in children with leukemia during the four years following diagnosis. J. Clin. Endocrinol. Metab. 2015, 100, 3408–3417. [Google Scholar] [CrossRef] [PubMed]
- Orgel, E.; Mueske, N.M.; Wren, T.A.; Gilsanz, V.; Butturini, A.M.; Freyer, D.R.; Mittelman, S.D. Early injury to cortical and cancellous bone from induction chemotherapy for adolescents and young adults treated for acute lymphoblastic leukemia. Bone 2016, 85, 131–137. [Google Scholar] [CrossRef]
- Van Atteveld, J.E.; Pluijm, S.M.F.; Ness, K.K.; Hudson, M.M.; Chemaitilly, W.; Kaste, S.C.; Robison, L.L.; Neggers, S.J.C.M.M.; Yasui, Y.; van den Heuvel-Eibrink, M.M.; et al. Prediction of Low and Very Low Bone Mineral Density Among Adult Survivors of Childhood Cancer. J. Clin. Oncol. 2019, 37, 2217–2225. [Google Scholar] [CrossRef] [PubMed]
- Kunstreich, M.; Kummer, S.; Laws, H.J.; Borkhardt, A.; Kuhlen, M. Osteonecrosis in children with acute lymphoblastic leukemia. Haematologica 2016, 101, 1295–1305. [Google Scholar] [CrossRef]
- Girard, P.; Auquier, P.; Barlogis, V.; Contet, A.; Poiree, M.; Demeocq, F.; Berbis, J.; Herrmann, I.; Villes, V.; Sirvent, N.; et al. Symptomatic osteonecrosis in childhood leukemia survivors: Prevalence, risk factors and impact on quality of life in adulthood. Haematologica 2013, 98, 1089–1097. [Google Scholar] [CrossRef]
- McAvoy, S.; Baker, K.S.; Mulrooney, D.; Blaes, A.; Arora, M.; Burns, L.J.; Majhail, N.S. Corticosteroid dose as a risk factor for avascular necrosis of the bone after hematopoietic cell transplantation. Biol. Blood Marrow Transplant. 2010, 16, 1231–1236. [Google Scholar] [CrossRef] [PubMed]
- Van den Heuvel-Eibrink, M.M.; Pieters, R. Steroids and risk of osteonecrosis in ALL: Take a break. Lancet Oncol. 2012, 13, 855–857. [Google Scholar] [CrossRef]
- Larsen, E.C.; Devidas, M.; Chen, S.; Salzer, W.L.; Raetz, E.A.; Loh, M.L.; Mattano, L.A., Jr.; Cole, C.; Eicher, A.; Haugan, M.; et al. Dexamethasone and High-Dose Methotrexate Improve Outcome for Children and Young Adults with High-Risk B-Acute Lymphoblastic Leukemia: A Report from Children’s Oncology Group Study AALL0232. J. Clin. Oncol. 2016, 34, 2380–2388. [Google Scholar] [CrossRef]
- Theill, L.E.; Boyle, W.J.; Penninger, J.M. RANK-L and RANK: T cells, bone loss, and mammalian evolution. Annu. Rev. Immunol. 2002, 20, 795–823. [Google Scholar] [CrossRef]
- Krishnan, V.; Bryant, H.U.; Mac Dougald, O.A. Regulation of bone mass by Wnt signaling. J. Clin. Investig. 2006, 116, 1202–1209. [Google Scholar] [CrossRef]
- Brunetti, G.; D’Amato, G.; Chiarito, M.; Tullo, A.; Colaianni, G.; Colucci, S.; Grano, M.; Faienza, M.F. An update on the role of RANKL-RANK/osteoprotegerin and WNT-β-catenin signaling pathways in pediatric diseases. World J. Pediatr. 2019, 15, 4–11. [Google Scholar] [CrossRef]
- Corrado, A.; Rotondo, C.; Mele, A.; Cici, D.; Maruotti, N.; Sanpaolo, E.; Colia, R.; Cantatore, F.P. Influence of glucocorticoid treatment on trabecular bone score and bone remodeling regulators in early rheumatoid arthritis. Arthritis Res. Ther. 2021, 23, 180. [Google Scholar] [CrossRef]
- Conter, V.; Bartram, C.R.; Valsecchi, M.G.; Schrauder, A.; Panzer-Grümayer, R.; Möricke, A.; Aricò, M.; Zimmermann, M.; Mann, G.; De Rossi, G.; et al. Molecular response to treatment redefines all prognostic factors in children and adolescents with B-cell precursor acute lymphoblastic leukemia: Results in 3184 patients of the AIEOP-BFM ALL 2000 study. Blood 2010, 115, 3206–3214. [Google Scholar] [CrossRef]
- Stewart, P.M.; Newell-Price, J.D.C. The adrenal cortex. In Williams Textbook of Endocrinology, 13th ed.; Melmed, S., Polonsky, K.S., Larsen, P.R., Kronenberg, H.M., Eds.; Saunders: Philadelphia, PA, USA, 2016; Chapter 15. [Google Scholar]
- Cacciari, E.; Milani, S.; Balsamo, A.; Spada, E.; Bona, G.; Cavallo, L.; Cerutti, F.; Gargantini, L.; Greggio, N.; Tonini, G.; et al. Italian cross-sectional growth charts for height, weight and BMI (2 to 20 yr). J. Endocrinol. Invetsig. 2006, 29, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Van Atteveld, J.E.; Mulder, R.L.; van den Heuvel-Eibrink, M.M.; Hudson, M.M.; Kremer, L.C.M.; Skinner, R.; Wallace, W.H.; Constine, L.S.; Higham, C.E.; Kaste, S.C.; et al. Bone mineral density surveillance for childhood, adolescent, and young adult cancer survivors: Evidence-based recommendations from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Diabetes Endocrinol. 2021, 9, 622–637. [Google Scholar] [CrossRef]
- Baron, R.; Kneissel, M. WNT signaling in bone homeostasis and disease: From human mutations to treatments. Nat. Med. 2013, 19, 179–192. [Google Scholar] [CrossRef]
- Qiang, Y.W.; Chen, Y.; Stephens, O.; Brown, N.; Chen, B.; Epstein, J.; Barlogie, B.; Shaughnessy, J.D., Jr. Myeloma-derived Dickkopf-1 disrupts Wnt-regulated osteoprotegerin and RANKL production by osteoblasts: A potential mechanism underlying osteolytic bone lesions in multiple myeloma. Blood 2008, 112, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Dalbeth, N.; Pool, B.; Smith, T.; Callon, K.E.; Lobo, M.; Taylor, W.J.; Jones, P.B.; Cornish, J.; McQueen, F.M. Circulating mediators of bone remodeling in psoriatic arthritis: Implications for disordered osteoclastogenesis and bone erosion. Arthritis Res. Ther. 2010, 12, R164. [Google Scholar] [CrossRef] [PubMed]
- Faienza, M.F.; D’Amato, G.; Chiarito, M.; Colaianni, G.; Colucci, S.; Grano, M.; Corbo, F.; Brunetti, G. Mechanisms Involved in Childhood Obesity-Related Bone Fragility. Front. Endocrinol. 2019, 10, 269. [Google Scholar] [CrossRef]
- Canalis, E.; Mazziotti, G.; Giustina, A.; Bilezikian, J.P. Glucocorticoid-induced osteoporosis: Pathophysiology and therapy. Osteoporos. Int. 2007, 18, 1319–1328. [Google Scholar] [CrossRef] [PubMed]
- Piemontese, M.; Xiong, J.; Fujiwara, Y.; Thostenson, J.D.; O’Brien, C.A. Cortical bone loss caused by glucocorticoid excess requires RANKL production by osteocytes and is associated with reduced OPG expression in mice. Am. J. Physiol. Endocrinol. Metab. 2016, 31, E587–E593. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, T.; Sabokbar, A.; Athanasou, N.A. Effect of corticosteroids on human osteoclast formation and activity. J. Endocrinol. 2002, 175, 155–163. [Google Scholar] [CrossRef]
- Conaway, H.H.; Henning, P.; Lie, A.; Tuckermann, J.; Lerner, U.H. Glucocorticoids employ the monomeric glucocorticoid receptor to potentiate vitamin D3 and parathyroid hormone-induced osteoclastogenesis. FASEB J. 2019, 33, 14394–14409. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, E.L.; Williams, J.H.; Davie, M.W.; Marshall, M.J. Effects of dissociated glucocorticoids on OPG and RANKL in osteoblastic cells. Bone 2006, 38, 652–661. [Google Scholar] [CrossRef]
- Dougall, W.C. Molecular pathways: Osteoclast-dependent and osteoclast-independent roles of the RANKL/RANK/OPG pathway in tumorigenesis and metastasis. Clin. Cancer Res. 2012, 18, 326–335. [Google Scholar] [CrossRef]
- Rajakumar, S.A.; Danska, J.S. Bad to the bone: B cell acute lymphoblastic leukemia cells mediate bone destruction. Mol. Cell. Oncol. 2020, 8, 1835423. [Google Scholar] [CrossRef]
- Halton, J.; Gaboury, I.; Grant, R.; Alos, N.; Cummings, E.A.; Matzinger, M.; Shenouda, N.; Lentle, B.; Abish, S.; Atkinson, S.; et al. Advanced vertebral fracture among newly diagnosed children with acute lymphoblastic leukemia: Results of the Canadian Steroid-Associated Osteoporosis in the Pediatric Population (STOPP) research program. J. Bone Miner. Res. 2009, 24, 1326–1334. [Google Scholar] [CrossRef] [PubMed]
- Mostoufi-Moab, S.; Halton, J. Bone morbidity in childhood leukemia: Epidemiology, mechanisms, diagnosis, and treatment. Curr. Osteoporos. Rep. 2014, 12, 300–312. [Google Scholar] [CrossRef]
- Vasikaran, S.D.; Miura, M.; Pikner, R.; Bhattoa, H.P.; Cavalier, E.; IOF-IFCC Joint Committee on Bone Metabolism (C-BM). Practical Considerations for the Clinical Application of Bone Turnover Markers in Osteoporosis. Calcif. Tissue Int. 2023, 112, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Maresova, K.B.; Pavelka, K.; Stepan, J.J. Acute effects of glucocorticoids on serum markers of osteoclasts, osteoblasts, and osteocytes. Calcif. Tissue Int. 2013, 92, 354–361. [Google Scholar] [CrossRef]
- Crofton, P.M.; Ahmed, S.F.; Wade, J.C.; Elmlinger, M.W.; Ranke, M.B.; Kelnar, C.J.; Wallace, W.H. Effects of a third intensification block of chemotherapy on bone and collagen turnover, insulin-like growth factor I, its binding proteins and short-term growth in children with acute lymphoblastic leukaemia. Eur. J. Cancer 1999, 35, 960–967. [Google Scholar] [CrossRef] [PubMed]
| Clinical Characteristics | Patients (n = 39) |
|---|---|
| Gender (male/female) | 21/18 |
| Age at recruitment—years | 7.64 ± 4.47 |
| Age at diagnosis—years | 6.24 ± 4.25 |
| Height (SDS) | −0.75 ± 1.26 |
| Weight (SDS) | −0.14 ± 1.18 |
| BMI (SDS) | 0.49 ± 0.91 |
| ALL phenotype: B-lineage | 32 |
| T-lineage | 5 |
| B-lineage t (9;22) | 2 |
| RISK GROUP: standard risk | 13 |
| Intermediate risk | 18 |
| High risk | 8 |
| Bone pain yes/no | 7/32 |
| Recurrent fever yes/no | 17/22 |
| Triglycerides mg/dL | 186.03 ± 176.94 |
| Corticosteroid dose mg/m2 | 20,472.53 ± 7023.68 |
| HD-MTX (10 g m−2/20 g m−2) | 7/32 |
| PEG-ASP UI/m2 7500 | 31 |
| 12,500 | 1 |
| 15,000 | 1 |
| 20,000 | 6 |
| Controls (n = 49) | Patients (n = 39) | p Value | |
|---|---|---|---|
| Osteocalcin (ng/mL) | 85.2 ± 46.4 | 114.4 ± 46.8 | 0.004 a |
| P1NP (ng/mL) | 692 (532∼948) | 751 (700∼852) | 0.305 b |
| bALP (µg/L) | 73.7 (55.9∼97.6) | 77.6 (54.6∼115.5) | 0.389 b |
| CTX (ng/mL) | 1.4 (1.0∼1.7) | 2.0 (1.7∼2.4) | <0.001 b |
| TRAcP5b (U/L) | 10.9 (8.4∼16.4) | 18.5 (11.8∼23.8) | 0.001 b |
| RANKL (pmol/L) | 1954 (350∼7258) | 3759 (1073∼24,976) | 0.029 b |
| OPG (pmol/L) | 3.7 ± 1.2 | 4.3 ± 1.2 | 0.020 a |
| RANKL/OPG ratio | 581 (89∼2345) | 762 (238∼4226) | 0.098 b |
| DKK1 (pg/mL) | 5537 (4335∼6287) | 5513 (4466∼7168) | 0.480 b |
| Sclerostin (pmol/L) | 17.8 (15.3∼19.9) | 16.9 (14.2∼19.3) | 0.252 b |
| DKK1 | Sclerostin | RANKL | OPG | 25OH Vit D | CTX | OC | bALP | TRAcP | P1NP | Ca | P | PTH | IL6 | Age at Diagnosis | Age at Recruitment | Weight | Height | BMI | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DKK-1 | 1 | ||||||||||||||||||
| Sclerostin | 0.18 | 1 | |||||||||||||||||
| RANKL | 0.15 | 0.01 | 1 | ||||||||||||||||
| OPG | 0.10 | 0.03 | −0.04 | 1 | |||||||||||||||
| 25OH Vit D | −0.23 | −0.04 | 0.07 | 0.11 | 1 | ||||||||||||||
| CTX | −0.01 | −0.02 | 0.15 | 0.06 | −0.10 | 1 | |||||||||||||
| OC | −0.05 | 0.05 | −0.06 | 0.12 | −0.19 | 0.55 *** | 1 | ||||||||||||
| bALP | −0.10 | −0.17 | −0.06 | 0.40 * | −0.02 | 0.24 | 0.38 * | 1 | |||||||||||
| TRAcP | −0.14 | −0.06 | 0.15 | 0.14 | −0.09 | 0.29 | 0.53 *** | 0.33 | 1 | ||||||||||
| P1NP | 0.01 | 0.16 | 0.08 | −0.09 | −0.16 | 0.50 * | 0.69 *** | 0.30 | 0.63 *** | 1 | |||||||||
| Ca | −0.06 | −0.20 | −0.22 | 0.27 | −0.08 | −0.16 | −0.05 | 0.04 | 0.05 | 0.032 | 1 | ||||||||
| P | −0.06 | −0.06 | −0.02 | 0.12 | −0.04 | 0.50 * | 0.51 *** | 0.14 | 0.33 * | 0.46 ** | 0.24 | 1 | |||||||
| PTH | −0.04 | −0.04 | −0.12 | −0.05 | −0.32 | 0.10 | 0.43 ** | −0.03 | 0.19 | 0.33 * | −0.12 | −0.01 | 1 | ||||||
| Age at diagnosis | −0.15 | 0.24 | 0.17 | −0.29 | −0.15 | 0.05 | −0.12 | −0.44 ** | 0.06 | −0.13 | −0.21 | −0.20 | 0.04 | −0.04 | 1 | ||||
| Age at recruitment | −0.33 * | 0.23 | 0.17 | −0.32 | −0.11 | −0.04 | −0.16 | −0.43 ** | 0.03 | −0.16 | −0.10 | −0.16 | 0.05 | −0.01 | 0.92 | 1 | |||
| Weight | −0.08 | 0.15 | −0.28 | 0.02 | −0.04 | −0.20 | −0.04 | −0.10 | 0.12 | 0.09 | −0.10 | −0.08 | −0.10 | −0.15 | 0.09 | 0.01 | 1 | ||
| Height | −0.03 | 0.04 | −0.06 | −0.23 | −0.26 | −0.06 | −0.09 | −0.27 | 0.09 | 0.02 | −0.13 | −0.20 | −0.07 | −0.33 | 0.40 | 0.28 | 0.73 | 1 | |
| BMI | 0.002 | 0.11 | −0.28 | 0.32 * | 0.16 | −0.33 * | −0.02 | 0.03 | −0.01 | 0.05 | 0.08 | 0.03 | −0.10 | −0.06 | −0.33 | −0.34 | 0.70 | 0.10 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muggeo, P.; Grassi, M.; D’Ascanio, V.; Brescia, V.; Fontana, A.; Piacente, L.; Di Serio, F.; Giordano, P.; Faienza, M.F.; Santoro, N. Bone Remodeling Markers in Children with Acute Lymphoblastic Leukemia after Intensive Chemotherapy: The Screenshot of a Biochemical Signature. Cancers 2023, 15, 2554. https://doi.org/10.3390/cancers15092554
Muggeo P, Grassi M, D’Ascanio V, Brescia V, Fontana A, Piacente L, Di Serio F, Giordano P, Faienza MF, Santoro N. Bone Remodeling Markers in Children with Acute Lymphoblastic Leukemia after Intensive Chemotherapy: The Screenshot of a Biochemical Signature. Cancers. 2023; 15(9):2554. https://doi.org/10.3390/cancers15092554
Chicago/Turabian StyleMuggeo, Paola, Massimo Grassi, Vito D’Ascanio, Vincenzo Brescia, Antonietta Fontana, Laura Piacente, Francesca Di Serio, Paola Giordano, Maria Felicia Faienza, and Nicola Santoro. 2023. "Bone Remodeling Markers in Children with Acute Lymphoblastic Leukemia after Intensive Chemotherapy: The Screenshot of a Biochemical Signature" Cancers 15, no. 9: 2554. https://doi.org/10.3390/cancers15092554
APA StyleMuggeo, P., Grassi, M., D’Ascanio, V., Brescia, V., Fontana, A., Piacente, L., Di Serio, F., Giordano, P., Faienza, M. F., & Santoro, N. (2023). Bone Remodeling Markers in Children with Acute Lymphoblastic Leukemia after Intensive Chemotherapy: The Screenshot of a Biochemical Signature. Cancers, 15(9), 2554. https://doi.org/10.3390/cancers15092554








