Comments on and Illustrations of the EFSUMB CEUS Guidelines: Transabdominal and Endoscopic Ultrasound Features of Intrapancreatic Metastases and the Role of Multiparametric Imaging and EUS-Guided Sampling in Rare Pancreatic Tumors
Abstract
Simple Summary
Abstract
1. Introduction
2. Disease Frequency
3. Features of Intrapancreatic Metastases
3.1. Clinical Presentation
3.2. Pathology
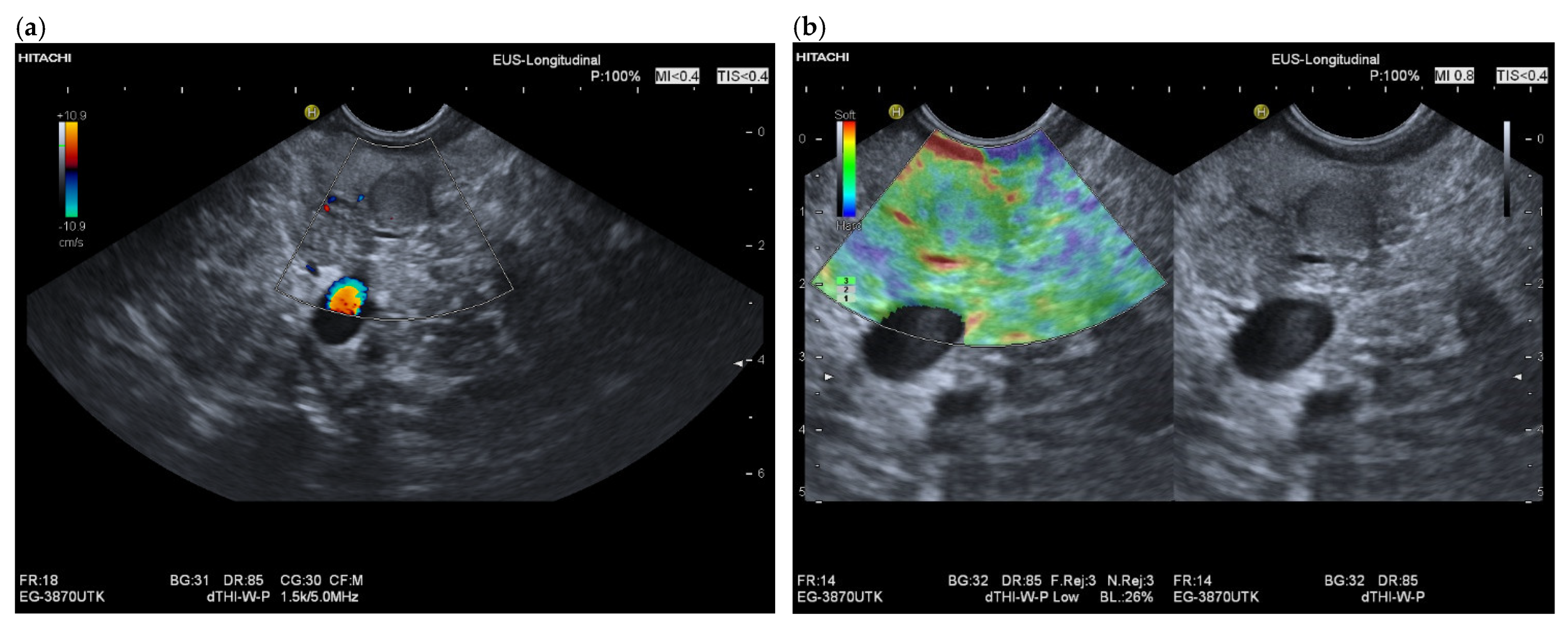
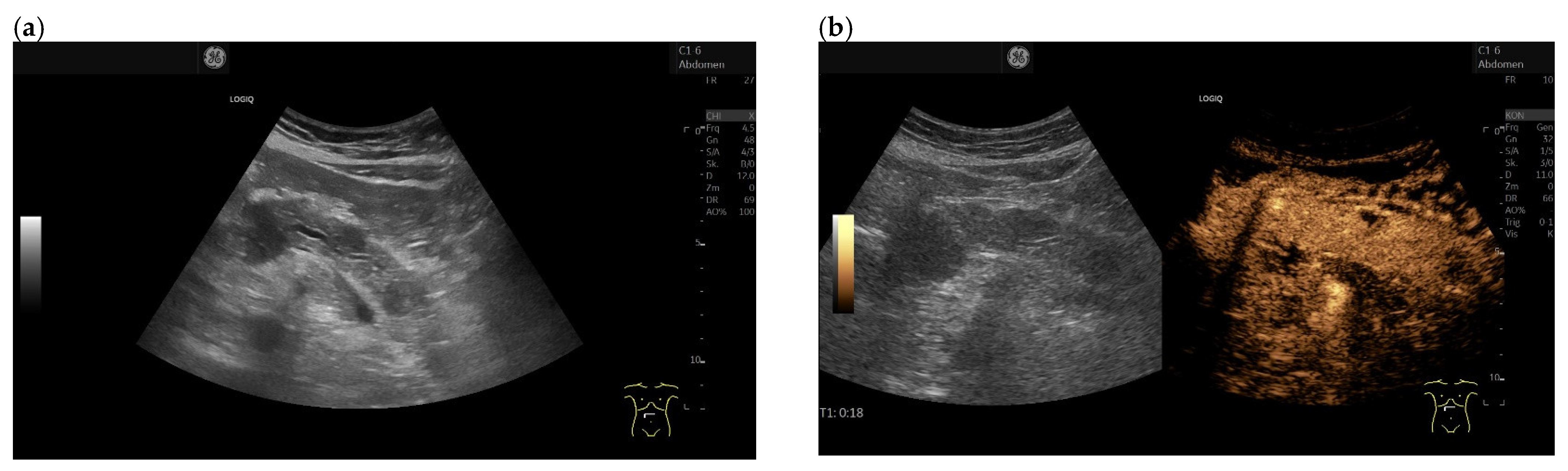
3.3. US and EUS
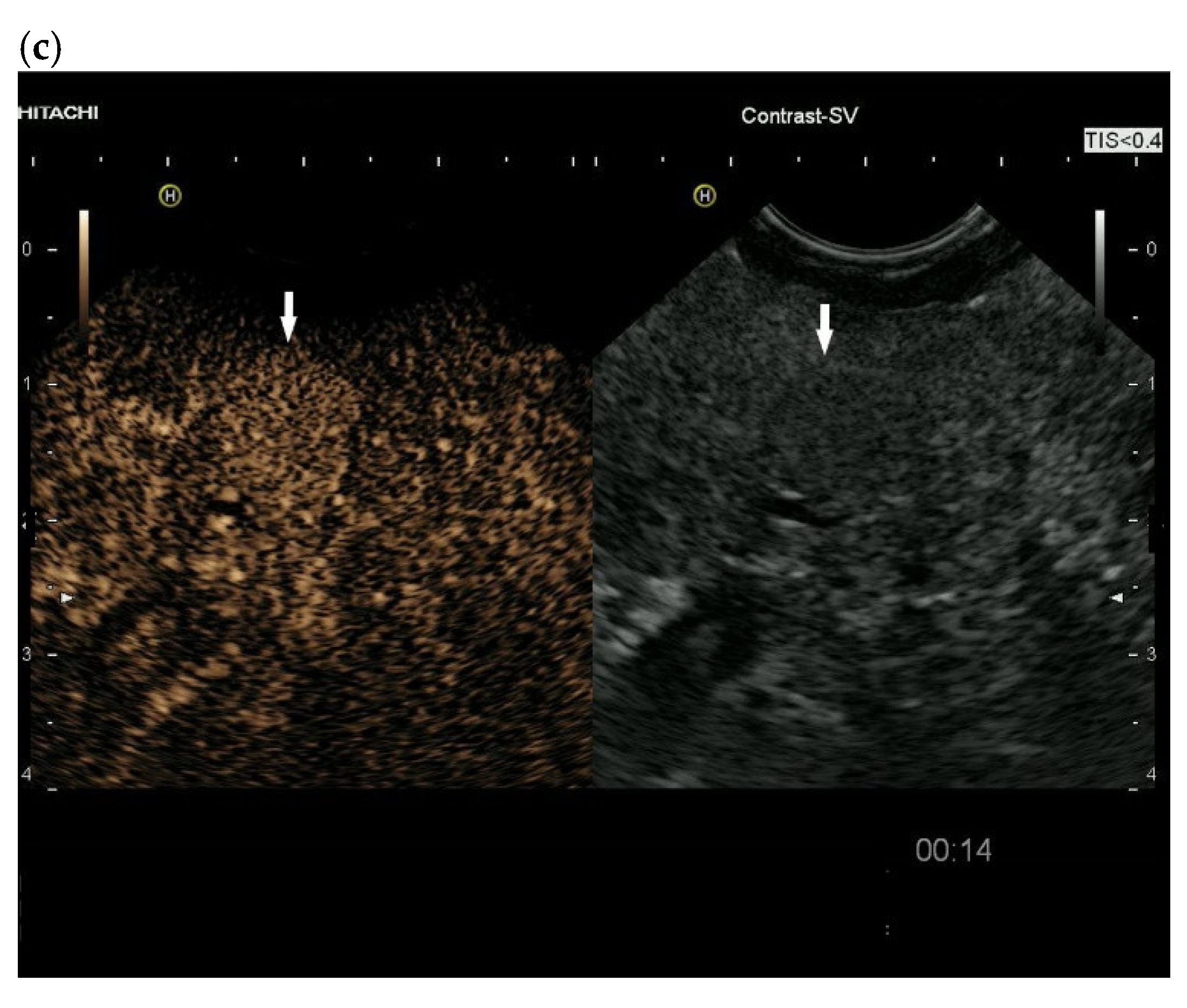
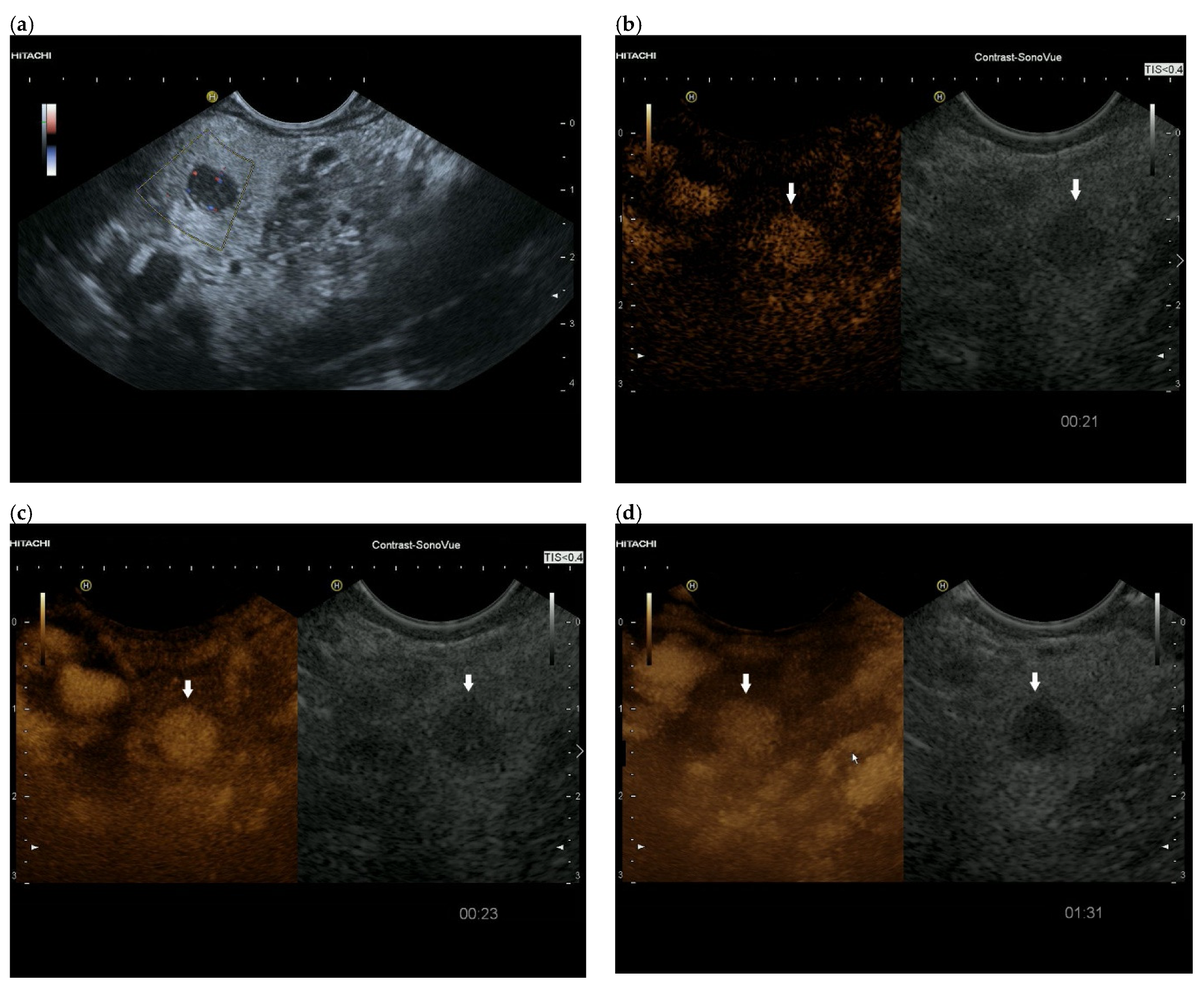
3.4. CEUS and CH-EUS
3.5. EUS-Guided Sampling
3.6. Computed Tomography (CT) and Magnetic Resonance Imaging (MRI)
4. Prognosis and Management of Pancreatic Metastasis
4.1. Renal Cell Carcinoma
4.2. Lung Cancer
4.3. Colorectal Carcinoma
4.4. Malignant Melanoma
4.5. Breast Cancer
4.6. Sarcoma
4.7. Other Tumors
4.8. Surgical Resection of Intrapancreatic Metastasis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dietrich, C.F.; Averkiou, M.; Nielsen, M.B.; Barr, R.G.; Burns, P.N.; Calliada, F.; Cantisani, V.; Choi, B.; Chammas, M.C.; Clevert, D.A.; et al. How to perform Contrast-Enhanced Ultrasound (CEUS). Ultrasound Int. Open 2018, 4, E2–E15. [Google Scholar] [CrossRef] [PubMed]
- Claudon, M.; Dietrich, C.F.; Choi, B.I.; Cosgrove, D.O.; Kudo, M.; Nolsoe, C.P.; Piscaglia, F.; Wilson, S.R.; Barr, R.G.; Chammas, M.C.; et al. Guidelines and good clinical practice recommendations for Contrast Enhanced Ultrasound (CEUS) in the liver-update 2012: A WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultrasound Med. Biol. 2013, 39, 187–210. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, C.F.; Nolsoe, C.P.; Barr, R.G.; Berzigotti, A.; Burns, P.N.; Cantisani, V.; Chammas, M.C.; Chaubal, N.; Choi, B.I.; Clevert, D.A.; et al. Guidelines and Good Clinical Practice Recommendations for Contrast-Enhanced Ultrasound (CEUS) in the Liver-Update 2020 WFUMB in Cooperation with EFSUMB, AFSUMB, AIUM, and FLAUS. Ultrasound Med. Biol. 2020, 46, 2579–2604. [Google Scholar] [CrossRef] [PubMed]
- Piscaglia, F.; Nolsoe, C.; Dietrich, C.F.; Cosgrove, D.O.; Gilja, O.H.; Bachmann Nielsen, M.; Albrecht, T.; Barozzi, L.; Bertolotto, M.; Catalano, O.; et al. The EFSUMB Guidelines and Recommendations on the Clinical Practice of Contrast Enhanced Ultrasound (CEUS): Update 2011 on non-hepatic applications. Ultraschall Med. 2012, 33, 33–59. [Google Scholar] [CrossRef]
- Sidhu, P.S.; Cantisani, V.; Dietrich, C.F.; Gilja, O.H.; Saftoiu, A.; Bartels, E.; Bertolotto, M.; Calliada, F.; Clevert, D.A.; Cosgrove, D.; et al. The EFSUMB Guidelines and Recommendations for the Clinical Practice of Contrast-Enhanced Ultrasound (CEUS) in Non-Hepatic Applications: Update 2017 (Long Version). Ultraschall Med. 2018, 39, e2–e44. [Google Scholar] [CrossRef]
- Kitano, M.; Yamashita, Y.; Kamata, K.; Ang, T.L.; Imazu, H.; Ohno, E.; Hirooka, Y.; Fusaroli, P.; Seo, D.W.; Napoleon, B.; et al. The Asian Federation of Societies for Ultrasound in Medicine and Biology (AFSUMB) Guidelines for Contrast-Enhanced Endoscopic Ultrasound. Ultrasound Med. Biol. 2021, 47, 1433–1447. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Hirche, T.O.; Ott, M.; Ignee, A. Real-time tissue elastography in the diagnosis of autoimmune pancreatitis. Endoscopy 2009, 41, 718–720. [Google Scholar] [CrossRef]
- Dong, Y.; D’Onofrio, M.; Hocke, M.; Jenssen, C.; Potthoff, A.; Atkinson, N.; Ignee, A.; Dietrich, C.F. Autoimmune pancreatitis: Imaging features. Endosc. Ultrasound 2018, 7, 196–203. [Google Scholar] [CrossRef]
- Hocke, M.; Ignee, A.; Dietrich, C.F. Contrast-enhanced endoscopic ultrasound in the diagnosis of autoimmune pancreatitis. Endoscopy 2011, 43, 163–165. [Google Scholar] [CrossRef]
- Hocke, M.; Ignee, A.; Dietrich, C.F. Three-dimensional contrast-enhanced endoscopic ultrasound for the diagnosis of autoimmune pancreatitis. Endoscopy 2011, 43, E381–E382. [Google Scholar] [CrossRef]
- Dong, Y.; Jurgensen, C.; Puri, R.; D’Onofrio, M.; Hocke, M.; Wang, W.P.; Atkinson, N.; Sharma, M.; Dietrich, C.F. Ultrasound imaging features of isolated pancreatic tuberculosis. Endosc. Ultrasound 2018, 7, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Barreiros, A.P.; Braden, B.; Schieferstein-Knauer, C.; Ignee, A.; Dietrich, C.F. Characteristics of intestinal tuberculosis in ultrasonographic techniques. Scand. J. Gastroenterol. 2008, 43, 1224–1231. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, C.F.; Sharma, M.; Chaubal, N.; Dong, Y.; Cui, X.W.; Schindler-Piontek, M.; Richter, J.; Radzina, M.; Sandouk, F.; Kucharzik, T.; et al. Ascariasis imaging: Pictorial essay. Z. Fur. Gastroenterol. 2017, 55, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Schindler-Piontek, M.; Chaubal, N.; Dehmani, S.; Cui, X.W.; Dong, Y.; Sharma, M.; Dietrich, C.F. Ascariasis, a review. Med. Ultrason. 2022, 24, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, C.F.; Douira-Khomsi, W.; Gharbi, H.; Sharma, M.; Cui, X.W.; Sparchez, Z.; Richter, J.; Kabaalioglu, A.; Atkinson, N.S.; Schreiber-Dietrich, D.; et al. Cystic and alveolar echinococcosis of the hepatobiliary tract-the role of new imaging techniques for improved diagnosis. Med. Ultrason. 2020, 22, 77–86. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Douira-Khomsi, W.; Gharbi, H.; Sharma, M.; Cui, X.W.; Sparchez, Z.; Richter, J.; Kabaalioglu, A.; Atkinson, N.S.; Schreiber-Dietrich, D.; et al. Cystic echinococcosis, review and illustration of non-hepatic manifestations. Med. Ultrason. 2020, 22, 319–324. [Google Scholar] [CrossRef]
- Gill, A.J.; Klimstra, D.S.; Lam, A.K.; Washington, M. Tumours of the Pancreas. In WHO Classification of Tumours, 5th ed.; Digestive System Tumours; WHO Classification of Tumours Editorial Board, Ed.; International Agency for Research on Cancer: Lyon, France, 2019. [Google Scholar]
- Klöppel, G.; Adsay, N.V.; Couvelard, A. Pancreatic neuroendocrine neoplasms: Introduction. In WHO Classification of Tumours, 5th ed.; Digestive System Tumours; WHO Classification of Tumours Editorial Board, Ed.; International Agency for Research on Cancer: Lyon, France, 2019; pp. 343–346. [Google Scholar]
- Krishna, S.G.; Bhattacharya, A.; Ross, W.A.; Ladha, H.; Porter, K.; Bhutani, M.S.; Lee, J.H. Pretest prediction and diagnosis of metastatic lesions to the pancreas by endoscopic ultrasound-guided fine needle aspiration. J. Gastroenterol. Hepatol. 2015, 30, 1552–1560. [Google Scholar] [CrossRef]
- Krishna, S.G.; Li, F.; Bhattacharya, A.; Ladha, H.; Porter, K.; Singh, A.; Ross, W.A.; Bhutani, M.S.; Lee, J.H. Differentiation of pancreatic ductal adenocarcinoma from other neoplastic solid pancreatic lesions: A tertiary oncology center experience. Gastrointest. Endosc. 2015, 81, 370–379. [Google Scholar] [CrossRef]
- Abdallah, M.A.; Bohy, K.; Singal, A.; Xie, C.; Patel, B.; Nelson, M.E.; Bleeker, J.; Askeland, R.; Abdullah, A.; Aloreidi, K.; et al. Metastatic tumors to the pancreas: Balancing clinical impression with cytology findings. Ann. Hepatobiliary Pancreat. Surg. 2022, 26, 91–97. [Google Scholar] [CrossRef]
- Sekulic, M.; Amin, K.; Mettler, T.; Miller, L.K.; Mallery, S.; Stewart, J.R. Pancreatic involvement by metastasizing neoplasms as determined by endoscopic ultrasound-guided fine needle aspiration: A clinicopathologic characterization. Diagn. Cytopathol. 2017, 45, 418–425. [Google Scholar] [CrossRef]
- Gagovic, V.; Spier, B.J.; DeLee, R.J.; Barancin, C.; Lindstrom, M.; Einstein, M.; Byrne, S.; Harter, J.; Agni, R.; Pfau, P.R.; et al. Endoscopic ultrasound fine-needle aspiration characteristics of primary adenocarcinoma versus other malignant neoplasms of the pancreas. Can. J. Gastroenterol. 2012, 26, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, C.F.; Sahai, A.V.; D’Onofrio, M.; Will, U.; Arcidiacono, P.G.; Petrone, M.C.; Hocke, M.; Braden, B.; Burmester, E.; Moller, K.; et al. Differential diagnosis of small solid pancreatic lesions. Gastrointest. Endosc. 2016, 84, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Cifuentes, N.; Pickren, J.W. Metastases from carcinoma of mammary gland: An autopsy study. J. Surg. Oncol. 1979, 11, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Abrams, H.L.; Spiro, R.; Goldstein, N. Metastases in carcinoma; analysis of 1000 autopsied cases. Cancer 1950, 3, 74–85. [Google Scholar] [CrossRef]
- Nakamura, E.; Shimizu, M.; Itoh, T.; Manabe, T. Secondary tumors of the pancreas: Clinicopathological study of 103 autopsy cases of Japanese patients. Pathol. Int. 2001, 51, 686–690. [Google Scholar] [CrossRef]
- Mao, C.; Domenico, D.R.; Kim, K.; Hanson, D.J.; Howard, J.M. Observations on the developmental patterns and the consequences of pancreatic exocrine adenocarcinoma. Findings of 154 autopsies. Arch. Surg. 1995, 130, 125–134. [Google Scholar] [CrossRef]
- Ardengh, J.C.; Lopes, C.V.; Kemp, R.; Venco, F.; de Lima-Filho, E.R.; dos Santos, J.S. Accuracy of endoscopic ultrasound-guided fine-needle aspiration in the suspicion of pancreatic metastases. BMC Gastroenterol. 2013, 13, 63. [Google Scholar] [CrossRef]
- Raymond, S.L.T.; Yugawa, D.; Chang, K.H.F.; Ena, B.; Tauchi-Nishi, P.S. Metastatic neoplasms to the pancreas diagnosed by fine-needle aspiration/biopsy cytology: A 15-year retrospective analysis. Diagn. Cytopathol. 2017, 45, 771–783. [Google Scholar] [CrossRef]
- Fritscher-Ravens, A.; Sriram, P.V.; Krause, C.; Atay, Z.; Jaeckle, S.; Thonke, F.; Brand, B.; Bohnacker, S.; Soehendra, N. Detection of pancreatic metastases by EUS-guided fine-needle aspiration. Gastrointest. Endosc. 2001, 53, 65–70. [Google Scholar] [CrossRef]
- Adsay, N.V.; Andea, A.; Basturk, O.; Kilinc, N.; Nassar, H.; Cheng, J.D. Secondary tumors of the pancreas: An analysis of a surgical and autopsy database and review of the literature. Virchows. Arch. 2004, 444, 527–535. [Google Scholar] [CrossRef]
- Sellner, F.; Thalhammer, S.; Klimpfinger, M. Tumour Evolution and Seed and Soil Mechanism in Pancreatic Metastases of Renal Cell Carcinoma. Cancers 2021, 13, 1342. [Google Scholar] [CrossRef] [PubMed]
- Hiotis, S.P.; Klimstra, D.S.; Conlon, K.C.; Brennan, M.F. Results after pancreatic resection for metastatic lesions. Ann. Surg. Oncol. 2002, 9, 675–679. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.; Edil, B.H.; Cameron, J.L.; Pawlik, T.M.; Herman, J.M.; Gilson, M.M.; Campbell, K.A.; Schulick, R.D.; Ahuja, N.; Wolfgang, C.L. Pancreatic resection of isolated metastases from nonpancreatic primary cancers. Ann. Surg. Oncol. 2008, 15, 3199–3206. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.; Wolfgang, C.L. The role of surgery in the management of isolated metastases to the pancreas. Lancet. Oncol. 2009, 10, 287–293. [Google Scholar] [CrossRef]
- Yoon, W.J.; Ryu, J.K.; Kim, Y.T.; Yoon, Y.B.; Kim, S.W.; Kim, W.H. Clinical features of metastatic tumors of the pancreas in Korea: A single-center study. Gut. Liver. 2011, 5, 61–64. [Google Scholar] [CrossRef]
- Madkhali, A.A.; Shin, S.H.; Song, K.B.; Lee, J.H.; Hwang, D.W.; Park, K.M.; Lee, Y.J.; Kim, S.C. Pancreatectomy for a secondary metastasis to the pancreas: A single-institution experience. Medicine 2018, 97, e12653. [Google Scholar] [CrossRef]
- Huang, Q.; Zhou, H.; Liu, C.; Jin, K.; Fan, K.; Cheng, H.; Fan, Z.; Yang, C.; Liu, L.; Long, J.; et al. Surgical Resection for Metastatic Tumors in the Pancreas: A Single-Center Experience and Systematic Review. Ann. Surg. Oncol. 2019, 26, 1649–1656. [Google Scholar] [CrossRef]
- Di Franco, G.; Gianardi, D.; Palmeri, M.; Furbetta, N.; Guadagni, S.; Bianchini, M.; Bonari, F.; Sbrana, A.; Vasile, E.; Pollina, L.E.; et al. Pancreatic resections for metastases: A twenty-year experience from a tertiary care center. Eur. J. Surg. Oncol. 2020, 46, 825–831. [Google Scholar] [CrossRef]
- Sellner, F.; Tykalsky, N.; De Santis, M.; Pont, J.; Klimpfinger, M. Solitary and multiple isolated metastases of clear cell renal carcinoma to the pancreas: An indication for pancreatic surgery. Ann. Surg. Oncol. 2006, 13, 75–85. [Google Scholar] [CrossRef]
- Sellner, F.; Thalhammer, S.; Klimpfinger, M. Isolated Pancreatic Metastases of Renal Cell Cancer: Genetics and Epigenetics of an Unusual Tumour Entity. Cancers 2022, 14, 1539. [Google Scholar] [CrossRef]
- Adler, H.; Redmond, C.E.; Heneghan, H.M.; Swan, N.; Maguire, D.; Traynor, O.; Hoti, E.; Geoghegan, J.G.; Conlon, K.C. Pancreatectomy for metastatic disease: A systematic review. Eur. J. Surg. Oncol. 2014, 40, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Konstantinidis, I.T.; Dursun, A.; Zheng, H.; Wargo, J.A.; Thayer, S.P.; Fernandez-del Castillo, C.; Warshaw, A.L.; Ferrone, C.R. Metastatic tumors in the pancreas in the modern era. J. Am. Coll. Surg. 2010, 211, 749–753. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.C.; Roh, M.H. Metastases to the Pancreas Encountered on Endoscopic Ultrasound-Guided, Fine-Needle Aspiration. Arch. Pathol. Lab. Med. 2015, 139, 1248–1252. [Google Scholar] [CrossRef] [PubMed]
- Sellner, F.; Thalhammer, S.; Klimpfinger, M. Isolated Pancreatic Metastases of Renal Cell Carcinoma-Clinical Particularities and Seed and Soil Hypothesis. Cancers 2023, 15, 339. [Google Scholar] [CrossRef] [PubMed]
- Malleo, G.; Salvia, R.; Maggino, L.; Marchegiani, G.; D’Angelica, M.; DeMatteo, R.; Kingham, P.; Pulvirenti, A.; Sereni, E.; Jarnagin, W.R.; et al. Long-term Outcomes After Surgical Resection of Pancreatic Metastases from Renal Clear-Cell Carcinoma. Ann. Surg. Oncol. 2021, 28, 3100–3108. [Google Scholar] [CrossRef] [PubMed]
- Sperti, C.; Moletta, L.; Patane, G. Metastatic tumors to the pancreas: The role of surgery. World J. Gastrointest. Oncol. 2014, 6, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Takada, R.; Omoto, S.; Tsuda, M.; Masuda, D.; Kato, H.; Matsumoto, T.; Moriyama, I.; Okabe, Y.; Shiomi, H.; et al. Analysis of Prognostic Factors in Pancreatic Metastases: A Multicenter Retrospective Analysis. Pancreas 2018, 47, 1033–1039. [Google Scholar] [CrossRef]
- Olson, M.T.; Wakely, P.E., Jr.; Ali, S.Z. Metastases to the pancreas diagnosed by fine-needle aspiration. Acta. Cytol. 2013, 57, 473–480. [Google Scholar] [CrossRef]
- DeWitt, J.; Jowell, P.; Leblanc, J.; McHenry, L.; McGreevy, K.; Cramer, H.; Volmar, K.; Sherman, S.; Gress, F. EUS-guided FNA of pancreatic metastases: A multicenter experience. Gastrointest. Endosc. 2005, 61, 689–696. [Google Scholar] [CrossRef]
- Layfield, L.J.; Hirschowitz, S.L.; Adler, D.G. Metastatic disease to the pancreas documented by endoscopic ultrasound guided fine-needle aspiration: A seven-year experience. Diagn. Cytopathol. 2012, 40, 228–233. [Google Scholar] [CrossRef]
- Waters, L.; Si, Q.; Caraway, N.; Mody, D.; Staerkel, G.; Sneige, N. Secondary tumors of the pancreas diagnosed by endoscopic ultrasound-guided fine-needle aspiration: A 10-year experience. Diagn. Cytopathol. 2014, 42, 738–743. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Shen, R.; Tonkovich, D.; Li, Z. Endoscopic ultrasound-guided fine-needle aspiration diagnosis of secondary tumors involving pancreas: An institution’s experience. J. Am. Soc. Cytopathol. 2018, 7, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.L.; Odronic, S.I.; Springer, B.S.; Reynolds, J.P. Solid tumor metastases to the pancreas diagnosed by FNA: A single-institution experience and review of the literature. Cancer Cytopathol. 2015, 123, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Fernandez, G.; Fondevila-Campo, C.; Sanjuanbenito, A.; Fabregat-Prous, J.; Secanella-Medayo, L.; Rotellar-Sastre, F.; Pardo-Sanchez, F.; Prieto-Calvo, M.; Marin-Ortega, H.; Sanchez-Cabus, S.; et al. Pancreatic metastases from renal cell carcinoma. Postoperative outcome after surgical treatment in a Spanish multicenter study (PANMEKID). Eur. J. Surg. Oncol. 2022, 48, 133–141. [Google Scholar] [CrossRef]
- Bassi, C.; Butturini, G.; Falconi, M.; Sargenti, M.; Mantovani, W.; Pederzoli, P. High recurrence rate after atypical resection for pancreatic metastases from renal cell carcinoma. Br. J. Surg. 2003, 90, 555–559. [Google Scholar] [CrossRef]
- Dousset, B.; Andant, C.; Guimbaud, R.; Roseau, G.; Tulliez, M.; Gaudric, M.; Palazzo, L.; Chaussade, S.; Chapuis, Y. Late pancreatic metastasis from renal cell carcinoma diagnosed by endoscopic ultrasonography. Surgery 1995, 117, 591–594. [Google Scholar] [CrossRef]
- Eloubeidi, M.A.; Jhala, D.; Chhieng, D.C.; Jhala, N.; Eltoum, I.; Wilcox, C.M. Multiple late asymptomatic pancreatic metastases from renal cell carcinoma: Diagnosis by endoscopic ultrasound-guided fine needle aspiration biopsy with immunocytochemical correlation. Dig. Dis. Sci. 2002, 47, 1839–1842. [Google Scholar] [CrossRef]
- El Hajj, I.I.; LeBlanc, J.K.; Sherman, S.; Al-Haddad, M.A.; Cote, G.A.; McHenry, L.; DeWitt, J.M. Endoscopic ultrasound-guided biopsy of pancreatic metastases: A large single-center experience. Pancreas 2013, 42, 524–530. [Google Scholar] [CrossRef]
- Fusaroli, P.; D’Ercole, M.C.; De Giorgio, R.; Serrani, M.; Caletti, G. Contrast harmonic endoscopic ultrasonography in the characterization of pancreatic metastases (with video). Pancreas 2014, 43, 584–587. [Google Scholar] [CrossRef]
- Okasha, H.H.; Pawlak, K.M.; Zorniak, M.; Wiechowska-Kozlowska, A.; Naga, Y.M.; ElHusseiny, R. EUS in the evaluation of metastatic lesions to the pancreas. Endosc. Ultrasound 2020, 9, 147–150. [Google Scholar] [CrossRef]
- Yuan, Z.; Yan, H.; Ling, W.; Luo, Y. Contrast-enhanced ultrasound of pancreatic melanoma: A case report and literature review. Front. Oncol. 2022, 12, 989638. [Google Scholar] [CrossRef] [PubMed]
- Atiq, M.; Bhutani, M.S.; Ross, W.A.; Raju, G.S.; Gong, Y.; Tamm, E.P.; Javle, M.; Wang, X.; Lee, J.H. Role of endoscopic ultrasonography in evaluation of metastatic lesions to the pancreas: A tertiary cancer center experience. Pancreas 2013, 42, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Ran, C.; Li, F.; Cheng, N. Melanoma of unknown primary in the pancreas: Should it be considered primary? BMC Surg. 2020, 20, 76. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.H.; Chiou, H.J.; Hong, T.M.; Tiu, C.M.; Chiou, S.Y.; Su, C.H.; Tsay, S.H. Solitary metastasis from renal cell carcinoma presenting as diffuse pancreatic enlargement. J. Clin. Ultrasound 2002, 30, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Ignee, A.; Jenssen, C.; Arcidiacono, P.G.; Hocke, M.; Moller, K.; Saftoiu, A.; Will, U.; Fusaroli, P.; Iglesias-Garcia, J.; Ponnudurai, R.; et al. Endoscopic ultrasound elastography of small solid pancreatic lesions: A multicenter study. Endoscopy 2018, 50, 1071–1079. [Google Scholar] [CrossRef]
- Napoleon, B.; Alvarez-Sanchez, M.V.; Gincoul, R.; Pujol, B.; Lefort, C.; Lepilliez, V.; Labadie, M.; Souquet, J.C.; Queneau, P.E.; Scoazec, J.Y.; et al. Contrast-enhanced harmonic endoscopic ultrasound in solid lesions of the pancreas: Results of a pilot study. Endoscopy 2010, 42, 564–570. [Google Scholar] [CrossRef]
- Kitano, M.; Kudo, M.; Yamao, K.; Takagi, T.; Sakamoto, H.; Komaki, T.; Kamata, K.; Imai, H.; Chiba, Y.; Okada, M.; et al. Characterization of small solid tumors in the pancreas: The value of contrast-enhanced harmonic endoscopic ultrasonography. Am. J. Gastroenterol. 2012, 107, 303–310. [Google Scholar] [CrossRef]
- Kitano, M.; Sakamoto, H.; Kudo, M. Contrast-enhanced endoscopic ultrasound. Dig. Endosc. Off. J. Jpn. Gastroenterol. Endosc. Soc. 2014, 26, 79–85. [Google Scholar] [CrossRef]
- Hocke, M.; Ignee, A.; Dietrich, C.F. Advanced endosonographic diagnostic tools for discrimination of focal chronic pancreatitis and pancreatic carcinoma—Elastography, contrast enhanced high mechanical index (CEHMI) and low mechanical index (CELMI) endosonography in direct comparison. Z. Fur. Gastroenterol. 2012, 50, 199–203. [Google Scholar] [CrossRef]
- Kitano, M.; Yamashita, Y. New Imaging Techniques for Endoscopic Ultrasonography: Contrast-Enhanced Endoscopic Ultrasonography. Gastrointest Endosc. Clin. N. Am. 2017, 27, 569–583. [Google Scholar] [CrossRef]
- Fusaroli, P.; Spada, A.; Mancino, M.G.; Caletti, G. Contrast harmonic echo-endoscopic ultrasound improves accuracy in diagnosis of solid pancreatic masses. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2010, 8, 629–634. [Google Scholar] [CrossRef] [PubMed]
- D‘Onofrio, M.; Biagioli, E.; Gerardi, C.; Canestrini, S.; Rulli, E.; Crosara, S.; De Robertis, R.; Floriani, I. Diagnostic performance of contrast-enhanced ultrasound (CEUS) and contrast-enhanced endoscopic ultrasound (ECEUS) for the differentiation of pancreatic lesions: A systematic review and meta-analysis. Ultraschall Med. 2014, 35, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Hocke, M.; Dietrich, C. Contrast-enhanced sonography and endoscopic ultrasound for the diagnosis of pancreatic diseases. Zent. Chir. 2014, 139, 301–307. [Google Scholar] [CrossRef]
- De Robertis, R.; D’Onofrio, M.; Crosara, S.; Dal Corso, F.; Barbi, E.; Canestrini, S.; Mucelli, R.P. Contrast-enhanced ultrasound of pancreatic tumours. Australas. J. Ultrasound Med. 2014, 17, 96–109. [Google Scholar] [CrossRef]
- D’Onofrio, M.; Barbi, E.; Dietrich, C.F.; Kitano, M.; Numata, K.; Sofuni, A.; Principe, F.; Gallotti, A.; Zamboni, G.A.; Mucelli, R.P. Pancreatic multicenter ultrasound study (PAMUS). Eur. J. Radiol. 2012, 81, 630–638. [Google Scholar] [CrossRef]
- Hocke, M.; Schulze, E.; Gottschalk, P.; Topalidis, T.; Dietrich, C.F. Contrast-enhanced endoscopic ultrasound in discrimination between focal pancreatitis and pancreatic cancer. World J. Gastroenterol. 2006, 12, 246–250. [Google Scholar] [CrossRef]
- Braden, B.; Jenssen, C.; D’Onofrio, M.; Hocke, M.; Will, U.; Moller, K.; Ignee, A.; Dong, Y.; Cui, X.W.; Saftoiu, A.; et al. B-mode and contrast-enhancement characteristics of small nonincidental neuroendocrine pancreatic tumors. Endosc. Ultrasound 2017, 6, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Serra, C.; Felicani, C.; Mazzotta, E.; Piscitelli, L.; Cipollini, M.L.; Tomassetti, P.; Pezzilli, R.; Casadei, R.; Morselli-Labate, A.M.; Stanghellini, V.; et al. Contrast-enhanced ultrasound in the differential diagnosis of exocrine versus neuroendocrine pancreatic tumors. Pancreas 2013, 42, 871–877. [Google Scholar] [CrossRef]
- Ota, T.; Ono, S. Intrapancreatic accessory spleen: Diagnosis using contrast enhanced ultrasound. Br. J. Radiol. 2004, 77, 148–149. [Google Scholar] [CrossRef]
- Ulrich, J.D.; Specht, K.; Schlitter, A.M.; Ceyhan, G.O.; Quante, M.; Schmid, R.M.; Schlag, C. A rare case of perivascular epithelioid cell tumor (PEComa) of the pancreas diagnosed by endoscopic ultrasound. Endosc. Int. Open 2020, 8, E25–E28. [Google Scholar] [CrossRef]
- Chen, X.; Hao, F.; Gui, Y.; Zhang, J.; Tan, L.; Xiao, M.; Zhang, Q.; Meng, H.; Li, J.; Jiang, Y.; et al. Enhancement patterns in the venous phase of contrast-enhanced ultrasounds: Diagnostic value for patients with solid pancreatic lesions. Quant. Imaging. Med. Surg. 2021, 11, 4321–4333. [Google Scholar] [CrossRef] [PubMed]
- Makino, Y.; Imai, Y.; Fukuda, K.; Seki, Y.; Kogita, S.; Sawai, Y.; Igura, T.; Kurokawa, M.; Takamura, M. Sonazoid-enhanced ultrasonography for the diagnosis of an intrapancreatic accessory spleen: A case report. J. Clin. Ultrasound 2011, 39, 344–347. [Google Scholar] [CrossRef] [PubMed]
- De Robertis, R.; D’Onofrio, M.; Manfrin, E.; Dal Bo, C.; Pozzi Mucelli, R. A rare case of pancreatic head splenosis diagnosed by contrast-enhanced ultrasound. Ultraschall Med. 2014, 35, 72–74. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.K.; Li, L.J.; He, Y.M.; Xu, Z.F. Misdiagnosis of pancreatic metastasis from renal cell carcinoma: A case report. World J. Clin. Cases. 2022, 10, 9012–9019. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Yamada, R.; Kaneko, M.; Naota, H.; Fujimura, Y.; Tabata, M.; Kobayashi, K.; Tanaka, K. Isolated pancreatic metastasis from malignant melanoma: A case report and literature review. Clin. J. Gastroenterol. 2019, 12, 626–636. [Google Scholar] [CrossRef]
- Endo, Y.; Noda, H.; Watanabe, F.; Kato, T.; Kakizawa, N.; Ichida, K.; Kasahara, N.; Rikiyama, T. A Retrospective Analysis of Preoperative Evaluation and Surgical Resection for Metastatic Tumors of the Pancreas. Indian J. Surg. Oncol. 2019, 10, 251–257. [Google Scholar] [CrossRef]
- Huang, J.; Yang, J.; Ding, J.; Zhou, J.; Yang, R.; Li, J.; Luo, Y.; Lu, Q. Development and validation of an ultrasound-based prediction model for differentiating between malignant and benign solid pancreatic lesions. Eur. Radiol. 2022, 32, 8296–8305. [Google Scholar] [CrossRef]
- Seufferlein, T.; Mayerle, J.; Böck, S.; Brunner, T.; Ettrich, T.J.; Grenacher, L.; Gress, T.M.; Hackert, T.; Heinemann, V.; Kestler, A.; et al. S3-Leitlinie zum exokrinen Pankreaskarzinom–Langversion 20–Dezember 2021–AWMF-Registernummer: 032/010OL. Z. Für. Gastroenterol. 2022, 60, e812–e909. [Google Scholar]
- Kovacevic, B. Vilmann PEUS tissue acquisition: From A to, B. Endosc. Ultrasound 2020, 9, 225–231. [Google Scholar] [CrossRef]
- Wang, K.X.; Ben, Q.W.; Jin, Z.D.; Du, Y.Q.; Zou, D.W.; Liao, Z.; Li, Z.S. Assessment of morbidity and mortality associated with EUS-guided FNA: A systematic review. Gastrointest. Endosc. 2011, 73, 283–290. [Google Scholar] [CrossRef]
- Mizuide, M.; Ryozawa, S.; Fujita, A.; Ogawa, T.; Katsuda, H.; Suzuki, M.; Noguchi, T.; Tanisaka, Y. Complications of En-doscopic Ultrasound-Guided Fine Needle Aspiration: A Narrative Review. Diagnostics 2020, 10, 964. [Google Scholar] [CrossRef] [PubMed]
- Möller, K.; Dietrich, C.F.; Faiss, S.; Mutze, S.; Goelz, L. Alternatives of histological material collection-When and how is histo-logical confirmation by ultrasound (US), computer tomography (CT) or endosonography (EUS) useful? Z Gastroenterol. 2022, 60, 937–958. [Google Scholar] [PubMed]
- Okasha, H.H.; Naga, M.I.; Esmat, S.; Naguib, M.; Hassanein, M.; Hassani, M.; El-Kassas, M.; Mahdy, R.E.; El-Gemeie, E.; Farag, A.H.; et al. Endoscopic Ultrasound-Guided Fine Needle Aspiration versus Percutaneous Ultrasound-Guided Fine Needle Aspiration in Diagnosis of Focal Pancreatic Masses. Endosc. Ultrasound 2013, 2, 190–193. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, F.C.; Levy, M.J.; Roden, A.C.; Boardman, L.A.; Sinicrope, F.A.; McWilliams, R.R.; Zhang, L. EUS fine-needle pancreatic core biopsy can determine eligibility for tumor-agnostic immunotherapy. Endosc. Int. Open 2018, 6, E1278–E1282. [Google Scholar] [CrossRef] [PubMed]
- Moller, K.; Papanikolaou, I.S.; Toermer, T.; Delicha, E.M.; Sarbia, M.; Schenck, U.; Koch, M.; Al-Abadi, H.; Meining, A.; Schmidt, H.; et al. EUS-guided FNA of solid pancreatic masses: High yield of 2 passes with combined histologic-cytologic analysis. Gastrointest. Endosc. 2009, 70, 60–69. [Google Scholar] [CrossRef]
- Barat, M.; Aldhaheri, R.; Dohan, A.; Fuks, D.; Kedra, A.; Hoeffel, C.; Oudjit, A.; Coriat, R.; Barret, M.; Terris, B.; et al. Utility of CT to Differentiate Pancreatic Parenchymal Metastasis from Pancreatic Ductal Adenocarcinoma. Cancers 2021, 13, 3103. [Google Scholar] [CrossRef]
- Palmowski, M.; Hacke, N.; Satzl, S.; Klauss, M.; Wente, M.N.; Neukamm, M.; Kleeff, J.; Hallscheidt, P. Metastasis to the pancreas: Characterization by morphology and contrast enhancement features on CT and MRI. Pancreatology 2008, 8, 199–203. [Google Scholar] [CrossRef]
- Tan, C.H.; Tamm, E.P.; Marcal, L.; Balachandran, A.; Charnsangavej, C.; Vikram, R.; Bhosale, P. Imaging features of hematogenous metastases to the pancreas: Pictorial essay. Cancer Imaging 2011, 11, 9–15. [Google Scholar] [CrossRef]
- Angelelli, G.; Mancini, M.; Pignataro, P.; Pedote, P.; Scardapane, A. Multidetector computed tomography in the study of pancreatic metastases. Radiol. Med. 2012, 117, 369–377. [Google Scholar] [CrossRef]
- Portale, T.R.; Di Benedetto, V.; Mosca, F.; Trovato, M.A.; Scuderi, M.G.; Puleo, S. Isolated pancreatic metastasis from melanoma. Case. Report. G Chir. 2011, 32, 135–137. [Google Scholar]
- Yamaguchi, H.; Kimura, Y.; Nagayama, M.; Imamura, M.; Tanaka, S.; Yoshida, M.; Yoshida, E.; Fujino, H.; Machiki, T.; Miyanishi, K.; et al. Central pancreatectomy in portal annular pancreas for metastatic renal cell carcinoma: A case report. World J. Surg. Oncol. 2019, 17, 76. [Google Scholar] [CrossRef] [PubMed]
- Chikhladze, S.; Lederer, A.K.; Kuhlbrey, C.M.; Hipp, J.; Sick, O.; Fichtner-Feigl, S.; Wittel, U.A. Curative-intent pancreas resection for pancreatic metastases: Surgical and oncological results. Clin. Exp. Metastasis. 2020, 37, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Rodger, F.E.; Brennan, P.T.; Nair, R.; Holroyd, D.J. The role of hepatic and pancreatic metastatectomy in the management of metastatic renal cell carcinoma: A systematic review. Surg. Oncol. 2022, 44, 101819. [Google Scholar] [CrossRef] [PubMed]
- Tanis, P.J.; van der Gaag, N.A.; Busch, O.R.; van Gulik, T.M.; Gouma, D.J. Systematic review of pancreatic surgery for metastatic renal cell carcinoma. Br. J. Surg. 2009, 96, 579–592. [Google Scholar] [CrossRef] [PubMed]
- Santoni, M.; Conti, A.; Partelli, S.; Porta, C.; Sternberg, C.N.; Procopio, G.; Bracarda, S.; Basso, U.; De Giorgi, U.; Derosa, L.; et al. Surgical resection does not improve survival in patients with renal metastases to the pancreas in the era of tyrosine kinase inhibitors. Ann. Surg. Oncol. 2015, 22, 2094–2100. [Google Scholar] [CrossRef]
- Figueiredo Ferreira, M.; Garces-Duran, R.; Eisendrath, P.; Deviere, J.; Deprez, P.; Monino, L.; Van Laethem, J.L.; Borbath, I. EUS-guided radiofrequency ablation of pancreatic/peripancreatic tumors and oligometastatic disease: An observational prospective multicenter study. Endosc. Int. Open 2022, 10, E1380–E1385. [Google Scholar] [CrossRef]
- Maeno, T.; Satoh, H.; Ishikawa, H.; Yamashita, Y.T.; Naito, T.; Fujiwara, M.; Kamma, H.; Ohtsuka, M.; Hasegawa, S. Patterns of pancreatic metastasis from lung cancer. Anticancer Res. 1998, 18, 2881–2884. [Google Scholar]
- Wilson, R.L.; Brown, R.K.; Reisman, D. Surgical resection for metastatic non-small cell lung cancer to the pancreas. Lung Cancer 2009, 63, 433–435. [Google Scholar] [CrossRef]
- Jaen-Torrejimeno, I.; Lopez-Guerra, D.; Rojas-Holguin, A.; De-Armas-Conde, N.; Blanco-Fernandez, G. Resection of isolated pancreatic metastases from pulmonary neoplasia: A systematic review. Updat. Surg. 2022, 74, 1817–1825. [Google Scholar] [CrossRef]
- Guerra, F.; Coletta, D.; Deutsch, G.B.; Giuliani, G.; Patriti, A.; Fischer, T.D.; Coratti, A.; Serafini, S.; Surjan, R.; Milanetto, A.C.; et al. The role of resection for melanoma metastases to the pancreas. HPB Off. J. Int. Hepato. Pancreato. Biliary. Assoc. 2022, 24, 2045–2052. [Google Scholar] [CrossRef]
- Fabbri, C.; Luigiano, C.; Collina, G.; Cennamo, V.; D’Imperio, N.; Jovine, E. EUS-FNA diagnosis of single pancreatic metastasis of liposarcoma. Gastrointest. Endosc. 2009, 69, 974–976. [Google Scholar] [CrossRef] [PubMed]
- Di Bari, N.; Vitale, N.; Marzullo, A.; Loizzo, T.; Marraudino, N.; Lionetti, G.; Milano, A.D. Deceptive appearance of a rapidly growing left atrial myxoid sarcoma with pancreatic metastasis. J. Card. Surg. 2020, 35, 3176–3178. [Google Scholar] [CrossRef] [PubMed]
- Sasajima, J.; Uehara, J.; Goto, T.; Fujibayashi, S.; Koizumi, K.; Mizukami, Y.; Ishida-Yamamoto, A.; Fujiya, M.; Okumura, T. Pancreatic metastasis of angiosarcoma (Stewart-Treves syndrome) diagnosed using endoscopic ultrasound-guided fine needle aspiration: A case report. Medicine 2016, 95, e4316. [Google Scholar] [CrossRef] [PubMed]
- Bhutani, M.S.; Arora, A.; Kundu, U.; Maru, D. A rare case of pancreatic metastasis of angiosarcoma: Diagnosed by endoscopic ultrasound-guided fine-needle aspiration and biopsy. Endosc. Ultrasound 2017, 6, 340–342. [Google Scholar] [CrossRef]
- Huddy, J.R.; Sodergren, M.H.; Deguara, J.; Thway, K.; Jones, R.L.; Mudan, S.S. Pancreaticoduodenectomy for the Management of Pancreatic or Duodenal Metastases from Primary Sarcoma. Anticancer Res. 2018, 38, 4041–4046. [Google Scholar] [CrossRef]
- Jobran, R.; Baloch, Z.W.; Aviles, V.; Rosato, E.F.; Schwartz, S.; LiVolsi, V.A. Tall cell papillary carcinoma of the thyroid: Metastatic to the pancreas. Thyroid 2000, 10, 185–187. [Google Scholar] [CrossRef]
- Siddiqui, A.A.; Olansky, L.; Sawh, R.N.; Tierney, W.M. Pancreatic metastasis of tall cell variant of papillary thyroid carcinoma: Diagnosis by endoscopic ultrasound-guided fine needle aspiration. JOP 2006, 7, 417–422. [Google Scholar]
- Chen, L.; Brainard, J.A. Pancreatic metastasis from papillary thyroid carcinoma diagnosed by endoscopic ultrasound-guided fine needle aspiration: A case report. Acta. Cytol. 2010, 54, 640–644. [Google Scholar] [CrossRef]
- Ren, H.; Ke, N.; Tan, C.; Wang, X.; Cao, W.; Liu, X. Unusual metastasis of papillary thyroid cancer to the pancreas, liver, and diaphragm: A case report with review of literature. BMC Surg. 2020, 20, 82. [Google Scholar] [CrossRef]
- Dokmak, S.; Cabral, C.; Couvelard, A.; Aussilhou, B.; Belghiti, J.; Sauvanet, A. Pancreatic metastasis from nephroblastoma: An unusual entity. JOP 2009, 10, 396–399. [Google Scholar]
- Lingamaneni, P.; Laswi, H.; Krbanjevic, A.; Moturi, K.; Katiyar, V.; Gupta, S. Merkel Cell Carcinoma With Isolated Pancreatic Metastasis. J. Investig. Med. High. Impact. Case. Rep. 2021, 9, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ghouri, Y.A.; Krishna, S.G.; Kundu, U.R.; Bhutani, M.S.; Lee, J.H.; Ross, W.A. A case series and literature review of Merkel cell carcinoma metastasizing to pancreas. Dig. Dis. Sci. 2015, 60, 1805–1812. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, N.; Haiart, S.; Kanhere, H.A.; Maddern, G.J. Merkel cell carcinoma metastasis to the pancreas: Report of a rare case and a review of the literature. JOP 2014, 15, 63–65. [Google Scholar] [CrossRef] [PubMed]
- Lucci, R.; Anna, M.D.; Marano, A.; Vigliar, E.; Avellino, M.; Napolitano, V.; Troncone, G.; Bellevicine, C. Morphological and immunocytochemical features of Merkel cell carcinoma metastatic to the pancreas diagnosed by endoscopic ultrasound-guided fine-needle aspiration. Diagn. Cytopathol. 2017, 45, 629–630. [Google Scholar] [CrossRef]
- Bernstein, J.; Adeniran, A.J.; Cai, G.; Theoharis, C.G.; Ustun, B.; Beckman, D.; Aslanian, H.R.; Harigopal, M. Endoscopic ultrasound-guided fine-needle aspiration diagnosis of Merkel cell carcinoma metastatic to the pancreas. Diagn. Cytopathol. 2014, 42, 247–252. [Google Scholar] [CrossRef]





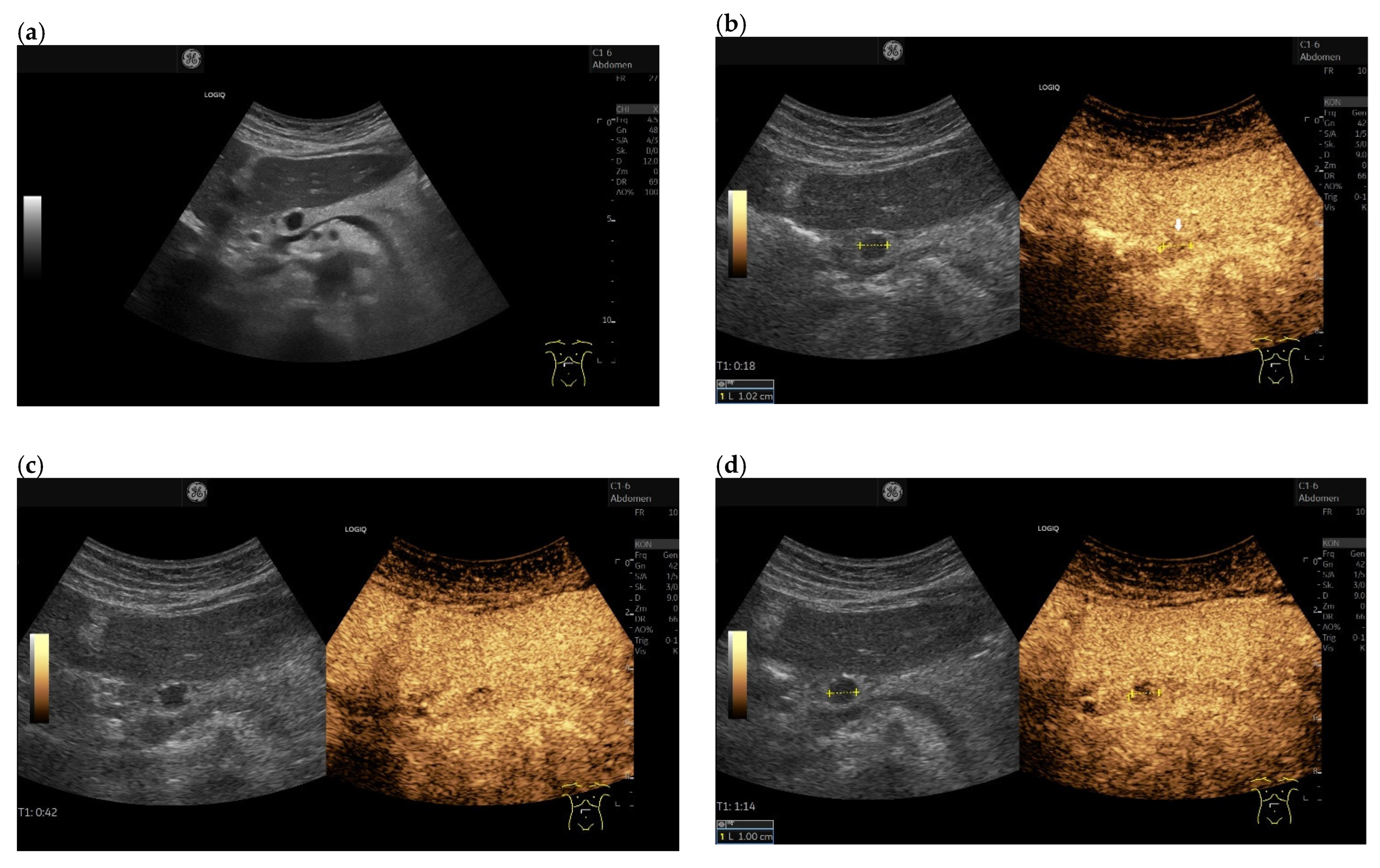
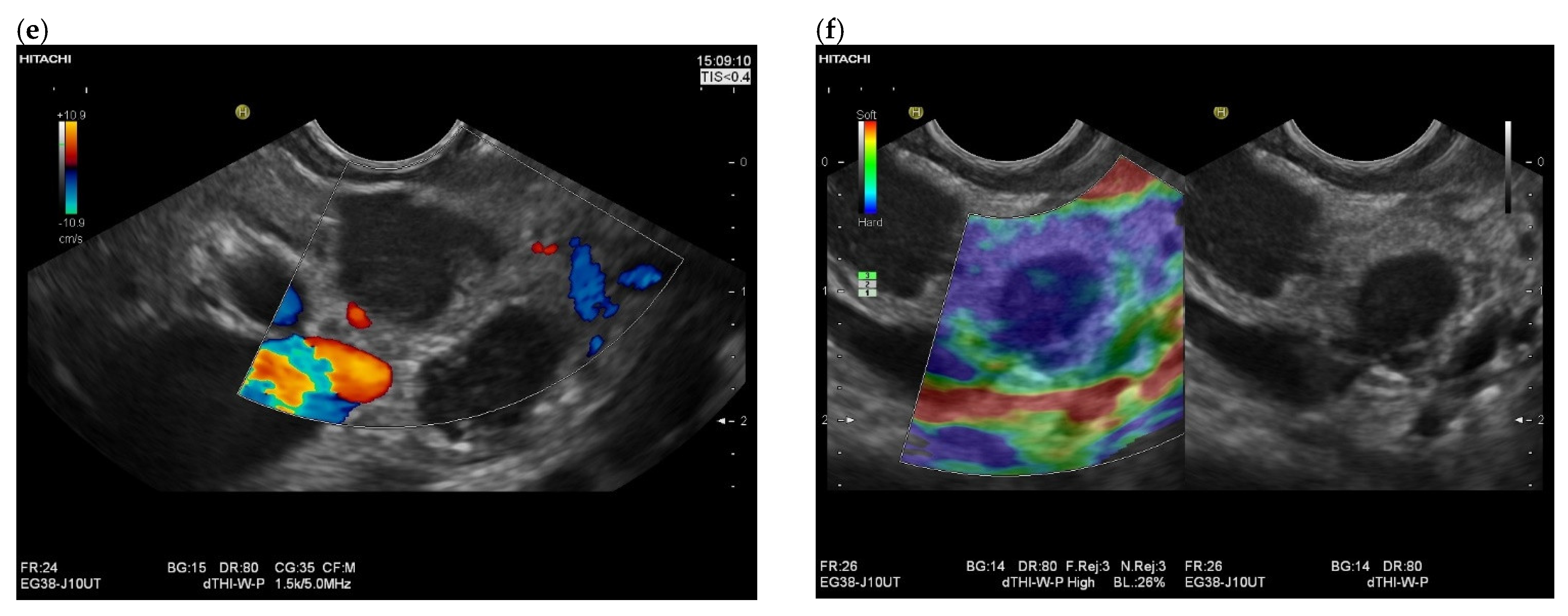


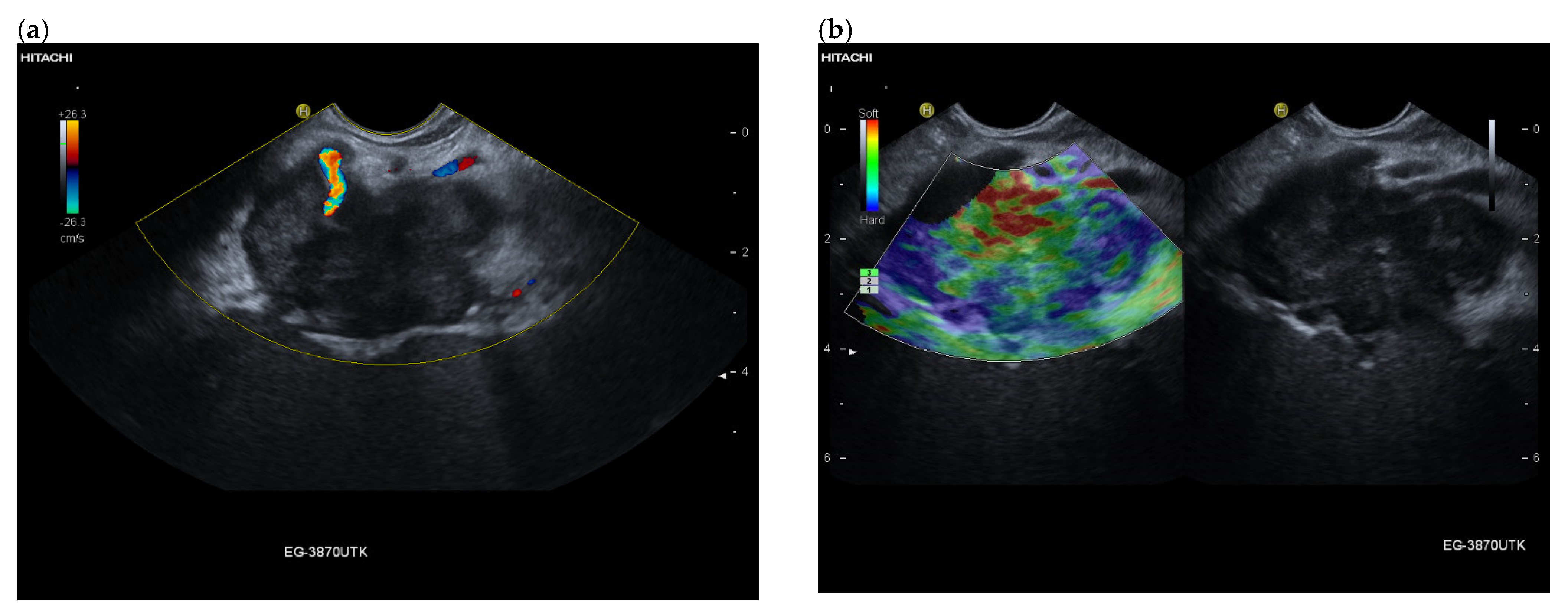

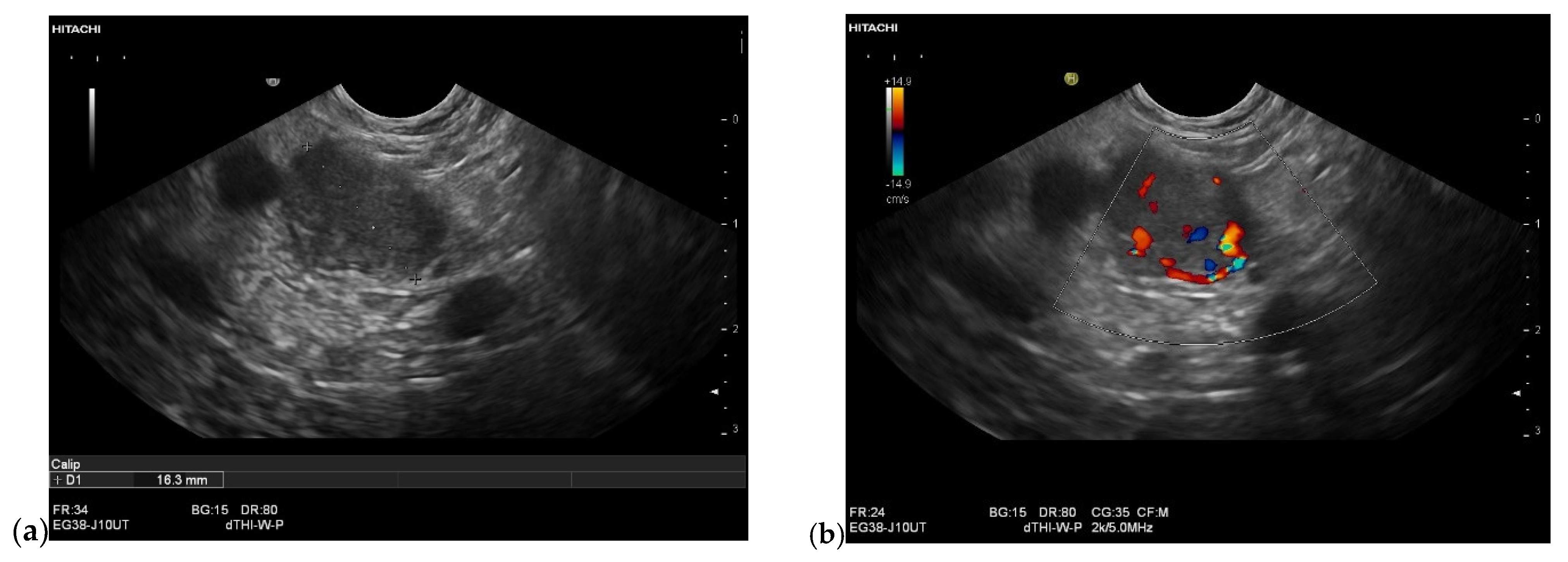

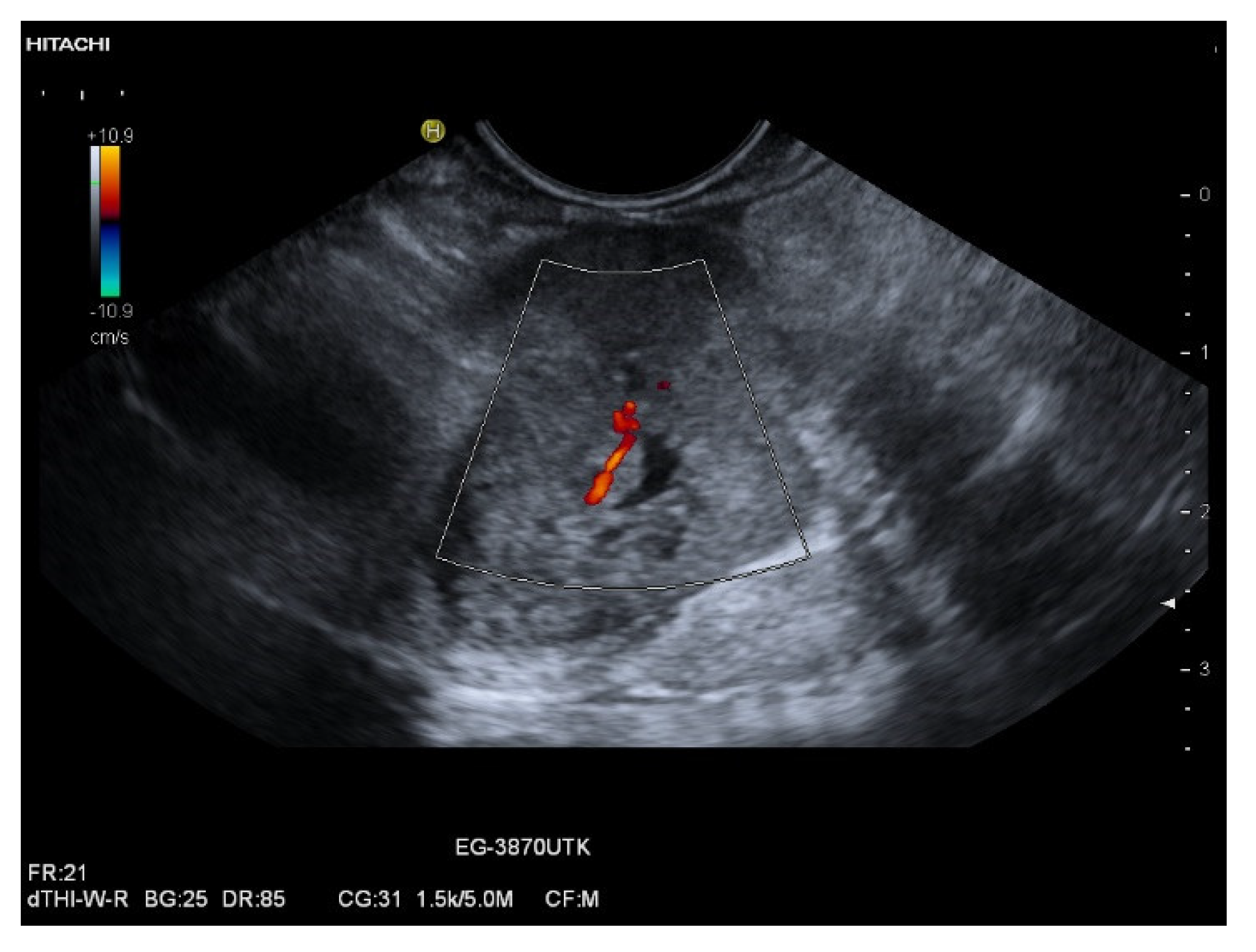

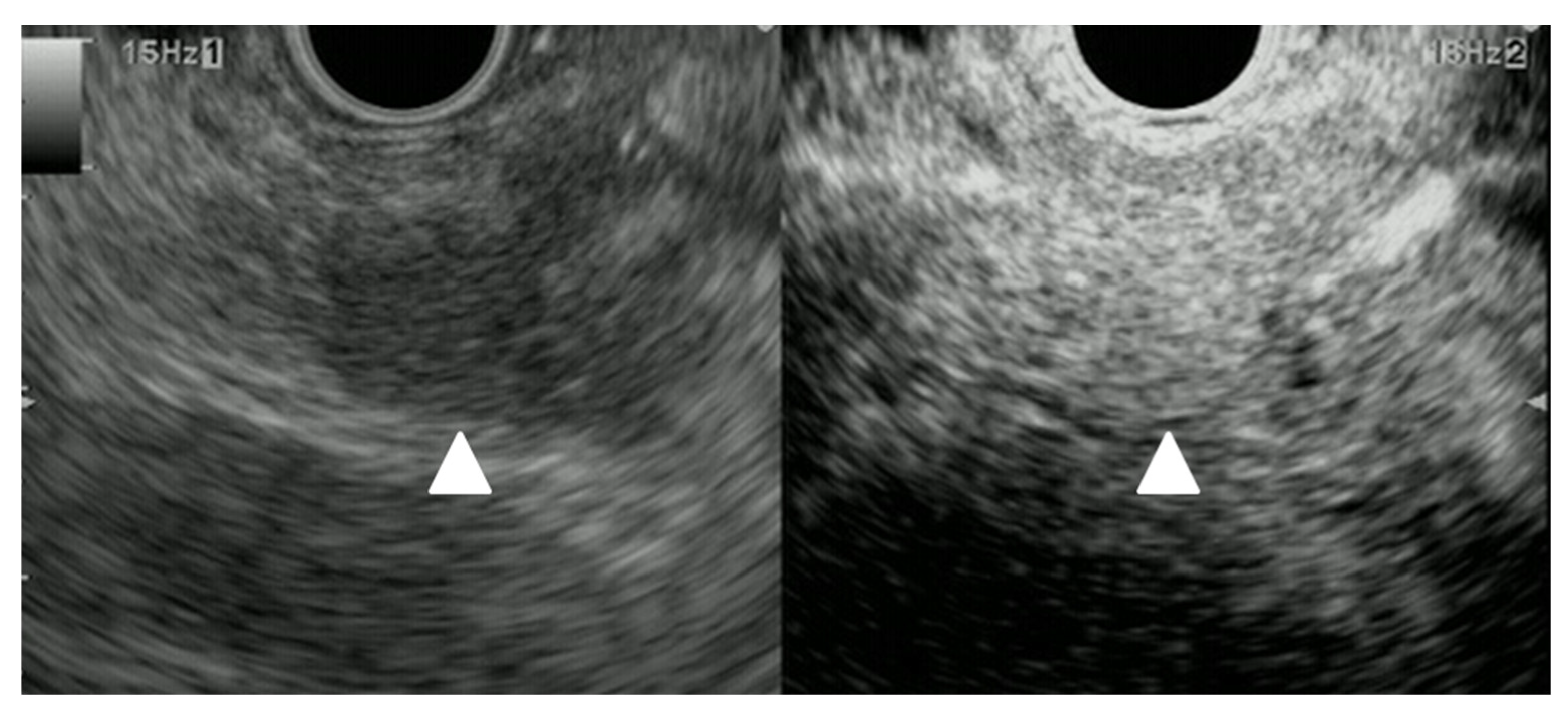
| Autopsy Study | Cases | Prevalence of Pancreatic Metastases Overall | Prevalence of Pancreatic Metastases in Relation to Primary Tumor Sites |
|---|---|---|---|
| Cifuentes 1979 [25] | n = 773 autopsy cases in patients with breast cancer | 13% | n.a. |
| Abrams 1950 [26] | n = 1000 autopsy cases with different carcinomas | 11.6% | Stomach 22.7% Ovary 16% Breast 13.8% Lung 10% Kidney 6% Colon 5.1% |
| Nakamura 2001 [27] | n = 1740 autopsies including 690 cases with malignant, non-primary pancreatic tumors and 103 cases with pancreatic metastases | 6% (103/1740) in all autopsies 15% in cases with malignant tumors | Papilla Vateri 75% Extrahepatic bile duct 50% Gallbladder 50% Stomach 35% Urinary bladder 25% Ovary 21% Lung 15% Thyroid 10% Breast 9% Kidney 9% Liver 5% Colorectum 5% Soft tissue 50% Retroperitoneum 20% Hematopoietic system 14% Skin 10% |
| Autopsy Study | All Cases | Secondary Pancreatic Tumors/Pancreatic Metastases | Proportion within the Secondary Pancreatic Tumor/Pancreatic Metastasis Group |
|---|---|---|---|
| Nakamura 2001 [27] | n = 1740 autopsies | n = 690 malignant tumors n = 103 pancreatic metastases | Stomach 20% Lung 17% Extrahepatic bile duct 13% Gall bladder 10% Liver 8% Breast 5% Ovary 3% Urinary bladder 3% Papilla Vateri 3% Colorectum 2% Kidney 1% Thyroid 1% Hematopoietic system 12% Leiomyosarcoma 2% Melanoma 1% |
| Adsay 2004 [32] | n = 4955 Autopsy cases with different indications, tumor and non-tumor patients | n = 190 cases with pancreatic tumors n = 81 cases (43%) with secondary pancreatic tumor/pancreatic metastases | Lung 42% Gastrointestinal tract 24.7% Kidney 5% Breast 3.7% Liver 2.5% Ovary 1.2% Urinary bladder 1.2% Hematopoietic origin 6% Melanoma 2% Sarcoma 2% Mesothelioma 2% Undetermined 5% |
| Study | Cases | Secondary Pancreatic Tumors/Pancreatic Metastases | Proportion within the Secondary Pancreatic Tumor/Pancreatic Metastasis Group |
|---|---|---|---|
| Cohort studies | |||
| Hiotis 2002 [34] | Surgical resection (Single institution experience) | n = 16 | RCC 62%; non-small-cell lung cancer 18.7%; sarcoma 6.3%; melanoma 6.3%; or transitional cell carcinoma of the bladder 6.3% |
| Adsay 2004 [32] | Surgical specimen n = 973 (Multicenter study) | n = 38 (3.9%) (n = 17 resections and n = 21 large-core needle biopsies) | Non-Hodgkin-Lymphoma 29%; stomach carcinoma 18.7%; renal cell carcinoma 15.7%; lung carcinoma 5.3%; prostate, liver, ovary, uterus, and Merkel cell carcinoma—2.6% for each one; malignant gastrointestinal stromal tumor 7.9%; leiomyosarcoma 2.6%; unknown origin 7.9% |
| Reddy 2008 [35] | Surgical resections in isolated pancreatic metastases (Single institution experience) | n = 49 | RCC 42.8%; gallbladder cancer 12.2%; lung cancer 8,2%; ovarian cancer 8.2%; sarcoma 8.2%; melanoma 6.1%; colon cancer 4.1%; breast cancer 4.1%; hepatocellular carcinoma 4.1%; seminoma 4.1%; Langerhans cell histiocytosis 4.1%; nonpancreatic endocrine cancer 4.1% |
| Reddy 2009 [36] | Review/metanalysis of surgical resections of isolated pancreatic metastases (Metaanalysis) | n = 243 | RCC 61.7%; colon cancer 7.8%; melanoma 4.9%; sarcoma 4.9%; lung cancer 3.3%, gastric cancer 3.3%; gall bladder cancer 3.3%; breast cancer 2.5%; ovarian cancer 2.1%; gastrointestinal stromal tumor 0.8%; esophageal cancer 0.8%; mesenteric fibromatosis 0.8%; schwannoma 0.8%; seminoma 0.4%; teratocarcinoma 0.4%; hemangiopericytoma 0.4%; urinary bladder cancer 0.4%; carcinoid 0.4%; non-pancreatic endocrine tumor 0.4%; hepatocellular carcinoma 0.4% |
| Yoon 2011 [37] | Surgical resection (Single institution study) | n = 53 Surgical resection of pancreatic metastasis | RCC 26.4%; gastric cancer 20.8%; colorectal cancer 9.4%; lymphoma 7.5%; non-small cell lung cancer 5.7%; gastrointestinal stromal tumor 3.8%; melanoma 3.8%; small cell lung cancer 3.8%; gallbladder cancer 3.8%; hepatocellular carcinoma 1.9%; thymic carcinoid 1.9%; liposarcoma 1.9%; cholangiocarcinoma 1.9%; osteosarcoma 1.9%; breast cancer 1.9%; duodenal cancer 1.9%; ovarian cancer 1.9% |
| Sperti 2014 [48] | Meta-analysis of surgical resection | n = 418 | RCC 70%; melanoma 9.1%; colorectal cancer 8.9%; breast cancer 4.5%; sarcoma 4.3%; lung cancer 3.1% |
| Dietrich 2016 [24] | EUS-guided sampling/surgery Tumors smaller than 15 mm, Multicenter study | n = 394 pancreatic tumors </= 15 mm n = 28 pancreatic metastases (7.1%) | RCC 42.9%; lung cancer 25%; melanoma 10.7%; breast and ovarian cancer each 7.1%; anal and thyroid cancer each 3.6% |
| Madkhali 2018 [38] | Surgical resection (Single institution experience) | n = 29 Surgical resection of pancreatic metastases | RCC 58.6%; colon cancer 17.2%; each one 3.4%: transitional cell carcinoma, hemangiopericytoma, spindle cell neoplasm, hepatocellular carcinoma, serous adenocarcinoma (peritoneum), cholangiocarcinoma (gall bladder), serous papillary adenocarcinoma (ovary) |
| Ito 2018 [49] | Surgical resection, (Multicenter analysis) | n = 159 Surgical resection of pancreatic metastases | RCC (38.4%); lung cancer (24.5%); colorectal cancer (11.3%); and sarcoma (6.3%) |
| DiFranco 2020 [40] | Surgical specimen n = 1000 pancreatic resections (Single institution experience) | n = 26 Surgical resection of pancreatic metastases | RCC 80.8%; lung tumors 7.7%; colon cancer, endometrial stromal sarcoma of uterus, and embryonal carcinoma of the testis—3.8% each one |
| Meta-Analyses | |||
| Adler 2014 [43] | Surgical resection (meta-analysis of 18 reports including 399 patients) | n = 399 Surgical resection of pancreatic metastasis | RCC62.6%; sarcoma 7.2%; colorectal cancer 6.2%; ovarian cancer 4.7%; melanoma 4%; lung 2%; adenocarcinoma 4.5%; other primary tumors 12.8% |
| Huang 2018 [39] | Surgical resection, (Single institution experience and meta-analysis | n = 414 Surgical resection of pancreatic metastasis | RCC54.3%; colorectal cancer 9.9%; sarcoma 4.3%; malignant melanoma 4.5%; lung cancer 5.4%; ovarian adenocarcinoma 4.5%; and gastric cancer 4.7%; 24 different kinds of tumors 12.4% |
| Study | Number of Cases | Proportion within the Secondary Pancreatic Tumor/Pancreatic Metastasis Group |
|---|---|---|
| US-guided sampling | ||
| Olson 2013 [50] | n = 2389 malignant aspirates of 5495 aspirates of US-guided pancreas FNA procedures, n = 42 metastases | Kidney (RCC, solitary fibrous tumor/hemangiopericytoma) (38.1%), skin (melanoma, Merkel cell, sebaceous) (19%), lung (adenocarcinoma, squamous cell, small cell) (14.3%), breast (ductal adenocarcinoma) (4.8%), liver (hepatocellular carcinoma) (4.8%), ovary (serous) (4.8%), soft tissue (pleomorphic sarcoma, malignant peripheral nerve sheath tumor) (4.8%), brain (solitary fibrous tumor/hemangiopericytoma) (2.4%), small intestine (gastrointestinal stromal tumor) (2.4%), larynx (squamous cell) (2.4%), thyroid (papillary thyroid carcinoma) (2.4%). |
| EUS-guided sampling | ||
| DeWitt 2005 [51] | n = 24 metastases | Kidney (41.7%), skin (25%), lung (16.7%), colon (8.3%), liver (4.2%), and stomach (4.2%) cancer |
| Layfield 2010 [52] | n = 2318 EUS-guided sampling of FPL, n = 17 metastases of 222 neoplasms of the pancreas | RCC 47%, medullary thyroid carcinoma 5.9%, lymphoma 23.5%, alveolar rhabdomyosarcoma 5.9%, squamous cell carcinoma of pulmonary origin 5.9%, small cell lung carcinoma 5.9% |
| Gagovic 2012 [23] | n = 230 FPL: PDAC n = 144, non-PDAC, non-metastases n = 38, metastases n = 10 | Melanoma (30%), small cell lung cancer (30%), high-grade soft tissue sarcoma (20%), papillary serous/metastatic ovarian cancer (10%), breast cancer (10%) |
| Waters 2014 [53] | n = 1406 EUS-guides samplings of FPL, n = 66 metastases | Renal cancer (41%), pulmonary (14%), skin (9%), breast (9%), colon cancer (7%), various other sites (20%) |
| Sekulic 2017 [22] | n = 25 metastases | Kidney (40%), colon (16%), ovary (12%), lung (8%, breast (4%), other (5%)) |
| Hou 2018 [54] | n = 30 metastases | RCC 37%, lung cancer 16,7%, melanoma 10%, sarcoma 10%, colon carcinoma 6.7%, breast cancer 6.7%, ovary cancer 3,3%, unknown 10%. |
| Pancreatic fine-needle aspiration (FNA) without specification of sampling method | ||
| Smith 2015 [55] | n = 22 metastases in n = 2327 pancreatic FNA | RCC 63.6%, colonic adenocarcinomas (9.1%), urothelial carcinoma (4.5%), non–small cell lung carcinoma (4.5%), ovarian serous carcinoma (4.5%), prostatic adenocarcinoma (4.5%), papillary thyroid carcinoma (4.5%), and mesenchymal chondrosarcoma (4.5%) |
| EUS-guided sampling or computerized tomographic-guided sampling | ||
| Raymond 2017 [30] | n = 16 metastases in 636 pancreatic samplings (60% EUS-guided, 40% CT-guided) | Lung cancer (adenocarcinoma, adenosquamous carcinoma, small cell carcinoma) (38%), RCC 19%, mucinous colon adenocarcinoma (12.5%), gastric adenocarcinoma (6.2%), malignant melanoma (6.2%), Merkel cell carcinoma (6.2%), gall bladder small cell carcinoma (6.2%), olfactory neuroblastoma 6.2% |
| Method | Pancreatic Metastases | PDAC | PanNENs (71) |
|---|---|---|---|
B-mode US/EUS
| Mostly hypoechoic, homogeneous, or heterogeneous More likely well-defined borders (46%) Anechoic and hyperechoic lesions are possible | Hypoechoic, typically heterogeneous, irregular borders | Hypoechoic, mostly homogeneous, smoothly bordered. Cystic components or cystic solid PanNENs are possible |
| Variable, in 80% no pancreatic duct dilatation | Pancreatic duct stenosis and pancreatic duct dilatation are an early and typical feature | No pancreatic duct dilatation |
| Mostly no infiltration into adjacent vessels | Infiltration around and into the vessels | No infiltration into adjacent vessels |
| Colour Doppler Imaging | RCC metastases are hypervascularized Most other pancreatic metastases are hypovascularized | No hypervascularization | Hypervascularized |
| Elastography (small lesions up to 15 mm) [67] | 41% softer or isoelastic, 59% stiffer compared to pancreatic parenchyma | 4% soft or isoelastic, 96% stiffer compared to pancreatic parenchyma | 64% soft or isoelastic, 36% stiffer compared to pancreatic parenchyma |
| Cases | CEUS | CH-EUS | ||
|---|---|---|---|---|
| Arterial Phase | Venous Phase | Arterial Phase | Venous Phase | |
| RCC metastases [83] (n = 4) | Hyperenhancement, Early | Hyperenhancement | ||
| RCC metastases [61] (n = 3) | Hyperenhancement, homogeneous pattern | Slow washout | ||
| RCC metastasis [86] (n = 1) | Hyperenhancement, Inhomogeneous pattern | No washout | ||
| Melanoma metastasis [63] (n = 1) | Iso- to slightly hypoenhanced | Hypoenhanced | ||
| Melanoma metastasis [87] (n = 1) | Isoenhanced | Hypoenhancement of the peripheral rim, central non-enhancement | ||
| Melanoma metastasis [61] (n = 1) | Isoenhanced, heterogeneous | Fast washout | ||
| SCLC metastasis [83] (n = 1) | Hyperenhancement | Rapid washout | ||
| Breast, ovarian, colon metastases, sarcoma metastases [61] (n = 6) | Hypoenhancement, homogeneous or heterogeneous | Fast or slow washout | ||
| Lymphoma metastasis [61] (n = 1) | Hyperenhancement, homogeneous pattern | Fast washout |
| Study and Number of All Patients with Pancreatic Metastases | Primary Tumor | Method | Correct Diagnosis and Problems |
|---|---|---|---|
| Atiq 2013 [64] n = 23 | Lung 21.7%, RCC 17.4%, colon 17.4%, lymphoma 17.4%, melanoma 13.0%, and other | Cytology, cell blocks, recovery of specimens for flow cytometry. Immunohistochemical staining as determined necessary by the cytopathologist | Accuracy 91.3%. 5/23—only suggestive of a malignant lesion. 2/23—nondiagnostic |
| El Hajj 2013 [60] n = 49 | RCC 42.8%, lung 16.3%, melanoma 12.2%, colon 8.2%, breast 6.1% and other | Cytomorphology alone in 63%, cytomorphology and immunocytochemistry in 33%, surgical pathology examination alone in 4% | EUS-TCB after EUS-FNA was performed in n = 2 with negative cytology. Diagnosis of RCC metastasis was confirmed in n = 1, n = 1 was again false negative. |
| Krishna 2015 [19] n = 53 | Kidney 28.3%, lung 18.9%, melanoma 9.4%, breast 7.8%, other | Cytology and immunohistochemical staining | Sensitivity 84.9%, specificity 100%, accuracy 98.8% False negative: 4 RCC, 1 gall bladder cancer, 1 breast cancer, 1 leiomyosarcoma, 1 unknow primary (lack of immunohistochemistry markers) |
| Hou 2018 [54] n = 30 | Kidney 36.7%, lung 16.7%, skin 10%, soft tissue 10%, colon 6.7%, breast 6.7%, ovary 3.3%, eye 3.3%, and 6.7% cases with unknown primary sites. | Combination of cytomorphology and ancillary studies. Immunohistochemistry was performed on cell block sections | 28/30 93.3% 2 misdiagnoses: 1 pleomorphic carcinoma, 1 liposarcoma. Lack of immunohistochemistry because of a lack of diagnostic materials in cell blocks. |
| Abdallah 2022 [21] n = 8 | 5 RCC, 1 breast carcinoma, 1 breast adenocarcinoma/small cell lung carcinoma, 1 gastric carcinoma | Cytology | Positive for malignancy in 62.5% (5 RCC), 25.0% atypical cytology, negative for malignancy in 12.5% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Möller, K.; Jenssen, C.; Braden, B.; Hocke, M.; Yamashita, Y.; Arcidiacono, P.G.; Ignee, A.; D’Onofrio, M.; Fusaroli, P.; Bhutani, M.S.; et al. Comments on and Illustrations of the EFSUMB CEUS Guidelines: Transabdominal and Endoscopic Ultrasound Features of Intrapancreatic Metastases and the Role of Multiparametric Imaging and EUS-Guided Sampling in Rare Pancreatic Tumors. Cancers 2023, 15, 2546. https://doi.org/10.3390/cancers15092546
Möller K, Jenssen C, Braden B, Hocke M, Yamashita Y, Arcidiacono PG, Ignee A, D’Onofrio M, Fusaroli P, Bhutani MS, et al. Comments on and Illustrations of the EFSUMB CEUS Guidelines: Transabdominal and Endoscopic Ultrasound Features of Intrapancreatic Metastases and the Role of Multiparametric Imaging and EUS-Guided Sampling in Rare Pancreatic Tumors. Cancers. 2023; 15(9):2546. https://doi.org/10.3390/cancers15092546
Chicago/Turabian StyleMöller, Kathleen, Christian Jenssen, Barbara Braden, Michael Hocke, Yasunobu Yamashita, Paolo Giorgio Arcidiacono, André Ignee, Mirko D’Onofrio, Pietro Fusaroli, Manoop S. Bhutani, and et al. 2023. "Comments on and Illustrations of the EFSUMB CEUS Guidelines: Transabdominal and Endoscopic Ultrasound Features of Intrapancreatic Metastases and the Role of Multiparametric Imaging and EUS-Guided Sampling in Rare Pancreatic Tumors" Cancers 15, no. 9: 2546. https://doi.org/10.3390/cancers15092546
APA StyleMöller, K., Jenssen, C., Braden, B., Hocke, M., Yamashita, Y., Arcidiacono, P. G., Ignee, A., D’Onofrio, M., Fusaroli, P., Bhutani, M. S., Dong, Y., Sun, S., Faiss, S., & Dietrich, C. F. (2023). Comments on and Illustrations of the EFSUMB CEUS Guidelines: Transabdominal and Endoscopic Ultrasound Features of Intrapancreatic Metastases and the Role of Multiparametric Imaging and EUS-Guided Sampling in Rare Pancreatic Tumors. Cancers, 15(9), 2546. https://doi.org/10.3390/cancers15092546








