Oral Microbiome Community Composition in Head and Neck Squamous Cell Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.1.1. UM SPORE Project 3
2.1.2. Case–Control Study
2.2. Measurements
2.2.1. Lifestyle Information, Risk Factors, and Nutritional Information
2.2.2. Oral Wash Samples
2.2.3. DNA Extraction and Quantification
2.2.4. 16S rRNA Sequencing
2.2.5. Sequence Processing
2.2.6. Statistical Analysis
3. Results
3.1. Study Population
3.2. Associations among Operation Taxonomic Units (OTU) and HNSCC
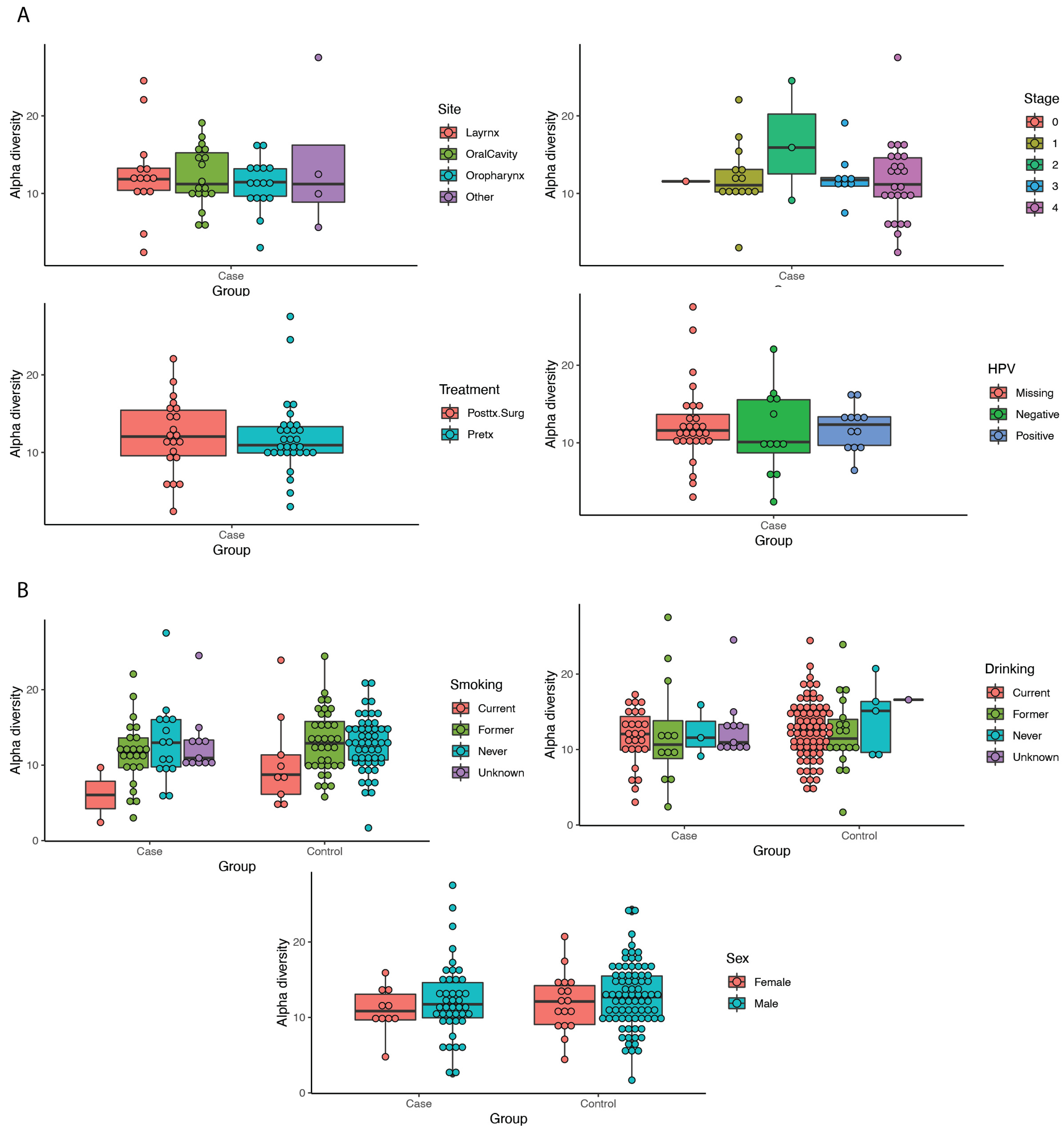
3.3. Diversity Metrics
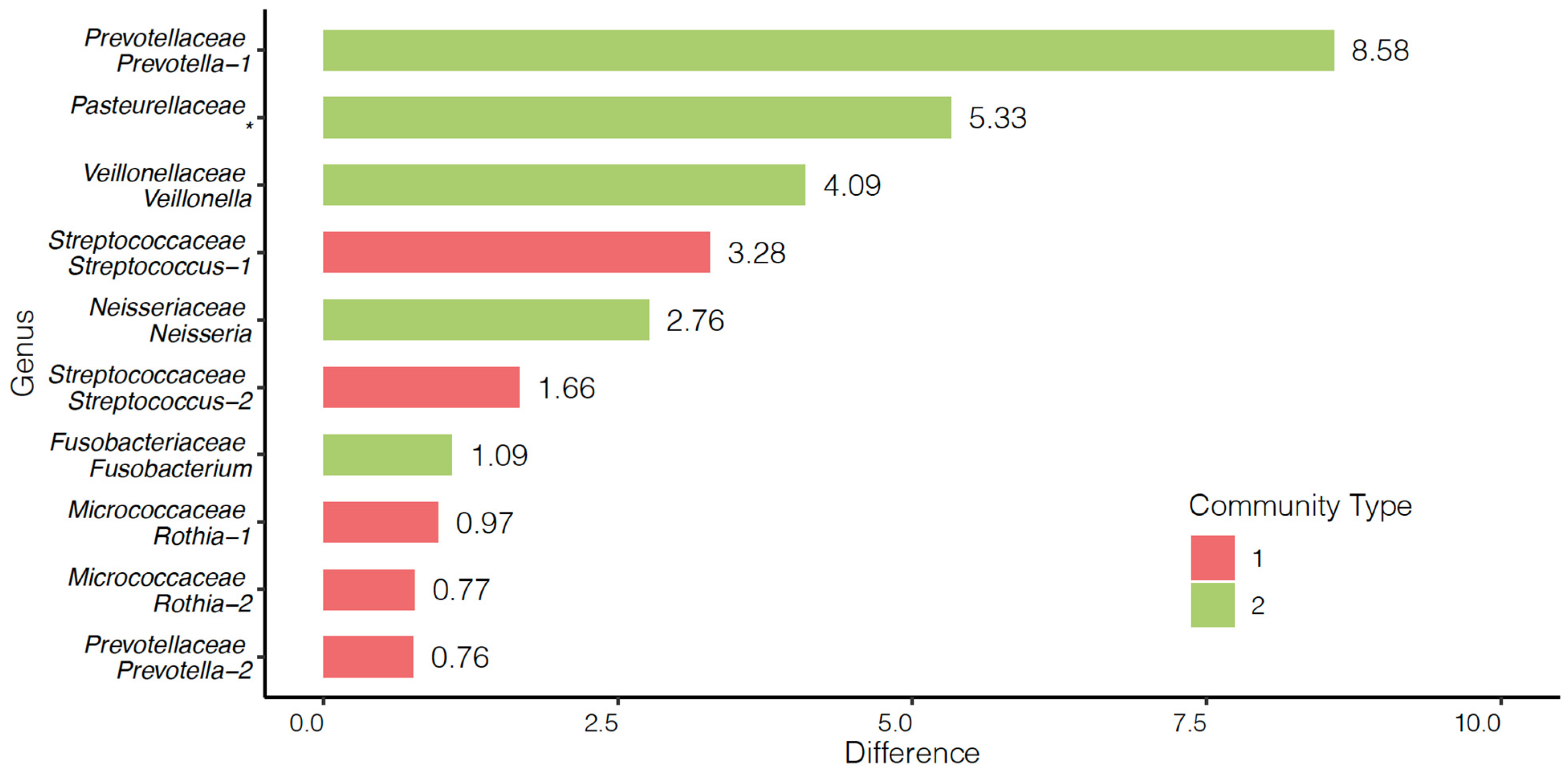
3.4. Community and Survival Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IARC. Cancer Fact Sheets: Number of New Cases and Deaths; IARC: Lyon, France, 2020. [Google Scholar]
- Burcher, K.M.; Burcher, J.T.; Inscore, L.; Bloomer, C.H.; Furdui, C.M.; Porosnicu, M. A Review of the Role of Oral Microbiome in the Development, Detection, and Management of Head and Neck Squamous Cell Cancers. Cancers 2022, 14, 4116. [Google Scholar] [CrossRef] [PubMed]
- Utter, D.R.; Borisy, G.G.; Eren, A.M.; Cavanaugh, C.M.; Mark Welch, J.L. Metapangenomics of the Oral Microbiome Provides Insights into Habitat Adaptation and Cultivar Diversity. Genome Biol. 2020, 21, 293. [Google Scholar] [CrossRef]
- Aas, J.A.; Paster, B.J.; Stokes, L.N.; Olsen, I.; Dewhirst, F.E. Defining the Normal Bacterial Flora of the Oral Cavity. J. Clin. Microbiol. 2005, 43, 5721–5732. [Google Scholar] [CrossRef]
- Dewhirst, F.E.; Chen, T.; Izard, J.; Paster, B.J.; Tanner, A.C.R.; Yu, W.H.; Lakshmanan, A.; Wade, W.G. The Human Oral Microbiome. J. Bacteriol. 2010, 192, 5002–5017. [Google Scholar] [CrossRef]
- Lamont, R.J.; Koo, H.; Hajishengallis, G. The Oral Microbiota: Dynamic Communities and Host Interactions. Nat. Rev. Microbiol. 2018, 16, 745–759. [Google Scholar] [CrossRef]
- Kamada, N.; Seo, S.U.; Chen, G.Y.; Núñez, G. Role of the Gut Microbiota in Immunity and Inflammatory Disease. Nat. Rev. Immunol. 2013, 13, 321–335. [Google Scholar] [CrossRef]
- Kamada, N.; Chen, G.Y.; Inohara, N.; Núñez, G. Control of Pathogens and Pathobionts by the Gut Microbiota. Nat. Immunol. 2013, 14, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Segers, S.; Hayes, R.B. Periodontal Disease, Porphyromonas gingivalis Serum Antibody Levels and Orodigestive Cancer Mortality. Carcinogenesis 2012, 33, 1055–1058. [Google Scholar] [CrossRef] [PubMed]
- Bolz, J.; Dosá, E.; Schubert, J.; Eckert, A.W. Bacterial Colonization of Microbial Biofilms in Oral Squamous Cell Carcinoma. Clin. Oral Investig. 2014, 18, 409–414. [Google Scholar] [CrossRef]
- Nagy, K.N.; Sonkodi, I.; Szöke, I.; Nagy, E.; Newman, H.N. The Microflora Associated with Human Oral Carcinomas. Oral Oncol. 1998, 34, 304–308. [Google Scholar] [CrossRef]
- Schmidt, B.L.; Kuczynski, J.; Bhattacharya, A.; Huey, B.; Corby, P.M.; Queiroz, E.L.S.; Nightingale, K.; Kerr, A.R.; DeLacure, M.D.; Veeramachaneni, R.; et al. Changes in Abundance of Oral Microbiota Associated with Oral Cancer. PLoS ONE 2014, 9, e98741. [Google Scholar] [CrossRef]
- He, J.; Li, Y.; Cao, Y.; Xue, J.; Zhou, X. The Oral Microbiome Diversity and Its Relation to Human Diseases. Folia Microbiol. 2015, 60, 69–80. [Google Scholar] [CrossRef]
- Han, Y.W.; Wang, X. Mobile Microbiome: Oral Bacteria in Extra-Oral Infections and Inflammation. J. Dent. Res. 2013, 92, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Hayes, R.B.; Ahn, J.; Fan, X.; Peters, B.A.; Ma, Y.; Yang, L.; Agalliu, I.; Burk, R.D.; Ganly, I.; Purdue, M.P.; et al. Association of Oral Microbiome with Risk for Incident Head and Neck Squamous Cell Cancer. JAMA Oncol. 2018, 4, 358–365. [Google Scholar] [CrossRef]
- Shillitoe, E.J. The Microbiome of Oral Cancer. Crit. Rev. Oncog. 2018, 23, 153–160. [Google Scholar] [CrossRef]
- Shin, J.M.; Luo, T.; Kamarajan, P.; Fenno, J.C.; Rickard, A.H.; Kapila, Y.L. Microbial Communities Associated with Primary and Metastatic Head and Neck Squamous Cell Carcinoma—A High Fusobacterial and Low Streptococcal Signature. Sci. Rep. 2017, 7, 9934. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Domingue, J.C.; Sears, C.L. Microbiota Dysbiosis in Select Human Cancers: Evidence of Association and Causality. Semin. Immunol. 2017, 32, 25–34. [Google Scholar] [CrossRef]
- Mager, D.L.; Haffajee, A.D.; Delvin, P.M.; Norris, C.M.; Posner, M.R.; Goodson, J.M. The Salivary Microbiota as a Diagnostic Indicator of Oral Cancer: A Descriptive, Non-Randomized Study of Cancer-Free and Oral Squamous Cell Carcinoma Subjects. J. Transl. Med. 2005, 3, 27. [Google Scholar] [CrossRef] [PubMed]
- Hooper, S.J.; Crean, S.J.; Fardy, M.J.; Lewis, M.A.O.; Spratt, D.A.; Wade, W.G.; Wilson, M.J. A Molecular Analysis of the Bacteria Present within Oral Squamous Cell Carcinoma. J. Med. Microbiol. 2007, 56, 1651–1659. [Google Scholar] [CrossRef] [PubMed]
- Ganly, I.; Yang, L.; Giese, R.A.; Hao, Y.; Nossa, C.W.; Morris, L.G.T.; Rosenthal, M.; Migliacci, J.; Kelly, D.; Tseng, W.; et al. Periodontal Pathogens Are a Risk Factor of Oral Cavity Squamous Cell Carcinoma, Independent of Tobacco and Alcohol and Human Papillomavirus. Int. J. Cancer 2019, 145, 775–784. [Google Scholar] [CrossRef]
- Whitmore, S.E.; Lamont, R.J. Oral Bacteria and Cancer. PLoS Pathog. 2014, 10, e1003933. [Google Scholar] [CrossRef]
- Atanasova, K.R.; Yilmaz, O. Looking in the Porphyromonas gingivalis Cabinet of Curiosities: The Microbium, the Host and Cancer Association. Mol. Oral Microbiol. 2014, 29, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Sahingur, S.E.; Yeudall, W.A. Chemokine Function in Periodontal Disease and Oral Cavity Cancer. Front. Immunol. 2015, 6, 214. [Google Scholar] [CrossRef]
- Cugini, C.; Klepac-Ceraj, V.; Rackaityte, E.; Riggs, J.E.; Davey, M.E. Porphyromonas gingivalis: Keeping the Pathos out of the Biont. J. Oral Microbiol. 2013, 5, 19804. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N. Oral Microbiome Metabolism: From “Who Are They?” to “What Are They Doing?”. J. Dent. Res. 2015, 94, 1628–1637. [Google Scholar] [CrossRef]
- Okuyama, K.; Yanamoto, S. Oral Bacterial Contributions to Gingival Carcinogenesis and Progression. Cancer Prev. Res. 2023, 16, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Pushalkar, S.; Ji, X.; Li, Y.; Estilo, C.; Yegnanarayana, R.; Singh, B.; Li, X.; Saxena, D. Comparison of Oral Microbiota in Tumor and Non-Tumor Tissues of Patients with Oral Squamous Cell Carcinoma. BMC Microbiol. 2012, 12, 144. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, J.; Feng, Q.; Chen, B.; Li, M.; Liang, C.; Li, M.; Li, Z.; Xu, Q.; Zhang, L.; et al. Compositional and Functional Analysis of the Microbiome in Tissue and Saliva of Oral Squamous Cell Carcinoma. Front. Microbiol. 2019, 10, 1439. [Google Scholar] [CrossRef]
- Banerjee, S.; Tian, T.; Wei, Z.; Peck, K.N.; Shih, N.; Chalian, A.A.; O’Malley, B.W.; Weinstein, G.S.; Feldman, M.D.; Alwine, J.; et al. Microbial Signatures Associated with Oropharyngeal and Oral Squamous Cell Carcinomas. Sci. Rep. 2017, 7, 4036. [Google Scholar] [CrossRef]
- Yang, S.F.; Lin, C.W.; Chuang, C.Y.; Lee, Y.C.; Chung, W.H.; Lai, H.C.; Chang, L.C.; Su, S.C. Host Genetic Associations with Salivary Microbiome in Oral Cancer. J. Dent. Res. 2022, 101, 590–598. [Google Scholar] [CrossRef]
- Neuzillet, C.; Marchais, M.; Vacher, S.; Hilmi, M.; Schnitzler, A.; Meseure, D.; Leclere, R.; Lecerf, C.; Dubot, C.; Jeannot, E.; et al. Prognostic Value of Intratumoral Fusobacterium nucleatum and Association with Immune-Related Gene Expression in Oral Squamous Cell Carcinoma Patients. Sci. Rep. 2021, 11, 7870. [Google Scholar] [CrossRef]
- Chan, J.Y.K.; Cheung, M.K.; Lan, L.; Ng, C.; Lau, E.H.L.; Yeung, Z.W.C.; Wong, E.W.Y.; Leung, L.; Qu, X.; Cai, L.; et al. Characterization of Oral Microbiota in HPV and Non-HPV Head and Neck Squamous Cell Carcinoma and Its Association with Patient Outcomes. Oral Oncol. 2022, 135, 106245. [Google Scholar] [CrossRef]
- Chan, J.Y.K.; Ng, C.W.K.; Lan, L.; Fung, S.; Li, J.W.; Cai, L.; Lei, P.; Mou, Q.; Meehan, K.; Lau, E.H.L.; et al. Restoration of the Oral Microbiota after Surgery for Head and Neck Squamous Cell Carcinoma Is Associated with Patient Outcomes. Front. Oncol. 2021, 11, 737843. [Google Scholar] [CrossRef]
- Jo, R.; Nishimoto, Y.; Umezawa, K.; Yama, K.; Aita, Y.; Ichiba, Y.; Murakami, S.; Kakizawa, Y.; Kumagai, T.; Yamada, T.; et al. Comparison of Oral Microbiome Profiles in Stimulated and Unstimulated Saliva, Tongue, and Mouth-Rinsed Water. Sci. Rep. 2019, 9, 16124. [Google Scholar] [CrossRef]
- Flemer, B.; Lynch, D.B.; Brown, J.M.R.; Jeffery, I.B.; Ryan, F.J.; Claesson, M.J.; O’Riordain, M.; Shanahan, F.; O’Toole, P.W. Tumour-Associated and Non-Tumour-Associated Microbiota in Colorectal Cancer. Gut 2017, 66, 633–643. [Google Scholar] [CrossRef]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.M.; et al. Enterotypes of the Human Gut Microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Piccirillo, J.F.; Tierney, R.M.; Costas, I.; Grove, L.; Spitznagel, E.L. Prognostic Importance of Comorbidity in a Hospital-Based Cancer Registry. J. Am. Med. Assoc. 2004, 291, 2441–2447. [Google Scholar] [CrossRef]
- Kozich, J.J.; Westcott, S.L.; Baxter, N.T.; Highlander, S.K.; Schloss, P.D. Development of a Dual-Index Sequencing Strategy and Curation Pipeline for Analyzing Amplicon Sequence Data on the Miseq Illumina Sequencing Platform. Appl. Environ. Microbiol. 2013, 79, 5112–5120. [Google Scholar] [CrossRef] [PubMed]
- Seekatz, A.M.; Theriot, C.M.; Molloy, C.T.; Wozniak, K.L.; Bergin, I.L.; Young, V.B. Fecal Microbiota Transplantation Eliminates Clostridium Difficile in a Murine Model of Relapsing Disease. Infect. Immun. 2015, 83, 3838–3846. [Google Scholar] [CrossRef] [PubMed]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing Mothur: Open-Source, Platform-Independent, Community-Supported Software for Describing and Comparing Microbial Communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef]
- Schloss, P.D. Reintroducing Mothur: 10 Years Later. Appl. Environ. Microbiol. 2020, 86, e02343-19. [Google Scholar] [CrossRef]
- Holmes, I.; Harris, K.; Quince, C. Dirichlet Multinomial Mixtures: Generative Models for Microbial Metagenomics. PLoS ONE 2012, 7, e30126. [Google Scholar] [CrossRef]
- Lee, S.; Sun, W.; Wright, F.A.; Zou, F. An Improved and Explicit Surrogate Variable Analysis Procedure by Coefficient Adjustment. Biometrika 2017, 104, 303–316. [Google Scholar] [CrossRef]
- Acharya, A.; Chen, T.; Chan, Y.; Watt, R.M.; Jin, L.; Mattheos, N. Species-Level Salivary Microbial Indicators of Well-Resolved Periodontitis: A Preliminary Investigation. Front. Cell. Infect. Microbiol. 2019, 9, 347. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, H.; Ochiai, K.; Suzuki, N.; Otsuka, K. Butyrate, a Bacterial Metabolite, Induces Apoptosis and Autophagic Cell Death in Gingival Epithelial Cells. J. Periodontal Res. 2010, 45, 626–634. [Google Scholar] [CrossRef]
- Kistler, J.O.; Booth, V.; Bradshaw, D.J.; Wade, W.G. Bacterial Community Development in Experimental Gingivitis. PLoS ONE 2013, 8, e71227. [Google Scholar] [CrossRef] [PubMed]
- Abusleme, L.; Dupuy, A.K.; Dutzan, N.; Silva, N.; Burleson, J.A.; Strausbaugh, L.D.; Gamonal, J.; Diaz, P.I. The Subgingival Microbiome in Health and Periodontitis and Its Relationship with Community Biomass and Inflammation. ISME J. 2013, 7, 1016–1025. [Google Scholar] [CrossRef]
- Lee, W.H.; Chen, H.M.; Yang, S.F.; Liang, C.; Peng, C.Y.; Lin, F.M.; Tsai, L.L.; Wu, B.C.; Hsin, C.H.; Chuang, C.Y.; et al. Bacterial Alterations in Salivary Microbiota and Their Association in Oral Cancer. Sci. Rep. 2017, 7, 16540. [Google Scholar] [CrossRef] [PubMed]
- Sheng, W.S.; Hsueh, P.R.; Hung, C.C.; Teng, L.J.; Chen, Y.C.; Luh, K.T. Clinical Features of Patients with Invasive Eikenella corrodens Infections and Microbiological Characteristics of the Causative Isolates. Eur. J. Clin. Microbiol. Infect. Dis. 2001, 20, 231–236. [Google Scholar] [CrossRef]
- Chen, C.K.C.; Wilson, M.E. Eikenella corrodens in Human Oral and Non-Oral Infections: A Review. J. Periodontol. 1992, 63, 941–953. [Google Scholar] [CrossRef]
- Surna, A.; Kubilius, R.; Sakalauskiene, J.; Vitkauskiene, A.; Jonaitis, J.; Saferis, V.; Gleiznys, A. Lysozyme and Microbiota in Relation to Gingivitis and Periodontitis. Med. Sci. Monit. 2009, 15, CR66–CR73. [Google Scholar] [PubMed]
- Chattopadhyay, I.; Verma, M.; Panda, M. Role of Oral Microbiome Signatures in Diagnosis and Prognosis of Oral Cancer. Technol. Cancer Res. Treat. 2019, 18, 1533033819867354. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.Y.; Yeh, Y.M.; Yu, H.Y.; Chin, C.Y.; Hsu, C.W.; Liu, H.; Huang, P.J.; Hu, S.N.; Liao, C.T.; Chang, K.P.; et al. Oral Microbiota Community Dynamics Associated with Oral Squamous Cell Carcinoma Staging. Front. Microbiol. 2018, 9, 862. [Google Scholar] [CrossRef]
- Nørskov-Lauritsen, N. Classification, Identification, and Clinical Significance of Haemophilus and Aggregatibacter Species with Host Specificity for Humans. Clin. Microbiol. Rev. 2014, 27, 214–240. [Google Scholar] [CrossRef] [PubMed]
- Furquim, C.P.; Soares, G.M.S.; Ribeiro, L.L.; Azcarate-Peril, M.A.; Butz, N.; Roach, J.; Moss, K.; Bonfim, C.; Torres-Pereira, C.C.; Teles, F.R.F. The Salivary Microbiome and Oral Cancer Risk: A Pilot Study in Fanconi Anemia. J. Dent. Res. 2017, 96, 292–299. [Google Scholar] [CrossRef]
- Guerrero-Preston, R.; Godoy-Vitorino, F.; Jedlicka, A.; Rodríguez-Hilario, A.; González, H.; Bondy, J.; Lawson, F.; Folawiyo, O.; Michailidi, C.; Dziedzic, A.; et al. 16S RRNA Amplicon Sequencing Identifies Microbiota Associated with Oral Cancer, Human Papilloma Virus Infection and Surgical Treatment. Oncotarget 2016, 7, 51320. [Google Scholar] [CrossRef]
- Hsiao, J.R.; Chang, C.C.; Lee, W.T.; Huang, C.C.; Ou, C.Y.; Tsai, S.T.; Chen, K.C.; Huang, J.S.; Wong, T.Y.; Lai, Y.H.; et al. The Interplay between Oral Microbiome, Lifestyle Factors and Genetic Polymorphisms in the Risk of Oral Squamous Cell Carcinoma. Carcinogenesis 2018, 39, 778–787. [Google Scholar] [CrossRef] [PubMed]
- Al-Hebshi, N.N.; Nasher, A.T.; Maryoud, M.Y.; Homeida, H.E.; Chen, T.; Idris, A.M.; Johnson, N.W. Inflammatory Bacteriome Featuring Fusobacterium nucleatum and Pseudomonas aeruginosa Identified in Association with Oral Squamous Cell Carcinoma. Sci. Rep. 2017, 7, 1834. [Google Scholar] [CrossRef]
- Bik, E.M.; Long, C.D.; Armitage, G.C.; Loomer, P.; Emerson, J.; Mongodin, E.F.; Nelson, K.E.; Gill, S.R.; Fraser-Liggett, C.M.; Relman, D.A. Bacterial Diversity in the Oral Cavity of 10 Healthy Individuals. ISME J. 2010, 4, 962–974. [Google Scholar] [CrossRef]
- Burcham, Z.M.; Garneau, N.L.; Comstock, S.S.; Tucker, R.M.; Knight, R.; Metcalf, J.L.; Miranda, A.; Reinhart, B.; Meyers, D.; Woltkamp, D.; et al. Patterns of Oral Microbiota Diversity in Adults and Children: A Crowdsourced Population Study. Sci. Rep. 2020, 10, 2133. [Google Scholar] [CrossRef]
- Kang, W.; Sun, T.; Tang, D.; Zhou, J.; Feng, Q. Time-Course Transcriptome Analysis of Gingiva-Derived Mesenchymal Stem Cells Reveals That Fusobacterium nucleatum Triggers Oncogene Expression in the Process of Cell Differentiation. Front. Cell Dev. Biol. 2020, 7, 359. [Google Scholar] [CrossRef] [PubMed]
- Harrandah, A.M.; Chukkapalli, S.S.; Bhattacharyya, I.; Progulske-Fox, A.; Chan, E.K.L. Fusobacteria Modulate Oral Carcinogenesis and Promote Cancer Progression. J. Oral Microbiol. 2021, 13, 1849493. [Google Scholar] [CrossRef]
- Doke, M.; Fukamachi, H.; Morisaki, H.; Arimoto, T.; Kataoka, H.; Kuwata, H. Nucleases from Prevotella intermedia Can Degrade Neutrophil Extracellular Traps. Mol. Oral Microbiol. 2017, 32, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Kirst, M.E.; Li, E.C.; Alfant, B.; Chi, Y.Y.; Walker, C.; Magnusson, I.; Wanga, G.P. Dysbiosis and Alterations in Predicted Functions of the Subgingival Microbiome in Chronic Periodontitis. Appl. Environ. Microbiol. 2015, 81, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Caselli, E.; Fabbri, C.; D’Accolti, M.; Soffritti, I.; Bassi, C.; Mazzacane, S.; Franchi, M. Defining the Oral Microbiome by Whole-Genome Sequencing and Resistome Analysis: The Complexity of the Healthy Picture. BMC Microbiol. 2020, 20, 120. [Google Scholar] [CrossRef]
- Hoesseini, A.; van Leeuwen, N.; Offerman, M.P.J.; Zhang, J.; Dronkers, E.A.C.; Sewnaik, A.; Lingsma, H.F.; Baatenburg de Jong, R.J. Predicting Survival in Head and Neck Cancer: External Validation and Update of the Prognostic Model OncologIQ in 2189 Patients. Head Neck 2021, 43, 2445–2456. [Google Scholar] [CrossRef]
- Gopinath, D.; Menon, R.K.; Wie, C.C.; Banerjee, M.; Panda, S.; Mandal, D.; Behera, P.K.; Roychoudhury, S.; Kheur, S.; Botelho, M.G.; et al. Differences in the Bacteriome of Swab, Saliva, and Tissue Biopsies in Oral Cancer. Sci. Rep. 2021, 11, 1181. [Google Scholar] [CrossRef]
- Gupta, V.K.; Paul, S.; Dutta, C. Geography, Ethnicity or Subsistence-Specific Variations in Human Microbiome Composition and Diversity. Front. Microbiol. 2017, 8, 1162. [Google Scholar] [CrossRef]

| Bacterial Genus (Phylum/Class/Order/Family) | LogFc | p-Value | FDR |
|---|---|---|---|
| Lactobacillus (Firmicutes/Bacilli/Lactobacillales/Lactobacillaceae) | −7.72 | 0.0001 | 0.0082 |
| Unclassified (Firmicutes/Clostridia/Clostridiales/Lachnospiraceae) | 3.92 | 0.0020 | 0.0939 |
| Bacillus (Firmicutes/Bacilli/Bacillales/Bacillaceae_1/) | −3.72 | 0.0022 | 0.0939 |
| Acinetobacter (Proteobacter/Gammaproteobacteria/Pseudomonadales/Moraxeaceae) | −3.62 | 2.71 × 10−13 | 7.1 × 10−11 |
| Eikenella (Proteobacteria/Betaproteobacteria/Neisseriales/Neisseriaceae) | 5.81 | 1.9 × 10−9 | 2.5 × 10−7 |
| Unclassified (Actinobacteria/Actinobacteria/Bifidobacteriales/Bifidobacteriaceae) | −4.02 | 1.5 × 10−6 | 0.0001 |
| Group 1 | Group 2 | Group 1 Median | Group 2 Median | p-Value | P-adj (Holm) |
|---|---|---|---|---|---|
| Cases vs. Cases | Controls vs. Controls | 0.57 | 0.45 | 9.2 × 10−27 | 2.6 × 10−26 |
| Cases vs. Cases | Cases vs. Controls | 0.57 | 0.49 | 2.0 × 10−9 | 4.0 × 10−9 |
| Controls vs. Controls | Cases vs. Controls | 0.45 | 0.49 | 1.3 × 10−3 | 1.3 × 10−3 |
| Community 1 | Community 2 | ||
|---|---|---|---|
| Characteristics | (n = 109) | (n = 45) | p-Value |
| Age (mean ± S.D) | 57.57 ± 11.18 | 62.67 ± 10.68 | 0.0100 |
| BMI no. (%) a | 0.7820 | ||
| <20 | 1 (1.02) | 0 (0.00) | |
| 20–25 | 21 (21.43) | 11 (28.21) | |
| 25–30 | 38 (38.78) | 13 (33.33) | |
| >30 | 38 (38.78) | 15 (38.46) | |
| Sex no. (%) b | 0.5372 | ||
| Female | 18 (16.67) | 9 (20.93) | |
| Male | 90 (83.33) | 34 (79.07) | |
| Alcohol Use no. (%) c | 0.0770 | ||
| Never | 5 (5.15) | 3 (7.69) | |
| Former | 17 (17.53) | 13 (33.33) | |
| Current | 75 (77.32) | 23 (58.97) | |
| Smoking Status no. (%) d | 0.0062 | ||
| Never | 55 (56.12) | 11 (28.21) | |
| Former | 38 (38.78) | 22 (56.41) | |
| Current | 5 (5.10) | 6 (15.38) | |
| Case status | 0.0035 | ||
| Case | 29 (26.61) | 23 (51.11) | |
| Control | 80 (73.39) | 22 (48.89) | |
| Site | 0.2341 | ||
| Larynx | 8 (27.59) | 6 (26.09) | |
| Oral Cavity | 7 (24.14) | 11 (47.83) | |
| Oropharynx | 11 (37.93) | 5 (21.74) | |
| Hypopharynx | 0 (0.00) | 1 (4.35) | |
| Nasal cavity, sinus, or skull | 1 (3.45) | 0 (0.00) | |
| Unknown primary | 2 (6.90) | 0 (0.00) | |
| HPV Proxy Variable | 11 (10.09) | 5 (11.11) | 0.8505 |
| Stage | 0.0822 | ||
| 1 and 2 | 13 (44.83) | 5 (21.74) | |
| 3 and 4 | 16 (55.17) | 18 (78.26) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benjamin, W.J.; Wang, K.; Zarins, K.; Bellile, E.; Blostein, F.; Argirion, I.; Taylor, J.M.G.; D’Silva, N.J.; Chinn, S.B.; Rifkin, S.; et al. Oral Microbiome Community Composition in Head and Neck Squamous Cell Carcinoma. Cancers 2023, 15, 2549. https://doi.org/10.3390/cancers15092549
Benjamin WJ, Wang K, Zarins K, Bellile E, Blostein F, Argirion I, Taylor JMG, D’Silva NJ, Chinn SB, Rifkin S, et al. Oral Microbiome Community Composition in Head and Neck Squamous Cell Carcinoma. Cancers. 2023; 15(9):2549. https://doi.org/10.3390/cancers15092549
Chicago/Turabian StyleBenjamin, William J., Kai Wang, Katherine Zarins, Emily Bellile, Freida Blostein, Ilona Argirion, Jeremy M. G. Taylor, Nisha J. D’Silva, Steven B. Chinn, Samara Rifkin, and et al. 2023. "Oral Microbiome Community Composition in Head and Neck Squamous Cell Carcinoma" Cancers 15, no. 9: 2549. https://doi.org/10.3390/cancers15092549
APA StyleBenjamin, W. J., Wang, K., Zarins, K., Bellile, E., Blostein, F., Argirion, I., Taylor, J. M. G., D’Silva, N. J., Chinn, S. B., Rifkin, S., Sartor, M. A., & Rozek, L. S. (2023). Oral Microbiome Community Composition in Head and Neck Squamous Cell Carcinoma. Cancers, 15(9), 2549. https://doi.org/10.3390/cancers15092549






