Risk Estimation in Non-Enhancing Glioma: Introducing a Clinical Score
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
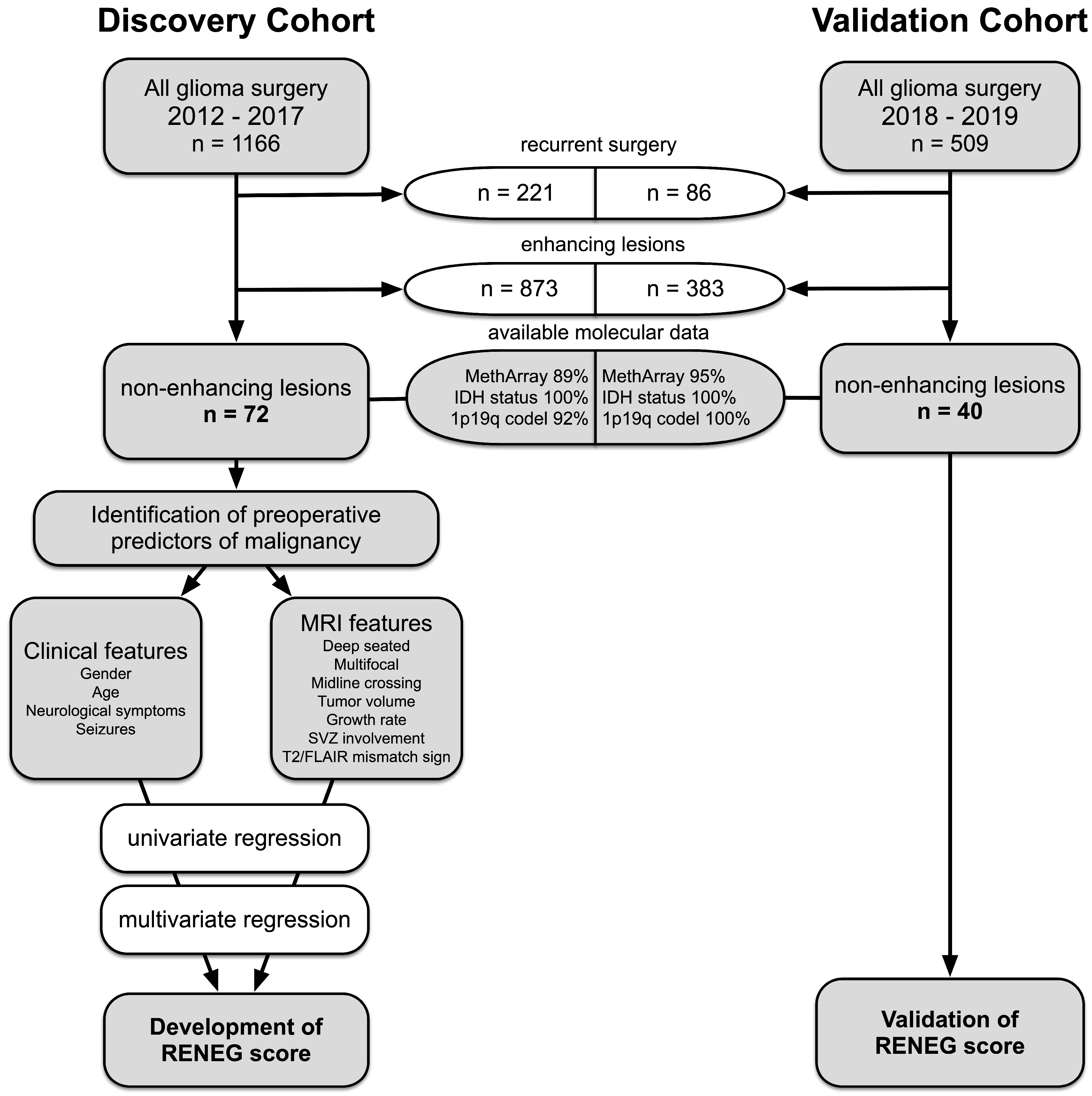
2.1. Patient Selection
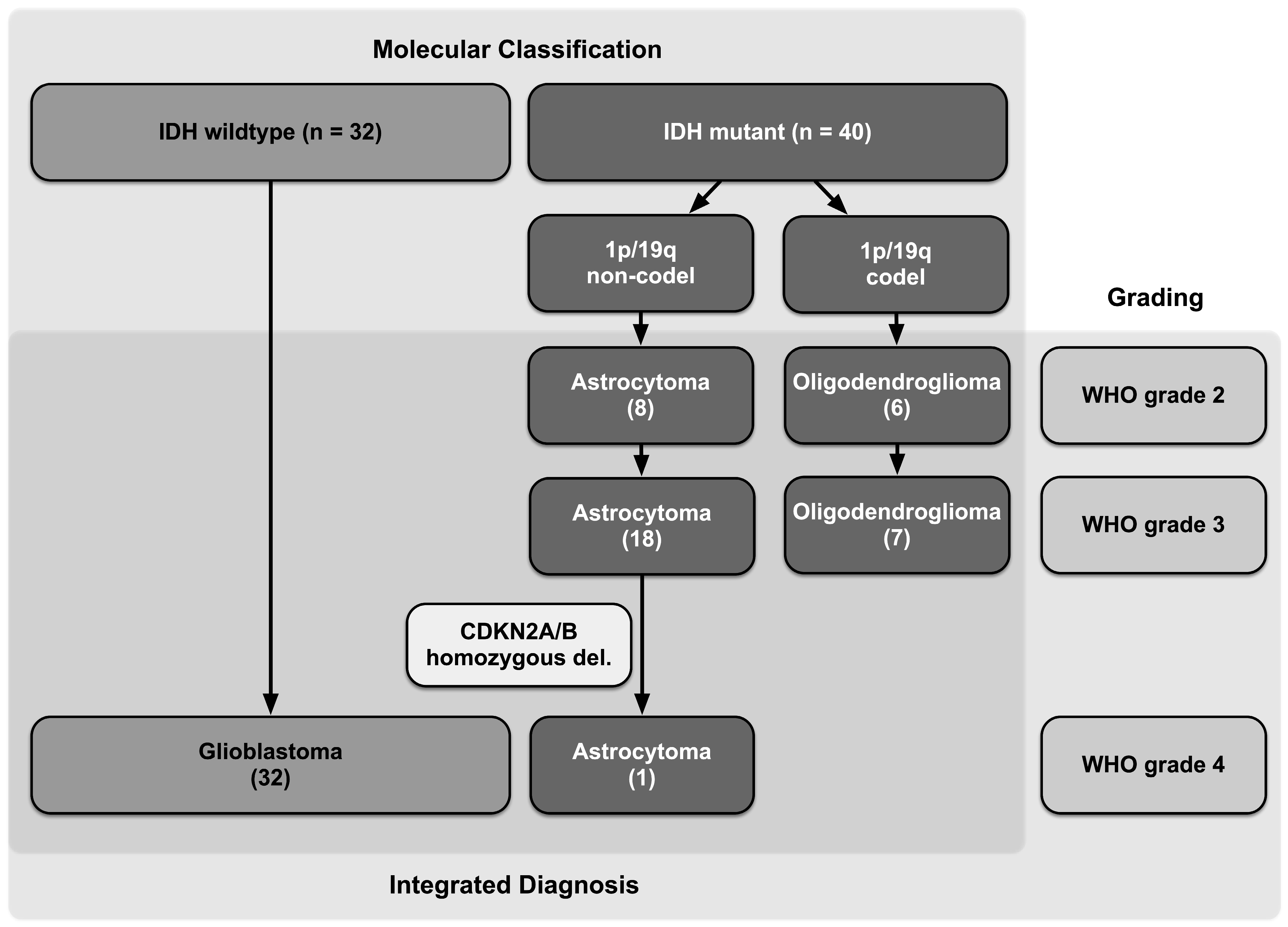
2.2. Histopathologic and Molecular Diagnosis
2.3. MRI Evaluation
2.4. Statistical Analysis
2.5. Development and Validation of a Risk Estimation Score
3. Results
3.1. Patient Characteristics of the Discovery Cohort
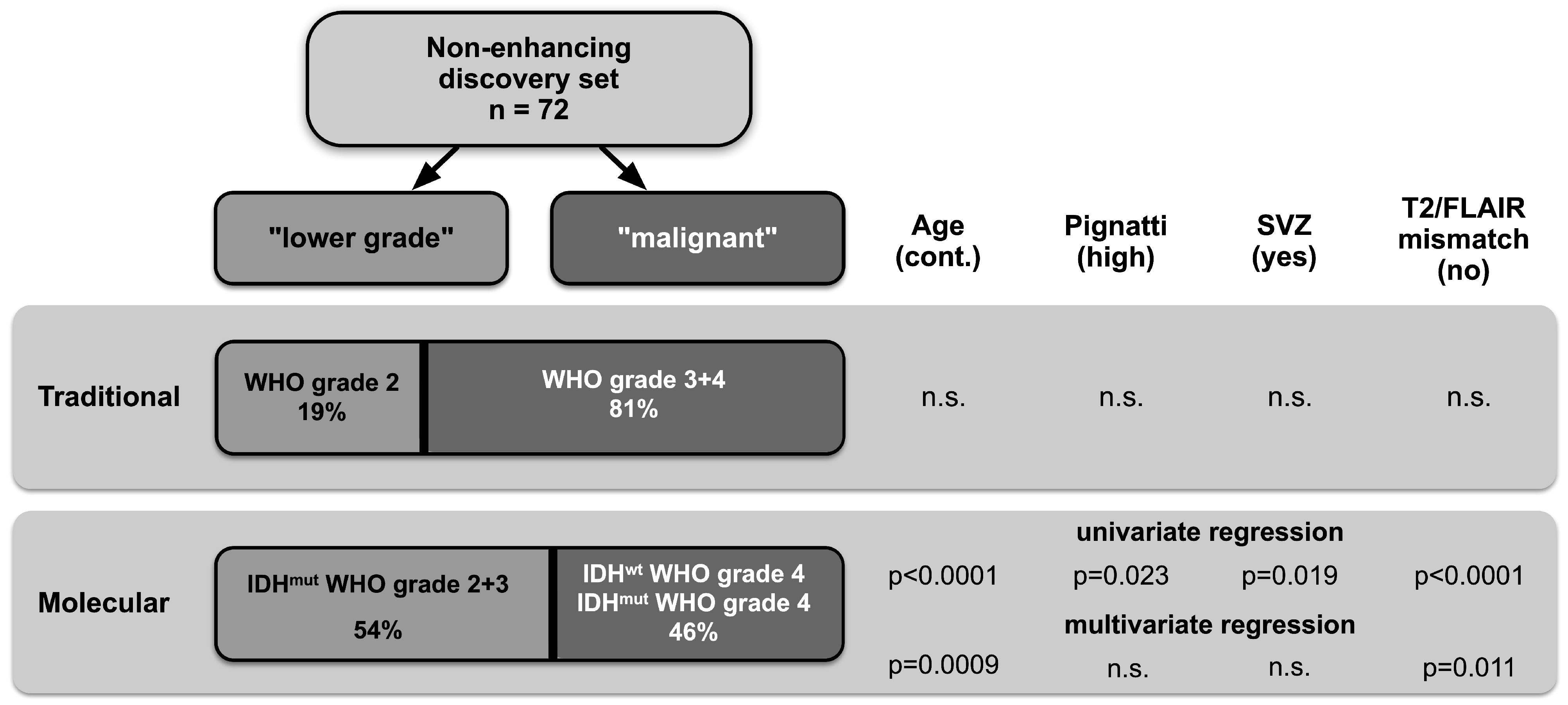
3.2. High Prevalence of Malignant Gliomas among Non-Enhancing Tumors
3.3. Identification of Preoperative Predictors of Malignancy and Risk Estimation
3.4. Development of a “Risk Estimation in Non-Enhancing Glioma” Score (RENEG Score)
3.5. Validation of the RENEG Score in an Independent Validation Cohort
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cancer Genome Atlas Research Network; Brat, D.J.; Verhaak, R.G.W.; Aldape, K.D.; Yung, W.K.A.; Salama, S.R.; Cooper, L.A.D.; Rheinbay, E.; Miller, C.R.; Vitucci, M.; et al. Comprehensive, Integrative Genomic Analysis of Diffuse Lower-Grade Gliomas. N. Engl. J. Med. 2015, 372, 2481–2498. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A Summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef]
- Hartmann, C.; Hentschel, B.; Wick, W.; Capper, D.; Felsberg, J.; Simon, M.; Westphal, M.; Schackert, G.; Meyermann, R.; Pietsch, T.; et al. Patients with IDH1 Wild Type Anaplastic Astrocytomas Exhibit Worse Prognosis than IDH1-Mutated Glioblastomas, and IDH1 Mutation Status Accounts for the Unfavorable Prognostic Effect of Higher Age: Implications for Classification of Gliomas. Acta Neuropathol. 2010, 120, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A Summary. Neuro Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Appay, R.; Dehais, C.; Maurage, C.-A.; Alentorn, A.; Carpentier, C.; Colin, C.; Ducray, F.; Escande, F.; Idbaih, A.; Kamoun, A.; et al. CDKN2A Homozygous Deletion Is a Strong Adverse Prognosis Factor in Diffuse Malignant IDH-Mutant Gliomas. Neuro Oncol. 2019, 21, 1519–1528. [Google Scholar] [CrossRef] [PubMed]
- Weller, M.; van den Bent, M.; Tonn, J.C.; Stupp, R.; Preusser, M.; Cohen-Jonathan-Moyal, E.; Henriksson, R.; Rhun, E.L.; Balana, C.; Chinot, O.; et al. European Association for Neuro-Oncology (EANO) Guideline on the Diagnosis and Treatment of Adult Astrocytic and Oligodendroglial Gliomas. Lancet Oncol. 2017, 18, e315–e329. [Google Scholar] [CrossRef]
- Paľa, A.; Coburger, J.; Scherer, M.; Ahmeti, H.; Roder, C.; Gessler, F.; Jungk, C.; Scheuerle, A.; Senft, C.; Tatagiba, M.; et al. To Treat or Not to Treat? A Retrospective Multicenter Assessment of Survival in Patients with IDH-Mutant Low-Grade Glioma Based on Adjuvant Treatment. J. Neurosurg. 2019, 1, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jungk, C.; Scherer, M.; Mock, A.; Capper, D.; Radbruch, A.; von Deimling, A.; Bendszus, M.; Herold-Mende, C.; Unterberg, A. Prognostic Value of the Extent of Resection in Supratentorial WHO Grade II Astrocytomas Stratified for IDH1 Mutation Status: A Single-Center Volumetric Analysis. J. Neurooncol. 2016, 129, 319–328. [Google Scholar] [CrossRef]
- Patel, S.H.; Bansal, A.G.; Young, E.B.; Batchala, P.P.; Patrie, J.T.; Lopes, M.B.; Jain, R.; Fadul, C.E.; Schiff, D. Extent of Surgical Resection in Lower-Grade Gliomas: Differential Impact Based on Molecular Subtype. AJNR Am. J. Neuroradiol. 2019, 40, 1149–1155. [Google Scholar] [CrossRef]
- Jakola, A.S.; Myrmel, K.S.; Kloster, R.; Torp, S.H.; Lindal, S.; Unsgård, G.; Solheim, O. Comparison of a Strategy Favoring Early Surgical Resection vs a Strategy Favoring Watchful Waiting in Low-Grade Gliomas. JAMA 2012, 308, 1881–1888. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.S.; Chang, E.F.; Lamborn, K.R.; Chang, S.M.; Prados, M.D.; Cha, S.; Tihan, T.; VandenBerg, S.; McDermott, M.W.; Berger, M.S. Role of Extent of Resection in the Long-Term Outcome of Low-Grade Hemispheric Gliomas. JCO 2008, 26, 1338–1345. [Google Scholar] [CrossRef]
- Aghi, M.K.; Nahed, B.V.; Sloan, A.E.; Ryken, T.C.; Kalkanis, S.N.; Olson, J.J. The Role of Surgery in the Management of Patients with Diffuse Low Grade Glioma. J. Neurooncol. 2015, 125, 503–530. [Google Scholar] [CrossRef]
- Scherer, M.; Ahmeti, H.; Roder, C.; Gessler, F.; Jungk, C.; Pala, A.; Mayer, B.; Senft, C.; Tatagiba, M.; Synowitz, M.; et al. Surgery for Diffuse WHO Grade II Gliomas: Volumetric Analysis of a Multicenter Retrospective Cohort From the German Study Group for Intraoperative Magnetic Resonance Imaging. Neurosurgery 2019, 86, E64–E74. [Google Scholar] [CrossRef]
- Whittle, I.R. What Is the Place of Conservative Management for Adult Supratentorial Low-Grade Glioma? Adv. Tech. Stand. Neurosurg. 2010, 35, 65–79. [Google Scholar] [PubMed]
- Recht, L.D.; Lew, R.; Smith, T.W. Suspected Low-Grade Glioma: Is Deferring Treatment Safe? Ann. Neurol. 1992, 31, 431–436. [Google Scholar] [CrossRef]
- Sahin, N.; Melhem, E.R.; Wang, S.; Krejza, J.; Poptani, H.; Chawla, S.; Verma, G. Advanced MR Imaging Techniques in the Evaluation of Nonenhancing Gliomas: Perfusion-Weighted Imaging Compared with Proton Magnetic Resonance Spectroscopy and Tumor Grade. Neuroradiol. J. 2013, 26, 531–541. [Google Scholar] [CrossRef]
- Ginsberg, L.E.; Fuller, G.N.; Hashmi, M.; Leeds, N.E.; Schomer, D.F. The Significance of Lack of MR Contrast Enhancement of Supratentorial Brain Tumors in Adults: Histopathological Evaluation of a Series. Surg. Neurol. 1998, 49, 436–440. [Google Scholar] [CrossRef] [PubMed]
- Eichberg, D.G.; Di, L.; Morell, A.A.; Shah, A.H.; Semonche, A.M.; Chin, C.N.; Bhatia, R.G.; Jamshidi, A.M.; Luther, E.M.; Komotar, R.J.; et al. Incidence of High Grade Gliomas Presenting as Radiographically Non-Enhancing Lesions: Experience in 111 Surgically Treated Non-Enhancing Gliomas with Tissue Diagnosis. J. Neurooncol. 2020, 147, 671–679. [Google Scholar] [CrossRef]
- Choi, C.; Ganji, S.K.; DeBerardinis, R.J.; Hatanpaa, K.J.; Rakheja, D.; Kovacs, Z.; Yang, X.-L.; Mashimo, T.; Raisanen, J.M.; Marin-Valencia, I.; et al. 2-Hydroxyglutarate Detection by Magnetic Resonance Spectroscopy in IDH-Mutated Patients with Gliomas. Nat. Med. 2012, 18, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Delfanti, R.L.; Piccioni, D.E.; Handwerker, J.; Bahrami, N.; Krishnan, A.; Karunamuni, R.; Hattangadi-Gluth, J.A.; Seibert, T.M.; Srikant, A.; Jones, K.A.; et al. Imaging Correlates for the 2016 Update on WHO Classification of Grade II/III Gliomas: Implications for IDH, 1p/19q and ATRX Status. J. Neurooncol. 2017, 135, 601–609. [Google Scholar] [CrossRef]
- Bahrami, N.; Hartman, S.J.; Chang, Y.-H.; Delfanti, R.; White, N.S.; Karunamuni, R.; Seibert, T.M.; Dale, A.M.; Hattangadi-Gluth, J.A.; Piccioni, D.; et al. Molecular Classification of Patients with Grade II/III Glioma Using Quantitative MRI Characteristics. J. Neurooncol. 2018, 139, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Juratli, T.A.; Tummala, S.S.; Riedl, A.; Daubner, D.; Hennig, S.; Penson, T.; Zolal, A.; Thiede, C.; Schackert, G.; Krex, D.; et al. Radiographic Assessment of Contrast Enhancement and T2/FLAIR Mismatch Sign in Lower Grade Gliomas: Correlation with Molecular Groups. J. Neurooncol. 2019, 141, 327–335. [Google Scholar] [CrossRef]
- Patel, S.H.; Poisson, L.M.; Brat, D.J.; Zhou, Y.; Cooper, L.; Snuderl, M.; Thomas, C.; Franceschi, A.M.; Griffith, B.; Flanders, A.E.; et al. T2-FLAIR Mismatch, an Imaging Biomarker for IDH and 1p/19q Status in Lower-Grade Gliomas: A TCGA/TCIA Project. Clin. Cancer Res. 2017, 23, 6078–6085. [Google Scholar] [CrossRef] [PubMed]
- Broen, M.P.G.; Smits, M.; Wijnenga, M.M.J.; Dubbink, H.J.; Anten, M.H.M.E.; Schijns, O.E.M.G.; Beckervordersandforth, J.; Postma, A.A.; van den Bent, M.J. The T2-FLAIR Mismatch Sign as an Imaging Marker for Non-Enhancing IDH-Mutant, 1p/19q-Intact Lower-Grade Glioma: A Validation Study. Neuro-Oncology 2018, 20, 1393–1399. [Google Scholar] [CrossRef] [PubMed]
- Pignatti, F.; van den Bent, M.; Curran, D.; Debruyne, C.; Sylvester, R.; Therasse, P.; Afra, D.; Cornu, P.; Bolla, M.; Vecht, C.; et al. Prognostic Factors for Survival in Adult Patients with Cerebral Low-Grade Glioma. J. Clin. Oncol. 2002, 20, 2076–2084. [Google Scholar] [CrossRef] [PubMed]
- Capper, D.; Weissert, S.; Balss, J.; Habel, A.; Meyer, J.; Jäger, D.; Ackermann, U.; Tessmer, C.; Korshunov, A.; Zentgraf, H.; et al. Characterization of R132H Mutation-Specific IDH1 Antibody Binding in Brain Tumors. Brain Pathol. 2010, 20, 245–254. [Google Scholar] [CrossRef]
- Hartmann, C.; Meyer, J.; Balss, J.; Capper, D.; Mueller, W.; Christians, A.; Felsberg, J.; Wolter, M.; Mawrin, C.; Wick, W.; et al. Type and Frequency of IDH1 and IDH2 Mutations Are Related to Astrocytic and Oligodendroglial Differentiation and Age: A Study of 1010 Diffuse Gliomas. Acta Neuropathol. 2009, 118, 469–474. [Google Scholar] [CrossRef]
- Capper, D.; Stichel, D.; Sahm, F.; Jones, D.T.W.; Schrimpf, D.; Sill, M.; Schmid, S.; Hovestadt, V.; Reuss, D.E.; Koelsche, C.; et al. Practical Implementation of DNA Methylation and Copy-Number-Based CNS Tumor Diagnostics: The Heidelberg Experience. Acta Neuropathol. 2018, 136, 181–210. [Google Scholar] [CrossRef]
- Jungk, C.; Warta, R.; Mock, A.; Friauf, S.; Hug, B.; Capper, D.; Abdollahi, A.; Debus, J.; Bendszus, M.; von Deimling, A.; et al. Location-Dependent Patient Outcome and Recurrence Patterns in IDH1-Wildtype Glioblastoma. Cancers 2019, 11, 122. [Google Scholar] [CrossRef]
- Jungk, C.; Mock, A.; Exner, J.; Geisenberger, C.; Warta, R.; Capper, D.; Abdollahi, A.; Friauf, S.; Lahrmann, B.; Grabe, N.; et al. Spatial Transcriptome Analysis Reveals Notch Pathway-Associated Prognostic Markers in IDH1 Wild-Type Glioblastoma Involving the Subventricular Zone. BMC Med. 2016, 14, 170. [Google Scholar] [CrossRef]
- Reuss, D.E.; Mamatjan, Y.; Schrimpf, D.; Capper, D.; Hovestadt, V.; Kratz, A.; Sahm, F.; Koelsche, C.; Korshunov, A.; Olar, A.; et al. IDH Mutant Diffuse and Anaplastic Astrocytomas Have Similar Age at Presentation and Little Difference in Survival: A Grading Problem for WHO. Acta Neuropathol. 2015, 129, 867–873. [Google Scholar] [CrossRef]
- Berzero, G.; Di Stefano, A.L.; Ronchi, S.; Bielle, F.; Villa, C.; Guillerm, E.; Capelle, L.; Mathon, B.; Laurenge, A.; Giry, M.; et al. IDH-Wildtype Lower-Grade Diffuse Gliomas: The Importance of Histological Grade and Molecular Assessment for Prognostic Stratification. Neuro Oncol. 2021, 23, 955–966. [Google Scholar] [CrossRef]
- Etxaniz, O.; Carrato, C.; de Aguirre, I.; Queralt, C.; Muñoz, A.; Ramirez, J.L.; Rosell, R.; Villà, S.; Diaz, R.; Estival, A.; et al. IDH Mutation Status Trumps the Pignatti Risk Score as a Prognostic Marker in Low-Grade Gliomas. J. Neurooncol. 2017, 135, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Geurts, M.; van den Bent, M.J. On High-risk, Low-grade Glioma: What Distinguishes High from Low? Cancer 2019, 125, 174–176. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Ohgaki, H.; Wiestler, O.D.; Cavenee, W.K.; Burger, P.C.; Jouvet, A.; Scheithauer, B.W.; Kleihues, P. The 2007 WHO Classification of Tumours of the Central Nervous System. Acta Neuropathol. 2007, 114, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Corell, A.; Ferreyra Vega, S.; Hoefling, N.; Carstam, L.; Smits, A.; Olsson Bontell, T.; Björkman-Burtscher, I.M.; Carén, H.; Jakola, A.S. The Clinical Significance of the T2-FLAIR Mismatch Sign in Grade II and III Gliomas: A Population-Based Study. BMC Cancer 2020, 20, 450. [Google Scholar] [CrossRef]
- Goyal, A.; Yolcu, Y.U.; Goyal, A.; Kerezoudis, P.; Brown, D.A.; Graffeo, C.S.; Goncalves, S.; Burns, T.C.; Parney, I.F. The T2-FLAIR-Mismatch Sign as an Imaging Biomarker for IDH and 1p/19q Status in Diffuse Low-Grade Gliomas: A Systematic Review with a Bayesian Approach to Evaluation of Diagnostic Test Performance. Neurosurg. Focus 2019, 47, E13. [Google Scholar] [CrossRef]
- Deguchi, S.; Oishi, T.; Mitsuya, K.; Kakuda, Y.; Endo, M.; Sugino, T.; Hayashi, N. Clinicopathological Analysis of T2-FLAIR Mismatch Sign in Lower-Grade Gliomas. Sci. Rep. 2020, 10, 10113. [Google Scholar] [CrossRef]
- Lee, M.K.; Park, J.E.; Jo, Y.; Park, S.Y.; Kim, S.J.; Kim, H.S. Advanced Imaging Parameters Improve the Prediction of Diffuse Lower-Grade Gliomas Subtype, IDH Mutant with No 1p19q Codeletion: Added Value to the T2/FLAIR Mismatch Sign. Eur. Radiol. 2020, 30, 844–854. [Google Scholar] [CrossRef]
- Zhao, K.; Sun, G.; Wang, Q.; Xue, Z.; Liu, G.; Xia, Y.; Yao, A.; Zhao, Y.; You, N.; Yang, C.; et al. The Diagnostic Value of Conventional MRI and CT Features in the Identification of the IDH1-Mutant and 1p/19q Co-Deletion in WHO Grade II Gliomas. Acad. Radiol. 2020, 28, e189–e198. [Google Scholar] [CrossRef]
- Muoio, B.; Giovanella, L.; Treglia, G. Recent Developments of 18F-FET PET in Neuro-Oncology. Curr. Med. Chem. 2018, 25, 3061–3073. [Google Scholar] [CrossRef]
- Lohmann, P.; Lerche, C.; Bauer, E.K.; Steger, J.; Stoffels, G.; Blau, T.; Dunkl, V.; Kocher, M.; Viswanathan, S.; Filss, C.P.; et al. Predicting IDH Genotype in Gliomas Using FET PET Radiomics. Sci. Rep. 2018, 8, 13328. [Google Scholar] [CrossRef]
- Verger, A.; Stoffels, G.; Bauer, E.K.; Lohmann, P.; Blau, T.; Fink, G.R.; Neumaier, B.; Shah, N.J.; Langen, K.-J.; Galldiks, N. Static and Dynamic 18F–FET PET for the Characterization of Gliomas Defined by IDH and 1p/19q Status. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Verburg, N.; Koopman, T.; Yaqub, M.M.; Hoekstra, O.S.; Lammertsma, A.A.; Barkhof, F.; Pouwels, P.J.W.; Reijneveld, J.C.; Heimans, J.J.; Rozemuller, A.J.M.; et al. Improved Detection of Diffuse Glioma Infiltration with Imaging Combinations: A Diagnostic Accuracy Study. Neuro Oncol. 2020, 22, 412–422. [Google Scholar] [CrossRef]
- Mistry, A.M.; Hale, A.T.; Chambless, L.B.; Weaver, K.D.; Thompson, R.C.; Ihrie, R.A. Influence of Glioblastoma Contact with the Lateral Ventricle on Survival: A Meta-Analysis. J. Neurooncol. 2017, 131, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Kappadakunnel, M.; Eskin, A.; Dong, J.; Nelson, S.F.; Mischel, P.S.; Liau, L.M.; Ngheimphu, P.; Lai, A.; Cloughesy, T.F.; Goldin, J.; et al. Stem Cell Associated Gene Expression in Glioblastoma Multiforme: Relationship to Survival and the Subventricular Zone. J. Neurooncol. 2010, 96, 359–367. [Google Scholar] [CrossRef]
- Jafri, N.F.; Clarke, J.L.; Weinberg, V.; Barani, I.J.; Cha, S. Relationship of Glioblastoma Multiforme to the Subventricular Zone Is Associated with Survival. Neuro-Oncology 2013, 15, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Chaichana, K.L.; McGirt, M.J.; Frazier, J.; Attenello, F.; Guerrero-Cazares, H.; Quinones-Hinojosa, A. Relationship of Glioblastoma Multiforme to the Lateral Ventricles Predicts Survival Following Tumor Resection. J. Neurooncol. 2008, 89, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Adeberg, S.; Bostel, T.; König, L.; Welzel, T.; Debus, J.; Combs, S.E. A Comparison of Long-Term Survivors and Short-Term Survivors with Glioblastoma, Subventricular Zone Involvement: A Predictive Factor for Survival? Radiat. Oncol. 2014, 9, 95. [Google Scholar] [CrossRef]
- Wenger, A.; Ferreyra Vega, S.; Kling, T.; Bontell, T.O.; Jakola, A.S.; Carén, H. Intratumor DNA Methylation Heterogeneity in Glioblastoma: Implications for DNA Methylation-Based Classification. Neuro Oncol. 2019, 21, 616–627. [Google Scholar] [CrossRef]

| Discovery Cohort n = 72 | Validation Cohort n = 40 | |
|---|---|---|
| Patient characteristics | ||
| Age at first diagnosis (mean ± SD; range) | 46 ± 16 (20–77) | 49 ± 16.6 (14–78) |
| Gender (female: male) | 26: 46 | 22: 18 |
| Mean follow-up (months ± SD; range) | 25 ± 19 (1–127) | 26 ± 35 (0–137) |
| Mean OS (months ± SD; range) | 20 ± 13 (2–45) | not reached |
| Mean PFS (months ± SD; range) | 14 ± 17 (0–67) | not reached |
| Progression (% of total) | 30 (43%) | 16 (40%) |
| Deaths (% of total) | 9 (13%) | 0 (0%) |
| Symptoms | (% of total) | |
| Seizures | 46 (64%) | 18 (45%) |
| Headache | 10 (14%) | 3 (8%) |
| Incidental finding | 7 (10%) | 9 (23%) |
| Vertigo | 5 (7%) | 0 (0%) |
| Motor deficits | 4 (6%) | 5 (13%) |
| Psychological disorder | 4 (6%) | 2 (5%) |
| Unspecific symptoms | 2 (3%) | 3 (8%) |
| Radiographic characteristics | ||
| Patients with preop follow-up MRI (% of total) | 40 (57%) | |
| Tumor volume (mL) at first diagnosis (mean ± SD; range) | 47.1 ± 41.0 (3–174) | |
| Tumor volume (mL) preop (mean ± SD; range) | 50.8 ± 41.4 (3–174) | |
| Mean growth rate (ml/month ± SD; range) | 1.22 ± 0.15 (0.1–15.9) | |
| SVZ involvement (% of total) | 38 (53%) | 25 (63%) |
| T2/FLAIR mismatch sign positive (% of total) | 23 (32%) | 8 (20%) |
| Localization | ||
| frontal lobe | 32% | |
| temporal lobe | 31% | |
| parietal lobe | 3% | |
| other | 6% | |
| Molecular diagnostics | (% of total) | |
| IDH mutation | 41 (57%) | 24 (60%) |
| 1p19q codeletion | 13 (18%) | 12 (30%) |
| CDKN2A/B homozygous deletion | 9 (12.5%) | 6 (15%) |
| Surgical procedure | (% of total) | |
| Gross/subtotal resection | 53 (73%) | 22 (55%) |
| Biopsy | 19 (26%) | 18 (45%) |
| Adjuvant treatment according to integrated diagnosis | 58 of 72 (81% of total) | 28 of 40 (70% of total) |
| Astro IDHmut WHO grade 2 | 6 of 14 (42%) | 5 of 11 (45%) |
| Astro IDHmut WHO grade 3 | 24 of 25 (96%) | 1 of 1 (100%) |
| Astro IDHmut WHO grade 4 | 1 of 1 (100%) | - |
| Glioblastoma IDHwt WHO grade 4 | 27 of 32 (84%) | 15 of 16 (94%) |
| Oligo IDHmut, 1p19q codel WHO grade 2 | 4 of 6 (67%) | 6 of 11 (55%) |
| Oligo IDHmut, 1p19q codel WHO grade 3 | 6 of 7 (86%) | 1 of 1 (100%) |
| no adjuvant treatment | 14 (19% of total) | 12 (30% of total) |
| WHO Grading | Molecular Classification | |||||||
|---|---|---|---|---|---|---|---|---|
| WHO Grade 2 and 3 vs. WHO Grade 4 | IDHmut WHO Grade 2 and 3 vs. IDHwt and IDHmut WHO Grade 4 | |||||||
| Univariate | Univariate | Multivariate | ||||||
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | OR | p-Value | |
| Clinical features | ||||||||
| Gender (m) | 0.85 | 0.26–2.56 | 0.777 | 0.84 | 0.32–2.21 | 0.723 | ||
| Age (cont.) | 1.02 | 0.99–1.06 | 0.240 | 1.12 | 1.07–1.18 | <0.0001 | 1.10 | 0.0009 |
| Specific symptoms a (yes) | 1.00 | 0.28–3.18 | 1.0 | 1.13 | 0.4–3.25 | 0.539 | ||
| Seizure (yes) | 1.27 | 0.41–3.81 | 0.669 | 0.95 | 0.36–2.54 | 0.815 | ||
| Pignatti risk (high) | 0.61 | 0.14–2.22 | 0.468 | 6.5 | 1.51–44.95 | 0.023 | ||
| Age (>40) | 2.33 | 0.78–7 | 0.132 | 10 | 3.34–34.76 | <0.0001 | ||
| Volume > 28 mL (yes) | 0.92 | 0.3–2.73 | 0.890 | 0.46 | 0.17–1.19 | 0.114 | ||
| Neurological deficit b (yes) | 0.64 | 0.11–4.9 | 0.625 | 2.4 | 0.44–18.18 | 0.331 | ||
| Midline crossing (yes) | 0.39 | 0.11–1.49 | 0.153 | 2.62 | 0.74–10.69 | 0.149 | ||
| Astrocytoma (yes) | 3.33 | 0.93–12.05 | 0.060 | - c | - c | |||
| Radiographic features | ||||||||
| Deep seated (yes) | 1.39 | 0.31–9.86 | 0.695 | 3.03 | 0.76–15.06 | 0.133 | ||
| Multifocal (yes) | 0.64 | 0.11–4.92 | 0.625 | 1.19 | 0.2–6.49 | 0.887 | ||
| Volume at first diagnosis (cont.) | 1.00 | 0.99–1.01 | 0.762 | 1.00 | 0.99–1.01 | 0.669 | ||
| Volume at surgery | 1.00 | 0.99–1.01 | 0.894 | 1.00 | 0.99–1.01 | 0.685 | ||
| Growth rate (mL/d) | 6.94 | 0.05–2016.8 | 0.454 | 14.3 | 0.2–4206.21 | 0.258 | ||
| SVZ involvement (yes) | 2.12 | 0.72–6.56 | 0.177 | 3.23 | 1.24–8.7 | 0.019 | ||
| T2/FLAIR mismatch sign (yes) | 1.30 | 0.42–4.56 | 0.662 | 0.05 | 0.01–0.2 | <0.0001 | 0.11 | 0.011 |
| RENEG Score (Cutoff Value: >0.59) | Pignatti Score (High Risk) | T2/FLAIR Mismatch (Absent) | |
|---|---|---|---|
| Sensitivity | 0.94 | 0.57 | 0.67 |
| Specificity | 0.75 | 0.82 | 1.00 |
| PPV | 0.71 | 0.81 | 1.00 |
| NPV | 0.95 | 0.58 | 0.67 |
| LR+ | 3.75 | 3.20 | 1.5 |
| LR− | 0.08 | 0.53 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dao Trong, P.; Kilian, S.; Jesser, J.; Reuss, D.; Aras, F.K.; Von Deimling, A.; Herold-Mende, C.; Unterberg, A.; Jungk, C. Risk Estimation in Non-Enhancing Glioma: Introducing a Clinical Score. Cancers 2023, 15, 2503. https://doi.org/10.3390/cancers15092503
Dao Trong P, Kilian S, Jesser J, Reuss D, Aras FK, Von Deimling A, Herold-Mende C, Unterberg A, Jungk C. Risk Estimation in Non-Enhancing Glioma: Introducing a Clinical Score. Cancers. 2023; 15(9):2503. https://doi.org/10.3390/cancers15092503
Chicago/Turabian StyleDao Trong, Philip, Samuel Kilian, Jessica Jesser, David Reuss, Fuat Kaan Aras, Andreas Von Deimling, Christel Herold-Mende, Andreas Unterberg, and Christine Jungk. 2023. "Risk Estimation in Non-Enhancing Glioma: Introducing a Clinical Score" Cancers 15, no. 9: 2503. https://doi.org/10.3390/cancers15092503
APA StyleDao Trong, P., Kilian, S., Jesser, J., Reuss, D., Aras, F. K., Von Deimling, A., Herold-Mende, C., Unterberg, A., & Jungk, C. (2023). Risk Estimation in Non-Enhancing Glioma: Introducing a Clinical Score. Cancers, 15(9), 2503. https://doi.org/10.3390/cancers15092503





