UVRAG Promotes Tumor Progression through Regulating SP1 in Colorectal Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Patients and Samples
2.2. Assessment of Tumor Downstaging
2.3. Cell Lines and Cell Culture
2.4. TMAs and IHC
2.5. Transient Transfection
2.6. Cell Cytotoxicity Assay
2.7. Cell Migration Assay
2.8. Clonogenic Survival Assay
2.9. Spheroid Formation Assay
2.10. Western Blot Analysis
2.11. Quantitative Reverse Transcription-PCR (RT-qPCR)
2.12. RNA-Seq Bulk Data Analysis
2.13. Single-Cell RNA-Seq (scRNA) Data Analysis
2.14. Statistical Analysis
3. Results
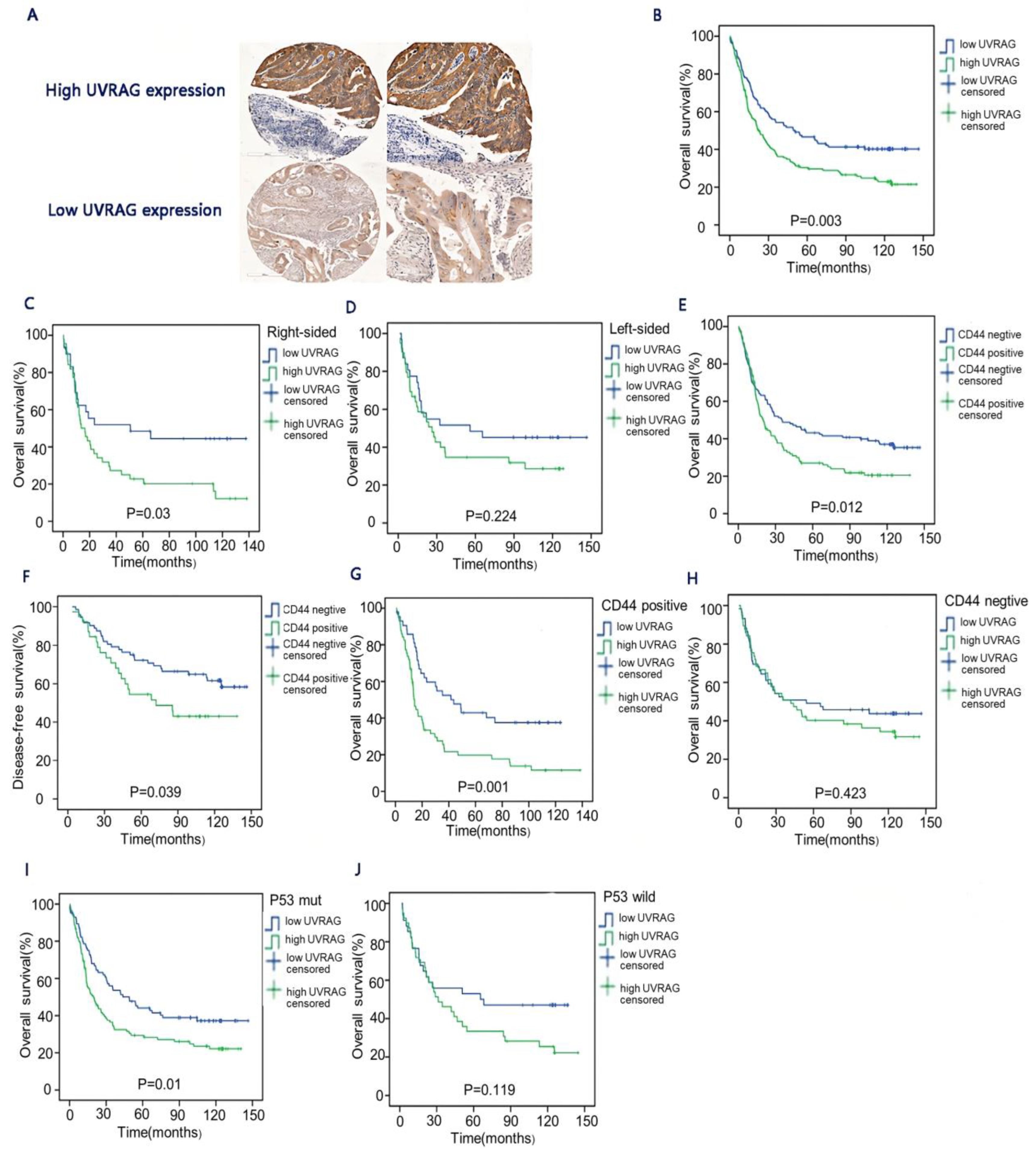
3.1. Correlation between UVRAG and Clinicopathological Features of CRC Patients without Neoadjuvant Treatment
3.2. The Relationship between UVRAG and Long-Term Survival in CRC
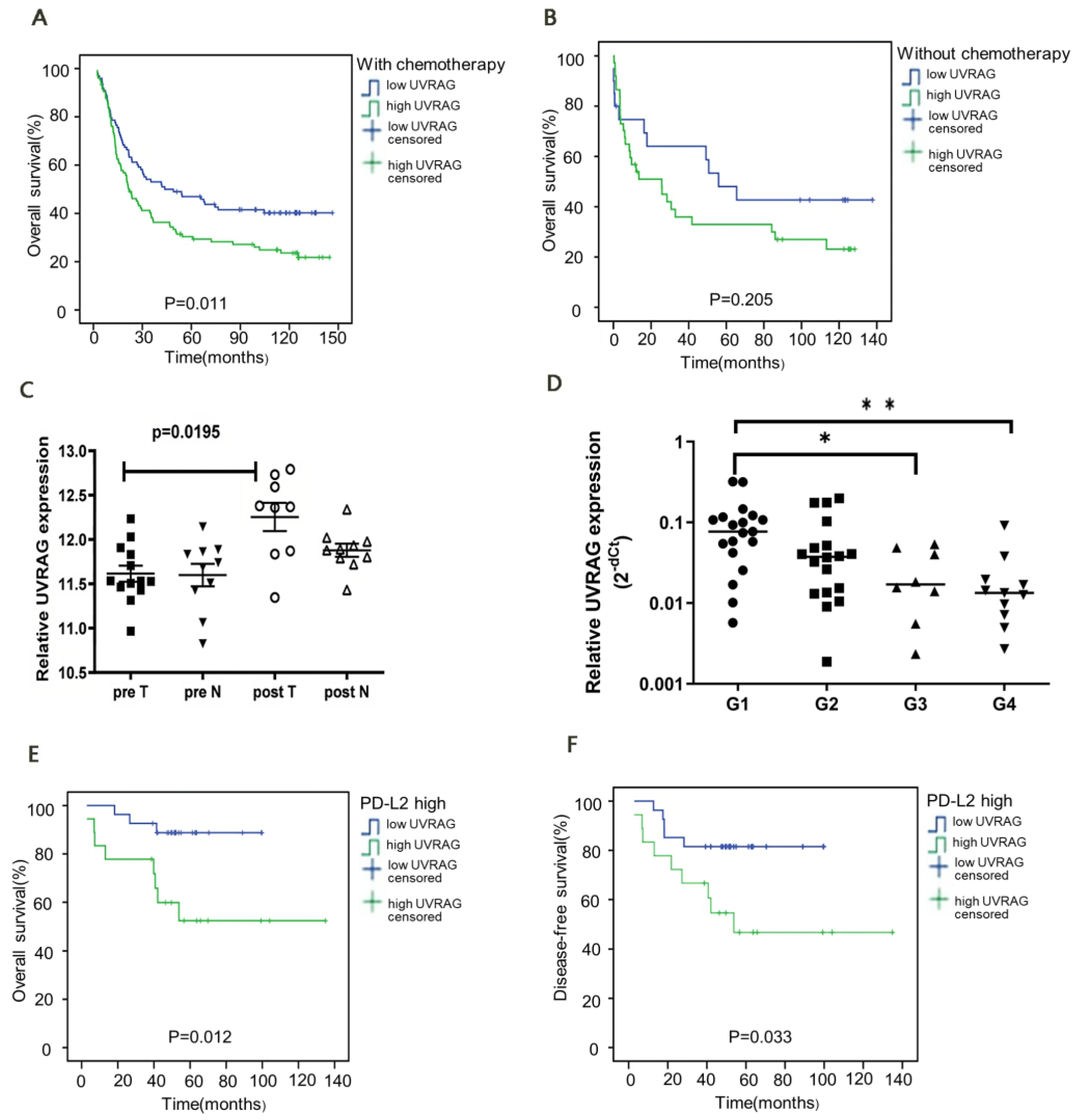
3.3. UVRAG Expression Was Correlated with the Efficacy of Adjuvant Chemotherapy
3.4. UVRAG Expression Was Correlated with the Efficacy of Radiotherapy and Prognosis following Neoadjuvant Radiotherapy
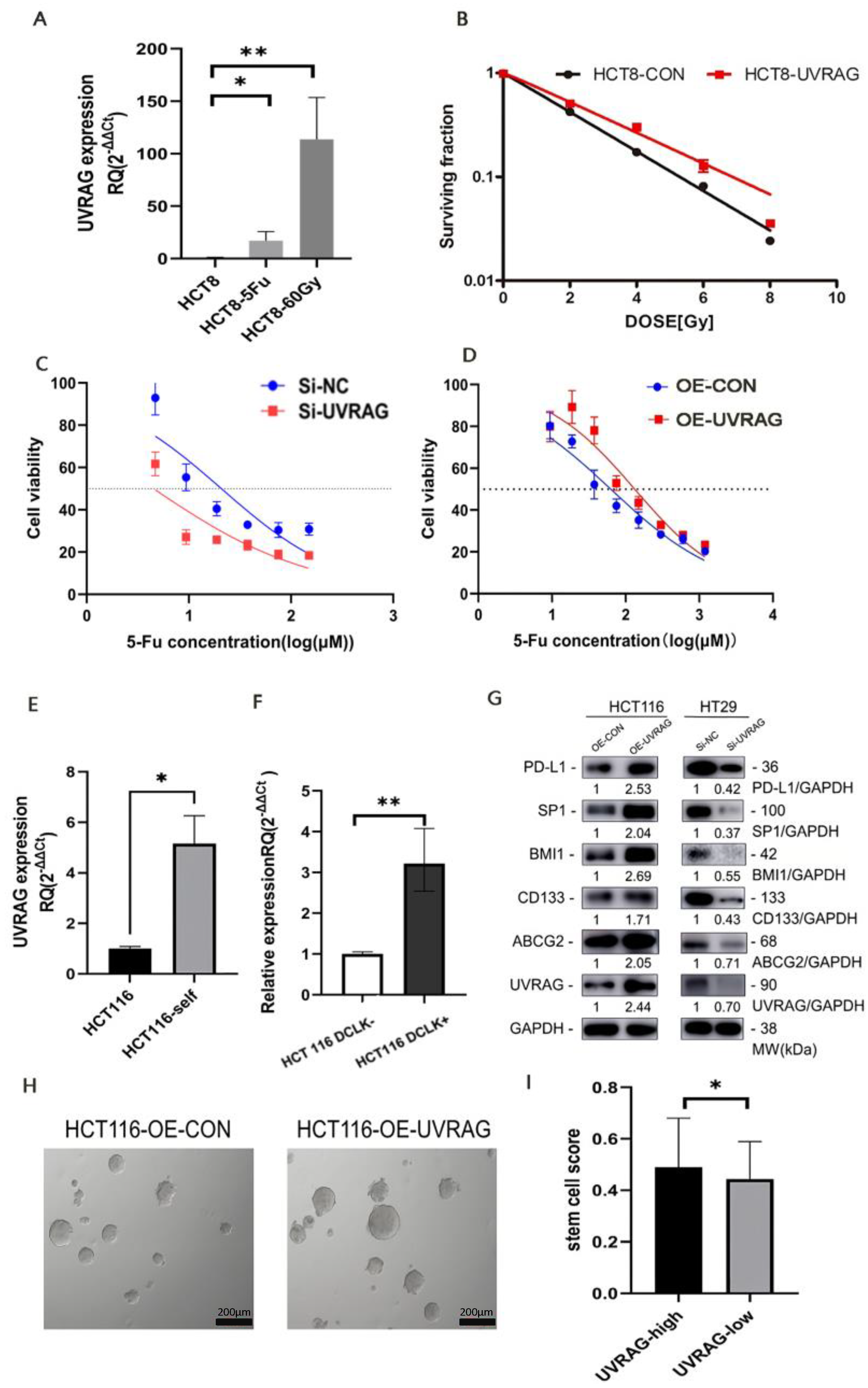
3.5. UVRAG Contributed to Resistance to Radiotherapy and Chemotherapy in CRC
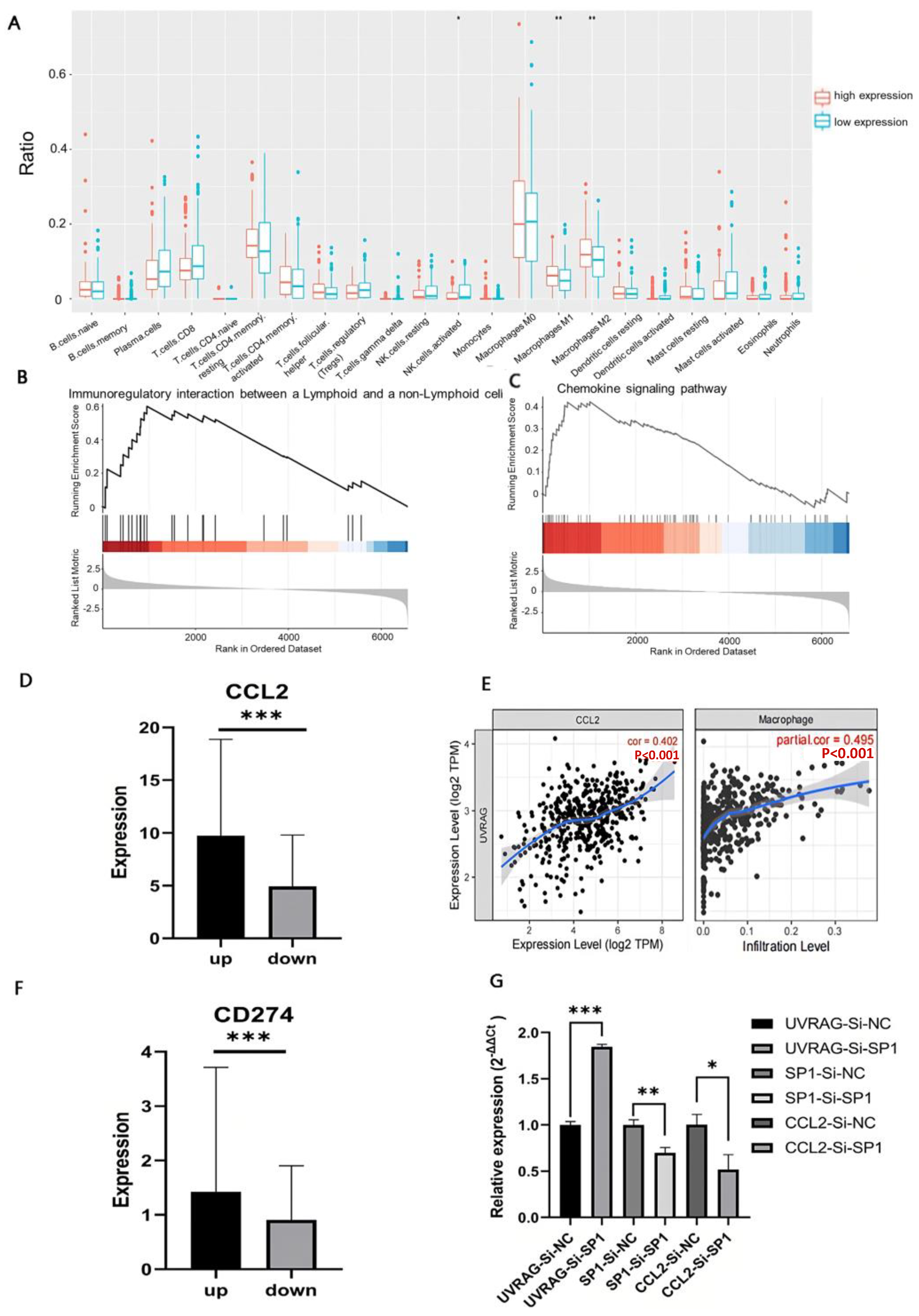
3.6. UVRAG Recruited Macrophages through CCL2 by Regulating Transcriptional Factor SP1
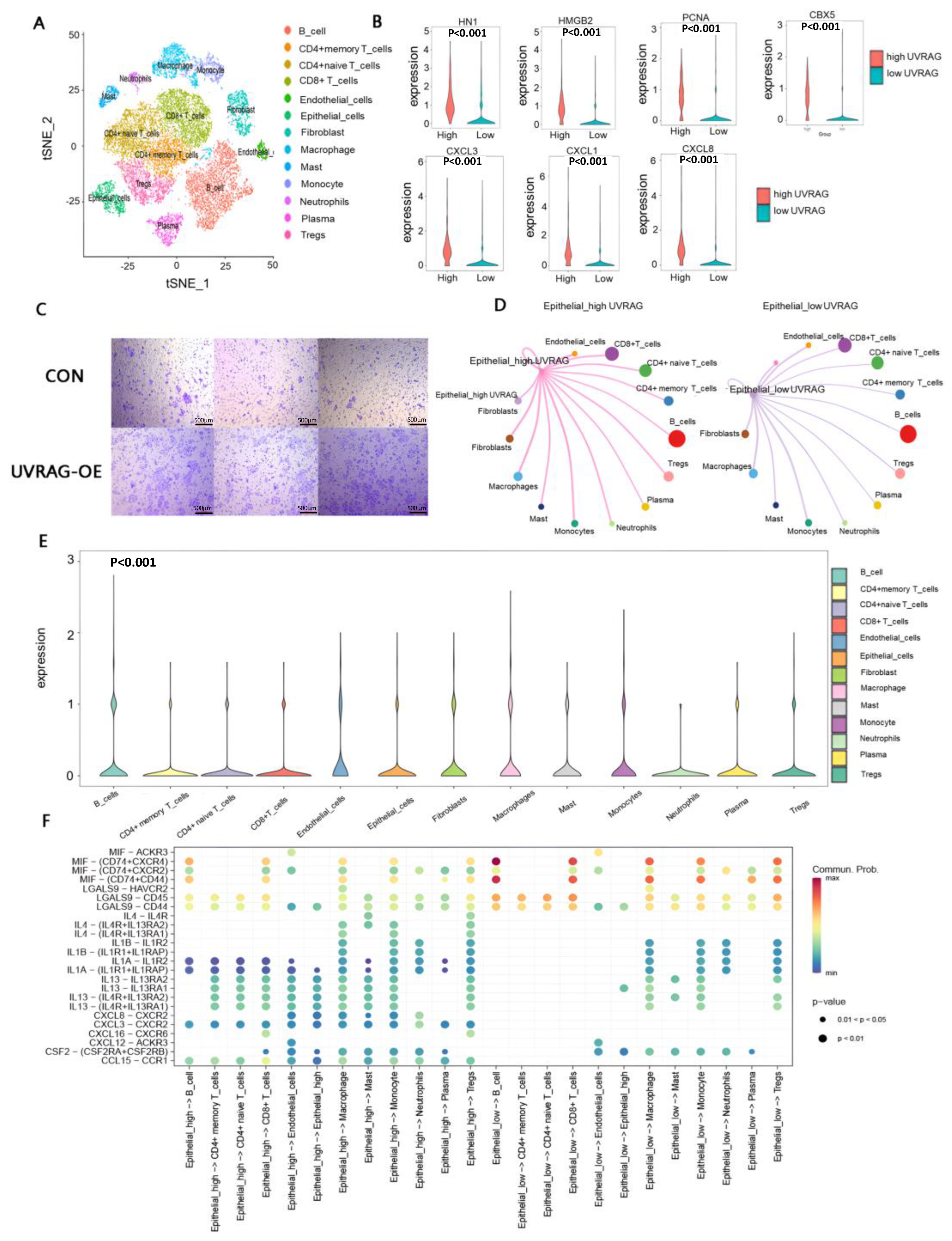
3.7. Single-Cell Transcriptome Analysis Revealed the Underlying Mechanisms of Immune Modulation in the High UVRAG Expression Group
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef] [PubMed]
- Perelman, B.; Dafni, N.; Naiman, T.; Eli, D.; Yaakov, M.; Feng, T.L.; Sinha, S.; Weber, G.; Khodaei, S.; Sancar, A.; et al. Molecular cloning of a novel human gene encoding a 63-kDa protein and its sublocalization within the 11q13 locus. Genomics 1997, 41, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Iida, A.; Emi, M.; Matsuoka, R.; Hiratsuka, E.; Okui, K.; Ohashi, H.; Inazawa, J.; Fukushima, Y.; Imai, T.; Nakamura, Y. Identification of a gene disrupted by inv (11)(q13. 5; q25) in a patient with left-right axis malformation. Hum. Genet. 2000, 106, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Cao, L.; Peng, Y.; Tan, Y.; Xie, M.; Kang, R.; Livesey, K.M.; Tang, D. A critical role for UVRAG in apoptosis. Autophagy 2011, 7, 1242–1244. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Feng, P.; Ku, B.; Oh, B.-H.; Jung, J.U. UVRAG: A new player in autophagy and tumor cell growth. Autophagy 2007, 3, 69–71. [Google Scholar] [CrossRef]
- Yin, X.; Cao, L.; Kang, R.; Yang, M.; Wang, Z.; Peng, Y.; Tan, Y.; Liu, L.; Xie, M.; Zhao, Y.; et al. UV irradiation re-sistance-associated gene suppresses apoptosis by interfering with BAX activation. EMBO Rep. 2011, 12, 727–734. [Google Scholar] [CrossRef]
- Zhao, Z.; Oh, S.; Li, D.; Ni, D.; Pirooz, S.D.; Lee, J.-H.; Yang, S.; Lee, J.-Y.; Ghozalli, I.; Costanzo, V.; et al. A dual role for UVRAG in maintaining chromosomal stability independent of autophagy. Dev. Cell 2012, 22, 1001–1016. [Google Scholar] [CrossRef]
- Xie, T.; Li, S.J.; Guo, M.R.; Wu, Y.; Wang, H.Y.; Zhang, K.; Zhang, X.; Ouyang, L.; Liu, J. Untangling knots between autophagic targets and candidate drugs, in cancer therapy. Cell Prolif. 2015, 48, 119–139. [Google Scholar] [CrossRef]
- Song, Y.; Quach, C.; Liang, C. UVRAG in autophagy, inflammation, and cancer. Autophagy 2020, 16, 387–388. [Google Scholar] [CrossRef]
- Liu, B.; Cheng, Y.; Liu, Q.; Bao, J.-K.; Yang, J.-M. Autophagic pathways as new targets for cancer drug development. Acta Pharmacol. Sin. 2010, 31, 1154–1164. [Google Scholar] [CrossRef]
- Sencan, S.; Tanriover, M.; Ulasli, M.; Karakas, D.; Ozpolat, B. UV radiation resistance-associated gene (UVRAG) promotes cell proliferation, migration, invasion by regulating cyclin-dependent kinases (CDK) and integrin-β/Src signaling in breast cancer cells. Mol. Cell. Biochem. 2021, 476, 2075–2084. [Google Scholar] [CrossRef] [PubMed]
- Dworak, O.; Keilholz, L.; Hoffmann, A. Pathological features of rectal cancer after preoperative radiochemotherapy. Int. J. Color. Dis. 1997, 12, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Pradhan, S.; Liu, C.; Le, L.Q. Skin-derived precursors as a source of progenitors for cutaneous nerve regeneration. Stem Cells 2012, 30, 2261–2270. [Google Scholar] [CrossRef]
- Yan, Y.; Zuo, X.; Wei, D. Concise Review: Emerging Role of CD44 in Cancer Stem Cells: A Promising Biomarker and Therapeutic Target. STEM CELLS Transl. Med. 2015, 4, 1033–1043. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhao, L.; Lin, Z.; Yu, D.; Jin, M.; Zhou, P.; Ren, J.; Cheng, J.; Yang, K.; Wu, G.; et al. Targeting DCLK1 over-comes 5-fluorouracil resistance in colorectal cancer through inhibiting CCAR1/β-catenin pathway-mediated cancer stemness. Clin. Transl. Med. 2022, 12, e743. [Google Scholar] [PubMed]
- Ji, D.; Zhan, T.; Li, M.; Yao, Y.; Jia, J.; Yi, H.; Qiao, M.; Xia, J.; Zhang, Z.; Ding, H.; et al. Enhancement of Sensitivity to Chemo/Radiation Therapy by Using miR-15b against DCLK1 in Colorectal Cancer. Stem Cell Rep. 2018, 11, 1506–1522. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Ni, D.; Ghozalli, I.; Pirooz, S.D.; Ma, B.; Liang, C. UVRAG: At the crossroad of autophagy and genomic stability. Autophagy 2012, 8, 1392–1393. [Google Scholar] [CrossRef]
- Liu, Q.; Li, A.; Tian, Y.; Wu, J.D.; Liu, Y.; Li, T.; Chen, Y.; Han, X.; Wu, K. The CXCL8-CXCR1/2 pathways in cancer. Cytokine Growth Factor Rev. 2016, 31, 61–71. [Google Scholar] [CrossRef]
- Van Damme, J.; Struyf, S.; Opdenakker, G. Chemokine–protease interactions in cancer. Semin. Cancer Biol. 2004, 14, 201–208. [Google Scholar] [CrossRef]
- Reyes, N.; Figueroa, S.; Tiwari, R.; Geliebter, J. CXCL3 Signaling in the Tumor Microenvironment. Single Mol. Single Cell Seq. 2021, 1302, 15–24. [Google Scholar] [CrossRef]
- Ning, Z.; Liu, K.; Xiong, H. Roles of BTLA in Immunity and Immune Disorders. Front. Immunol. 2021, 12, 654960. [Google Scholar] [CrossRef]
- Itatani, Y.; Kawada, K.; Fujishita, T.; Kakizaki, F.; Hirai, H.; Matsumoto, T.; Iwamoto, M.; Inamoto, S.; Hatano, E.; Hasegawa, S.; et al. Loss of SMAD4 from colorectal cancer cells promotes CCL15 expression to recruit CCR1+ myeloid cells and facilitate liver metastasis. Gastroenterology 2013, 145, 1064–1075.e11. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Kawada, K.; Itatani, Y.; Inamoto, S.; Okamura, R.; Iwamoto, M.; Miyamoto, E.; Chen-Yoshikawa, T.F.; Hirai, H.; Hasegawa, S.; et al. Loss of SMAD4 Promotes Lung Metastasis of Colorectal Cancer by Accumulation of CCR1+ Tumor-Associated Neutrophils through CCL15-CCR1 Axis. Clin. Cancer Res. 2017, 23, 833–844. [Google Scholar] [CrossRef] [PubMed]
- Gelfo, V.; Romaniello, D.; Mazzeschi, M.; Sgarzi, M.; Grilli, G.; Morselli, A.; Manzan, B.; Rihawi, K.; Lauriola, M. Roles of IL-1 in Cancer: From Tumor Progression to Resistance to Targeted Therapies. Int. J. Mol. Sci. 2020, 21, 6009. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, R.; Yu, Q.; Dong, L.; Bi, Y.; Liu, G. Metabolic reprogramming of macrophages during infections and cancer. Cancer Lett. 2019, 452, 14–22. [Google Scholar] [CrossRef]
- Chabot, V.; Martin, L.; Meley, D.; Sensebé, L.; Baron, C.; Lebranchu, Y.; Dehaut, F.; Velge-Roussel, F. Unexpected impairment of TNF-α-induced maturation of human dendritic cells in vitro by IL-4. J. Transl. Med. 2016, 14, 93. [Google Scholar] [CrossRef] [PubMed]
- Christian, L.S.; Wang, L.; Lim, B.; Deng, D.; Wu, H.; Wang, X.-F.; Li, Q.-J. Resident memory T cells in tumor-distant tissues fortify against metastasis formation. Cell Rep. 2021, 35, 109118. [Google Scholar] [CrossRef]
- Noe, J.T.; Mitchell, R.A. MIF-Dependent Control of Tumor Immunity. Front. Immunol. 2020, 11, 609948. [Google Scholar] [CrossRef]
- Liang, C.; Feng, P.; Ku, B.; Dotan, I.; Canaani, D.; Oh, B.-H.; Jung, J.U. Autophagic and tumour suppressor activity of a novel Beclin1-binding protein UVRAG. Nature 2006, 8, 688–698. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.; Kroemer, G. Autophagy in the pathogenesis of disease. Cell 2008, 132, 27–42. [Google Scholar] [CrossRef]
- White, E.; Mehnert, J.M.; Chan, C.S. Autophagy, Metabolism, and Cancer. Clin. Cancer Res. 2015, 21, 5037–5046. [Google Scholar] [CrossRef]
- Zhong, Z.; Sanchez-Lopez, E.; Karin, M. Autophagy, Inflammation, and Immunity: A Troika Governing Cancer and Its Treatment. Cell 2016, 166, 288–298. [Google Scholar] [CrossRef]
- Li, X.; He, S.; Ma, B. Autophagy and autophagy-related proteins in cancer. Mol. Cancer 2020, 19, 12. [Google Scholar] [CrossRef]
- Zhang, X.; Qi, Z.; Yin, H.; Yang, G. Interaction between p53 and Ras signaling controls cisplatin resistance via HDAC4- and HIF-1α-mediated regulation of apoptosis and autophagy. Theranostics 2019, 9, 1096–1114. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, S.; Xie, P.; Zhang, B.; Yu, M.; Yan, J.; Jin, L.; Zhang, W.; Zhou, B.; Li, X.; et al. Tumor-derived PD1 and PD-L1 could promote hepatocellular carcinoma growth through autophagy induction in vitro. Biochem. Biophys. Res. Commun. 2022, 605, 82–89. [Google Scholar] [CrossRef]
- Leon, G.; MacDonagh, L.; Finn, S.P.; Cuffe, S.; Barr, M.P. Cancer stem cells in drug resistant lung cancer: Targeting cell surface markers and signaling pathways. Pharmacol. Ther. 2015, 158, 71–90. [Google Scholar] [CrossRef]
- Pavlinov, I.; Salkovski, M.; Aldrich, L.N. Beclin 1–ATG14L Protein–Protein Interaction Inhibitor Selectively Inhibits Autophagy through Disruption of VPS34 Complex I. J. Am. Chem. Soc. 2020, 142, 8174–8182. [Google Scholar] [CrossRef]
- Whelan, K.A.; Chandramouleeswaran, P.M.; Tanaka, K.; Natsuizaka, M.; Guha, M.; Srinivasan, S.; Darling, D.S.; Kita, Y.; Natsugoe, S.; Winkler, J.D.; et al. Autophagy supports generation of cells with high CD44 expression via modulation of oxidative stress and Parkin-mediated mitochondrial clearance. Oncogene 2017, 36, 4843–4858. [Google Scholar] [CrossRef]
- Saygin, C.; Matei, D.; Majeti, R.; Reizes, O.; Lathia, J.D. Targeting Cancer Stemness in the Clinic: From Hype to Hope. Cell Stem Cell 2018, 24, 25–40. [Google Scholar] [CrossRef]
- Lei, X.; He, Q.; Li, Z.; Zou, Q.; Xu, P.; Yu, H.; Ding, Y.; Zhu, W. Cancer stem cells in colorectal cancer and the association with chemotherapy resistance. Med. Oncol. 2021, 38, 43. [Google Scholar] [CrossRef]
- Bai, X.; Ni, J.; Beretov, J.; Graham, P.; Li, Y. Cancer stem cell in breast cancer therapeutic resistance. Cancer Treat. Rev. 2018, 69, 152–163. [Google Scholar] [CrossRef]
- Steinbichler, T.B.; Dudas, J.; Skvortsov, S.; Ganswindt, U.; Riechelmann, H.; Skvortsova, I.-I. Therapy resistance mediated by cancer stem cells. Semin. Cancer Biol. 2018, 53, 156–167. [Google Scholar] [CrossRef]
- Robainas, M.; Otano, R.; Bueno, S.; Ait-Oudhia, S. Understanding the role of PD-L1/PD1 pathway blockade and autophagy in cancer therapy. OncoTargets Ther. 2017, 10, 1803. [Google Scholar] [CrossRef]
- Afzal, S.; Hao, Z.; Itsumi, M.; Abouelkheer, Y.; Brenner, D.; Gao, Y.; Wakeham, A.; Hong, C.; Li, W.Y.; Sylvester, J.; et al. Autophagy-independent functions of UVRAG are essential for peripheral naive T-cell homeostasis. Proc. Natl. Acad. Sci. USA 2015, 112, 1119–1124. [Google Scholar] [CrossRef]
- Masud, S.; Prajsnar, T.K.; Torraca, V.; Lamers, G.E.; Benning, M.; Van Der Vaart, M.; Meijer, A.H. Macrophages target Salmonella by Lc3-associated phagocytosis in a systemic infection model. Autophagy 2019, 15, 796–812. [Google Scholar] [CrossRef]
- Aubert, N.; Brunel, S.; Olive, D.; Marodon, G. Blockade of HVEM for Prostate Cancer Immunotherapy in Humanized Mice. Cancers 2021, 13, 3009. [Google Scholar] [CrossRef]
- Liu, L.-Z.; Zhang, Z.; Zheng, B.-H.; Shi, Y.; Duan, M.; Ma, L.-J.; Wang, Z.-C.; Dong, L.-Q.; Dong, P.-P.; Shi, J.-Y.; et al. CCL15 Recruits Suppressive Monocytes to Facilitate Immune Escape and Disease Progression in Hepatocellular Carcinoma. Hepatology 2019, 69, 143–159. [Google Scholar] [CrossRef]
- Kaplanov, I.; Carmi, Y.; Kornetsky, R.; Shemesh, A.; Shurin, G.V.; Shurin, M.R.; Dinarello, C.A.; Voronov, E.; Apte, R.N. Blocking IL-1β reverses the immunosuppression in mouse breast cancer and synergizes with anti–PD-1 for tumor abrogation. Proc. Natl. Acad. Sci. USA 2019, 116, 1361–1369. [Google Scholar] [CrossRef]
- Patel, N.H.; Bloukh, S.; Alwohosh, E.; Alhesa, A.; Saleh, T.; Gewirtz, D.A. Autophagy and senescence in cancer therapy. Adv. Cancer Res. 2021, 150, 1–74. [Google Scholar] [CrossRef]
| Clinical Pathological Data | UVRAG |
|---|---|
| differentiation | |
| R | 0.153 |
| p value | 0.016 |
| T stage | |
| r | 0.047 |
| p value | 0.445 |
| N stage | |
| r | 0.056 |
| p value | 0.367 |
| M stage | |
| r | 0.246 |
| p value | <0.0001 |
| TNM stage | |
| r | 0.163 |
| p value | 0.008 |
| Histological type | |
| U | 0.6687 |
| p value | 0.5369 |
| Vascular cancer embolus | |
| U | 1.57 |
| p value | 0.1155 |
| Location (colon & rectal) | |
| U | 1.45 |
| p value | 0.14 |
| PD-1 | |
| r | 0.128 |
| p value | 0.041 |
| PD-L1 | |
| r | 0.236 |
| p value | 0.000 |
| PD-L2 | |
| r | 0.278 |
| p value | 0.000 |
| Clinical Pathological Data | Location (Colon & Rectal) |
|---|---|
| UVRAG | |
| U | 1.45 |
| p value | 0.14 |
| PD-L2 | |
| U | 1.99 |
| p value | 0.046 |
| PD-L1 | |
| U | 1.78 |
| p value | 0.074 |
| PD-1 | |
| U | 0.78 |
| p value | 0.43 |
| Clinical Pathological Data | UVARG (Colon) |
|---|---|
| PD-L2 | |
| r | 0.319 |
| p value | 0.002 |
| PD-L1 | |
| r | 0.315 |
| p value | 0.000 |
| PD-1 | |
| r | 0.332 |
| p value | 0.001 |
| Clinical Pathological Data | UVRAG (Rectal) |
|---|---|
| PD-L2 | |
| r | 0.232 |
| p value | 0.004 |
| PD-L1 | |
| r | 0.124 |
| p value | 0.12 |
| PD-1 | |
| r | −0.023 |
| p value | 0.78 |
| Clinical Pathological Data | UVRAG |
|---|---|
| PD-L2 | |
| r | 0.222 |
| p value | 0.001 |
| PD-L1 | |
| r | 0.228 |
| p value | 0.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, M.; An, G.; Chen, N.; Jia, J.; Cui, X.; Zhan, T.; Ji, D. UVRAG Promotes Tumor Progression through Regulating SP1 in Colorectal Cancer. Cancers 2023, 15, 2502. https://doi.org/10.3390/cancers15092502
Shi M, An G, Chen N, Jia J, Cui X, Zhan T, Ji D. UVRAG Promotes Tumor Progression through Regulating SP1 in Colorectal Cancer. Cancers. 2023; 15(9):2502. https://doi.org/10.3390/cancers15092502
Chicago/Turabian StyleShi, Mengyuan, Guo An, Nan Chen, Jinying Jia, Xinxin Cui, Tiancheng Zhan, and Dengbo Ji. 2023. "UVRAG Promotes Tumor Progression through Regulating SP1 in Colorectal Cancer" Cancers 15, no. 9: 2502. https://doi.org/10.3390/cancers15092502
APA StyleShi, M., An, G., Chen, N., Jia, J., Cui, X., Zhan, T., & Ji, D. (2023). UVRAG Promotes Tumor Progression through Regulating SP1 in Colorectal Cancer. Cancers, 15(9), 2502. https://doi.org/10.3390/cancers15092502






