Proton Beam Therapy in the Oligometastatic/Oligorecurrent Setting: Is There a Role? A Literature Review
Abstract
Simple Summary
Abstract
1. Introduction
- -
- Synchronous oligometastatic disease: includes patients found to have metastatic disease at the time of initial diagnosis.
- -
- Metachronous oligometastatic disease (often used interchangeably with oligorecurrence): refers to patients initially treated with definitive therapy to cure their malignancy who subsequently (>3 months later) develop limited disease recurrence.
- -
- Oligoprogressive disease: represents patients with known metastatic disease who exhibit few isolated areas of progression in a background of otherwise stable disease.
- -
- Oligopersistent disease: persistent disease after systemic therapy.
2. Materials and Methods
3. Results
3.1. Various Anatomical Sites
3.2. Lungs and Thoracic Lymph Nodes
3.3. Liver
3.4. Bone
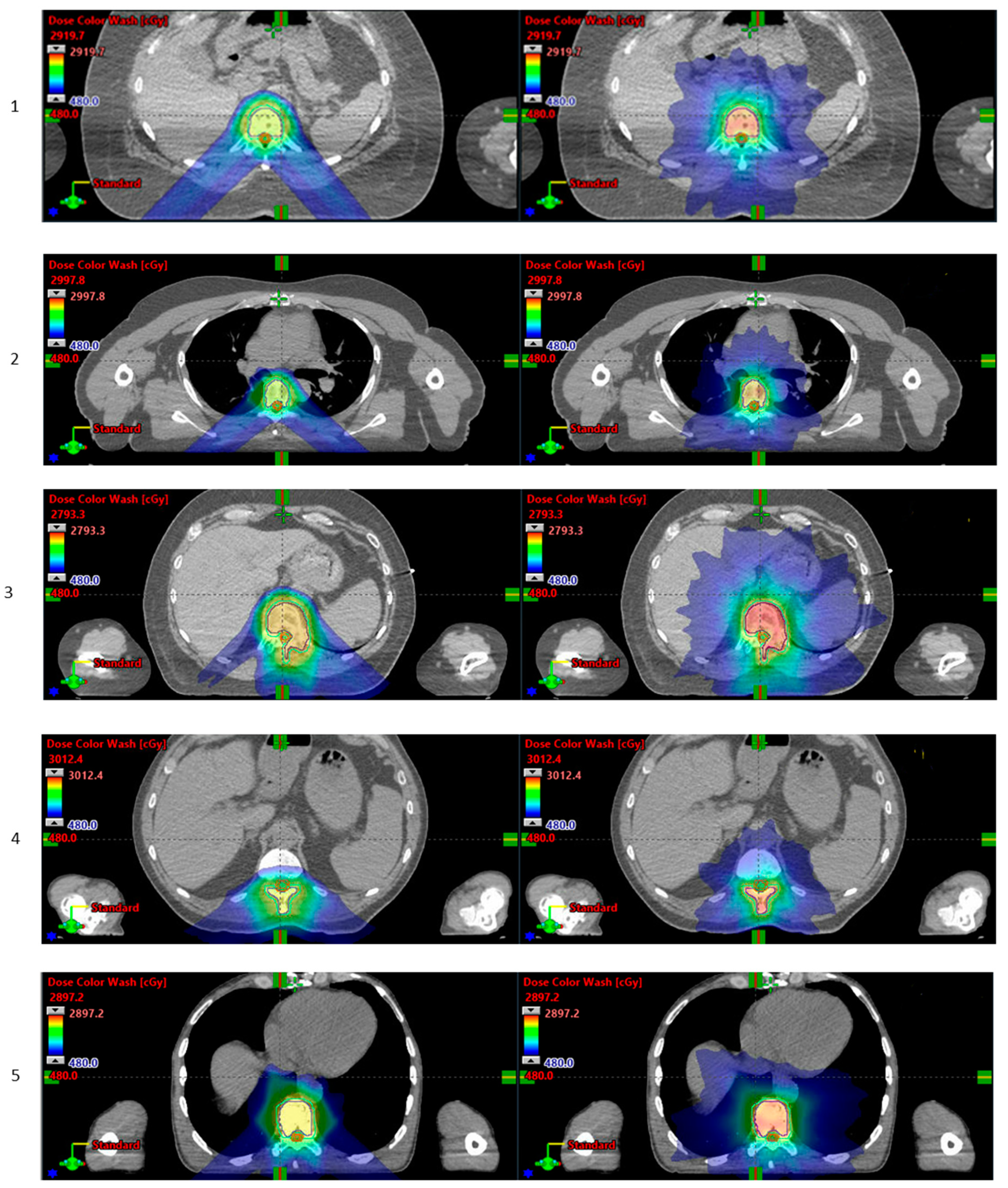
3.5. Pelvis
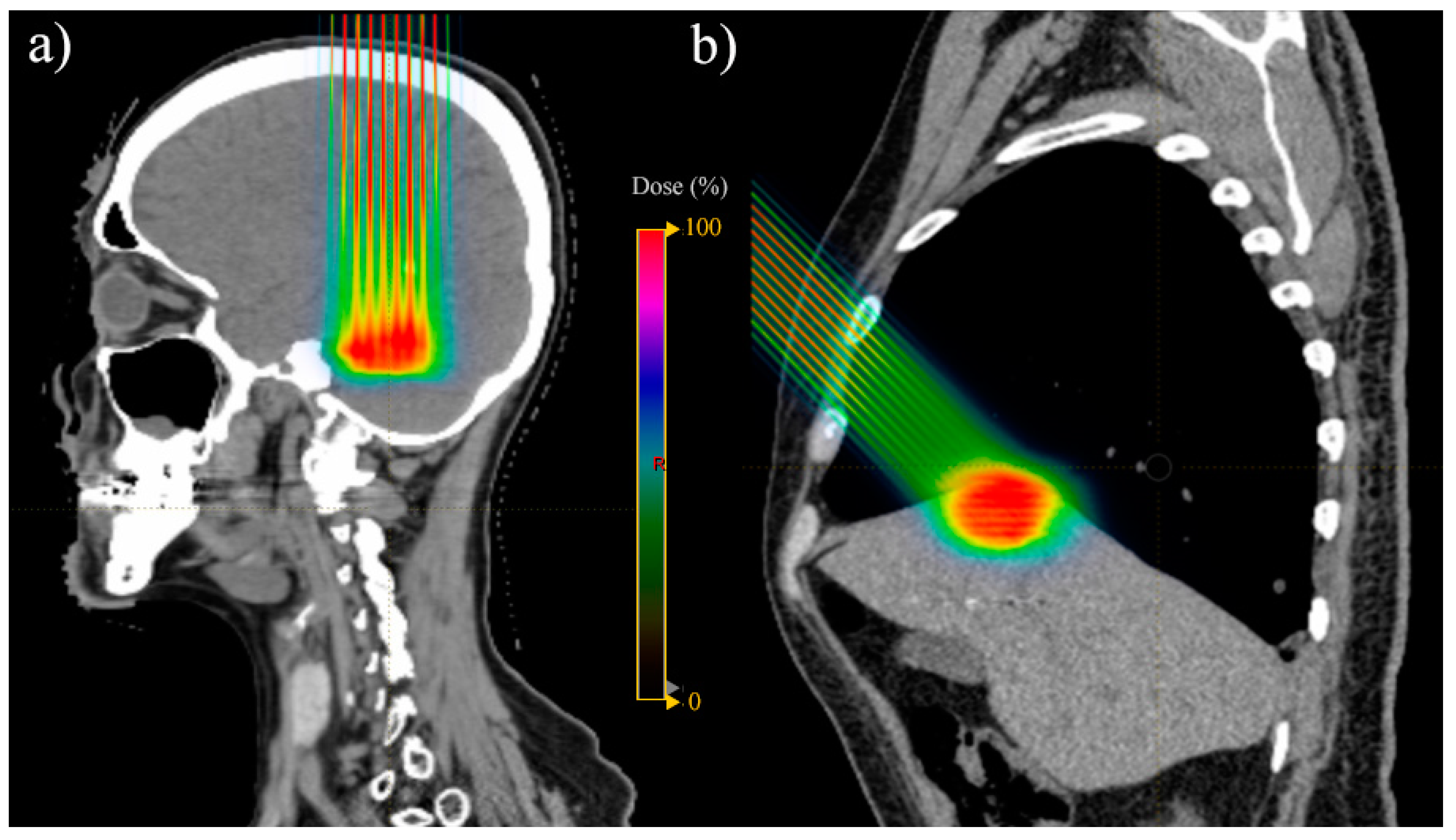
3.6. Brain
| N. | Author | Year | Country | Primary Tumour | Site of Mets | No. of | Setting | Article Type | Type of Study | Total Dose (GyRBE) | No. of Fractions | Dose per Fraction (GyRBE) | Local Control/Overall Survival Rates | Acute and Late Toxicities |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients/Lesions | 1. Abstract | |||||||||||||
| 2. Article | ||||||||||||||
| 1 | Rans et al. [22] | 2022 | Belgium | Prostate | Various | 12/25 | Oligomets | 1 | Retrospective cohort ◊ | 36 | 12 | N/A | N/A | |
| 2 | Nakajima et al. [33] | 2019 | Japan | Gastric/colorectal | Liver | 43/53 | Oligomets | 1 | Retrospective cohort | 66 or 72.6 | 6.6 | 1- and 2- year OS: 87% and 63%, respectively; 1- and 2-year LC: 73% and 70%, respectively | N/A | |
| 3 | Hoyer et al. [40] | 2018 | Denmark | N/A | N/A | Oligomets | 1 | Descriptive abstract * | N/A | N/A | N/A | N/A | ||
| 4 | Contreras et al. [28] | 2017 | USA | Various | Lung | 25 patients | Recurrence | 1 | Retrospective cohort | Median 60 (40–62.5) | Median 2 (2–10) | 1-year OS: 82%; 1-year LC: 74.8%, | AT: 12% (G > 3); LT: 20% (G > 3) | |
| 5 | Sufficool et al. [34] | 2018 | USA | Various | Liver | 06/09 | Oligomets | 1 | Phase II trial | 60 | 20 | Median follow-up 9.8 months (1–33): LC 100% | AT: 1 patient G1 fatigue | |
| 6 | Bakhtiar et al. [27] | 2021 | USA | Various | Various | 182 patients | Oligomets/recurrence | 1 | Retrospective cohort | Median 50 (15–80) | 2 | Median OS: 139 days (1–363) | AT: 85% (any grade); LT: 17% (any grade) | |
| 7 | Sulaiman et al. [29] | 2014 | Japan | Various | Lung | 47/59 | Oligomets | 2 | Retrospective cohort | Median 60 (52.8–70.2) | Median 8 (4–26) | 1- and 2-year OS: 72.7% and 54%, respectively; 1- and 2-year LC: 88.4% and 79%, respectively | AT *: G1 23, G2 1, G3 2; LT *: G1 19, G2 6, G3 4 | |
| 8 | Johnson et al. [38] | 2022 | USA | Breast | Sternum | 4 patients | Oligomets | 2 | Case series ◊ | 60 (sternum); 45–50.4 (other targets) | 2–2.4 (sternum); 1.8–2 (other targets) | Median follow-up 28 months: LC 100% | AT *:5 G2 | |
| 9 | Aibe et al. [30] | 2021 | Japan | Various | Lung | 118/141 | Oligomets | 2 | Retrospective cohort | Median 64 (52.8–89.6) | Median 10 (4–40) | 6.6 (2–13.2) | 1- and 2-year OS: 79% and 67.8%, respectively; 1- and 2-year LC: 92.2% and 86.3%, respectively | AT: 7% (G ≥ 2); LT: 8% (G2) |
| 10 | Ishikawa et al. [38] | 2022 | Japan | Breast | Sternum | 01/01 | Oligomets | 2 | Case report | 70 | 2.5 | NED at 3-years follow-up | AT: 1 G2 | |
| 11 | Kawamata et al. [31] | 2020 | Japan | Breast | Lymph nodes | 01/01 | Oligomets | 2 | Case report | 60 | 2 | NED at last follow-up | Nil | |
| 12 | Gill et al. [35] | 2018 | UK | Colorectal | Liver | Oligomets | 2 | Review | N/A | N/A | N/A | N/A | ||
| 13 | Nakamura et al. [32] | 2020 | Japan | Lung | Lymph nodes | 33 patients | Recurrence | 2 | Retrospective cohort | Median 70 (66–76) | 2 | 3-year OS: 63.8%; 3-year LC: 79.7%, | AT: 11 patients G2 (33%), 1 G3 (3%); LT: 1 G3 (3%) | |
| 14 | Hong T et al. [36] | 2017 | USA | Various | Liver | 89 patients | Oligomets | 2 | Phase II trial | Median 40 (30–50) | Median 8 (6–10) | Median OS: 18.1 months; 1- and 3-year LC: 71.9% and 61.2%, respectively | AT: 87.6% G2 ≤ 2 | |
| 15 | Atkins et al. [21] | 2018 | USA | Various | Brain | 370/815 | Oligomets | 2 | Retrospective cohort | Median 18 (8–28) | 1 | Median follow-up 9.2 months; 6- and 12-month local failure: 4.3% and 8.5%, respectively; 6- and 12-month OS: 76.0% and 51.5%, respectively | LT: 3.6% radionecrosis at 12 months | |
| 16 | Chuter et al. [20] | 2022 | UK | Various | Pelvis | 10/10 | Recurrence | 2 | Retrospective cohort ◊ | 30 | 5 | 6 | N/A | N/A |
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hellman, S.; Weichselbaum, R.R. Oligometastases. J. Clin. Oncol. 1995, 13, 8–10. [Google Scholar] [CrossRef]
- Milano, M.T.; Katz, A.W.; Zhang, H.; Okunieff, P. Oligometastases Treated with Stereotactic Body Radiotherapy: Long-Term Follow-Up of Prospective Study. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, 878–886. [Google Scholar] [CrossRef] [PubMed]
- Niibe, Y.; Hayakawa, K. Oligometastases and oligo-recurrence: The new era of cancer therapy. Jpn. J. Clin. Oncol. 2010, 40, 107–111. [Google Scholar] [CrossRef]
- Deek, M.P.; Tran, P.T. Oligometastatic and Oligoprogression Disease and Local Therapies in Prostate Cancer. Cancer J. 2020, 26, 137–143. [Google Scholar] [CrossRef]
- Lievens, Y.; Guckenberger, M.; Gomez, D.; Hoyer, M.; Iyengar, P.; Kindts, I.; Romero, A.M.; Nevens, D.; Palma, D.; Park, C.; et al. Defining oligometastatic disease from a radiation oncology perspective: An ESTRO-ASTRO consensus document. Radiother. Oncol. 2020, 148, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Alfonso, J.C.L.; Poleszczuk, J.; Walker, R.; Kim, S.; Pilon-Thomas, S.; Conejo-Garcia, J.; Soliman, H.; Czerniecki, B.; Harrison, L.B.; Enderling, H. Immunologic Consequences of Sequencing Cancer Radiotherapy and Surgery. JCO Clin. Cancer Inform. 2019, 3, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Pastorino, U.; Buyse, M.; Friedel, G.; Ginsberg, R.J.; Girard, P.; Goldstraw, P.; Johnston, M.; McCormack, P.; Pass, H.; Putnam, J.B., Jr.; et al. Long-term results of lung metastasectomy: Prognostic analyses based on 5206 cases. The International Registry of Lung Metastases. J. Thorac. Cardiovasc. Surg. 1997, 113, 37–49. [Google Scholar] [CrossRef]
- Feuer, Z.; Taylor, J.I.; Huang, W.C. The contemporary role of metastasectomy in the management of metastatic RCC. J. Cancer Metastasis Treat. 2021, 7, 68. [Google Scholar] [CrossRef]
- Chandy, E.; Taylor, H.; Gaito, S.; Wells, E.; Jones, C.; Meehan, C.; Burland, H.; Stone, J.; Snowball, C.; Mashru, J.; et al. Hypofractionated Stereotactic Ablative Radiotherapy for Recurrent or Oligometastatic Tumours in Children and Young Adults. Clin. Oncol. 2020, 32, 316–326. [Google Scholar] [CrossRef]
- Lehrer, E.J.; Singh, R.; Wang, M.; Chinchilli, V.M.; Trifiletti, D.M.; Ost, P.; Siva, S.; Meng, M.-B.; Tchelebi, L.; Zaorsky, N.G. Safety and Survival Rates Associated with Ablative Stereotactic Radiotherapy for Patients with Oligometastatic Cancer: A Systematic Review and Meta-analysis. JAMA Oncol. 2021, 7, 92. [Google Scholar] [CrossRef]
- Palma, D.; Tree, A.; Guckenberger, M. Should stereotactic radiotherapy be the preferred treatment for oligometastatic disease? Lancet Oncol. 2021, 22, 1067–1068. [Google Scholar] [CrossRef]
- Palma, D.A.; Olson, R.; Harrow, S.; Gaede, S.; Louie, A.V.; Haasbeek, C.; Mulroy, L.; Lock, M.; Rodrigues, G.B.; Yaremko, B.P.; et al. Stereotactic ablative radiotherapy for the comprehensive treatment of oligometastatic cancers: Long-term results of the SABR-COMET Phase II randomized trial. J. Clin. Oncol. 2020, 38, 2830–2838. [Google Scholar] [CrossRef]
- Gagliardi, F.; De Domenico, P.; Snider, S.; Roncelli, F.; Pompeo, E.; Barzaghi, L.R.; Bulotta, A.; Gregorc, V.; Lazzari, C.; Cascinu, S. Role of stereotactic radiosurgery for the treatment of brain metastasis in the era of immunotherapy: A systematic review on current evidences and predicting factors. Crit. Rev. Oncol. Hematol. 2021, 165, 103431. [Google Scholar] [CrossRef] [PubMed]
- Paganetti, H. Relative biological effectiveness (RBE) values for proton beam therapy. Variations as a function of biological endpoint, dose, and linear energy transfer. Phys. Med. Biol. 2014, 59, R419–72. [Google Scholar] [CrossRef]
- Hwang, E.; Gaito, S.; France, A.; Crellin, A.; Thwaites, D.; Ahern, V.; Indelicato, D.; Timmermann, B.; Smith, E. Outcomes of Patients Treated in the UK Proton Overseas Programme: Non-central Nervous System Group. Clin. Oncol. 2023, 35, 292–300. [Google Scholar] [CrossRef]
- Gaito, S.; Hwang, E.; France, A.; Aznar, M.; Burnet, N.; Crellin, A.; Holtzman, A.; Indelicato, D.; Timmerman, B.; Whitfield, G.; et al. Outcomes of Patients Treated in the UK Proton Overseas Programme: Central Nervous System Group. Clin. Oncol. 2023, 35, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Grau, C.; Durante, M.; Georg, D.; Langendijk, J.A.; Weber, D.C. Particle therapy in Europe. Mol. Oncol. 2020, 14, 1492–1499. [Google Scholar] [CrossRef] [PubMed]
- PBT Facilities n.d. Available online: https://www.ptcog.ch/index.php/facilities-in-operation-restricted (accessed on 2 December 2022).
- Sackett, D.L. How to Practice and Teach EBM; Evidence-Based Medicine: New York, NY, USA, 2000. [Google Scholar]
- Chuter, R.; Glassborow, E.; Speight, R.; Clarke, M.; Murray, L.; Radhakrishna, G.; Lavin, V.; Aspin, L.; Aldred, M.; Gregory, S.; et al. A treatment planning comparison of photon stereotactic ablative radiotherapy and proton beam therapy for the re-irradiation of pelvic cancer recurrence. Phys. Imaging Radiat. Oncol. 2022, 21, 78–83. [Google Scholar] [CrossRef]
- Atkins, K.M.; Pashtan, I.M.; Bussière, M.R.; Kang, K.H.; Niemierko, A.; Daly, J.E.; Botticello, T.M.; Hurd, M.C.; Chapman, P.H.; Oh, K.; et al. Proton Stereotactic Radiosurgery for Brain Metastases: A Single-Institution Analysis of 370 Patients. Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 820–829. [Google Scholar] [CrossRef] [PubMed]
- Rans, K.; De Meerleer, G.; Berghen, C.; Poels, K. Protons versus photons for oligometastatic prostate cancer: A planning comparison. Radiother. Oncol. 2022, 170, S1272. [Google Scholar] [CrossRef]
- Gaito, S.; Abravan, A.; Richardson, J.; Lowe, M.; Indelicato, D.; Burnet, N.; Smith, E. Skin Toxicity Profile of Photon Radiotherapy versus Proton Beam Therapy in Paediatric and Young Adult Patients with Sarcomas. Clin. Oncol. 2021, 33, 507–516. [Google Scholar] [CrossRef]
- Gaito, S.; Burnet, N.; Aznar, M.; Crellin, A.; Indelicato, D.; Ingram, S.; Pan, S.; Price, G.; Hwang, E.; France, A.; et al. Normal Tissue Complication Probability Modelling for Toxicity Prediction and Patient Selection in Proton Beam Therapy to the Central Nervous System: A Literature Review. Clin. Oncol. 2022, 34, e225–e237. [Google Scholar] [CrossRef] [PubMed]
- Gaito, S.; France, A.; Foden, P.; Abravan, A.; Burnet, N.; Garcez, K.; Kota, V.; Lee, L.; Price, J.; Sykes, A.; et al. A Predictive Model for Reactive Tube Feeding in Head and Neck Cancer Patients Undergoing Definitive (Chemo) Radiotherapy. Clin. Oncol. 2021, 33, e433–e441. [Google Scholar] [CrossRef] [PubMed]
- Langendijk, J.A.; Lambin, P.; De Ruysscher, D.; Widder, J.; Bos, M.; Verheij, M. Selection of patients for radiotherapy with protons aiming at reduction of side effects: The model-based approach. Radiother. Oncol. 2013, 107, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Bakhtiar, M.; Butala, A.A.; Taunk, N.K.; Lukens, J.N.; Jones, J.A.; Paydar, I. Factors Associated With and Characteristics of Proton Radiotherapy Use at the End of Life. Int. J. Radiat. Oncol. Biol. Phys. 2021, 111, e494–e495. [Google Scholar] [CrossRef]
- Contreras, J.; Chundury, A.; Reynoso, F.; Zhao, T.; Sun, B.; Roach, M.; Robinson, C.; Bradley, J. Proton Therapy Reirradiation for Thoracic Recurrences: Toxicity and Outcomes. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, E448. [Google Scholar] [CrossRef]
- Sulaiman, N.S.; Fujii, O.; Demizu, Y.; Terashima, K.; Niwa, Y.; Akagi, T.; Daimon, T.; Murakami, M.; Sasaki, R.; Fuwa, N. Particle beam radiation therapy using carbon ions and protons for oligometastatic lung tumors. Radiat. Oncol. 2014, 9, 183. [Google Scholar] [CrossRef]
- Aibe, N.; Ogino, H.; Teramukai, S.; Yamazaki, H.; Iwata, H.; Matsuo, Y.; Okimoto, T.; Murakami, M.; Suzuki, M.; Arimura, T.; et al. Multi-Institutional Retrospective Analysis of the Outcomes of Proton Beam Therapy for Patients with 1 to 3 Pulmonary Oligometastases From Various Primary Cancers. Adv. Radiat. Oncol. 2021, 6, 100690. [Google Scholar] [CrossRef]
- Kawamata, A.; Hikino, H.; Makino, Y.; Murata, Y. Importance of Local Therapy for Oligometastatic Breast Cancer-A Case Report. Gan Kagaku Ryoho 2020, 47, 2397–2399. [Google Scholar]
- Nakamura, M.; Ohnishi, K.; Ishikawa, H.; Nakazawa, K.; Shiozawa, T.; Okumura, T.; Sekine, I.; Sato, Y.; Hizawa, N.; Sakurai, H. Salvage photon or proton radiotherapy for oligo-recurrence in regional lymph nodes after surgery for non-small cell lung cancer. In Vivo 2020, 34, 1883–1892. [Google Scholar] [CrossRef]
- Nakajima, K.; Iwata, H.; Hattori, Y.; Hashimoto, S.; Hayashi, K.; Toshito, T.; Baba, F.; Sasaki, S.; Mizoe, J.; Ogino, H.; et al. Image-guided Proton Therapy (IGPT) for Oligometastatic Liver Tumors from Gastric/Colorectal Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, E224–E225. [Google Scholar] [CrossRef]
- Sufficool, D.C.; Kang, J.I.; Hsueh, C.-T.; Wroe, A.J.; Patyal, B.; Reeves, M.E.; Slater, J.D.; Yang, G.Y. Interim Results of a Phase I/II Trial of Proton Stereotactic Body Radiation Therapy (SBRT) for Liver Metastases. Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, E4. [Google Scholar] [CrossRef]
- Gill, S.; Liu, D.M.; Green, H.M.; Sharma, R.A. Beyond the Knife: The Evolving Nonsurgical Management of Oligometastatic Colorectal Cancer. Am. Soc. Clin. Oncol. Educ. Book 2018, 38, 209–219. [Google Scholar] [CrossRef]
- Hong, T.S.; Wo, J.Y.; Borger, D.R.; Yeap, B.Y.; McDonnell, E.I.; Willers, H.; Blaszkowsky, L.S.; Kwak, E.L.; Allen, J.N.; Clark, J.W.; et al. Phase II Study of Proton-Based Stereotactic Body Radiation Therapy for Liver Metastases: Importance of Tumor Genotype. J. Natl. Cancer Inst. 2017, 109, djx031. [Google Scholar] [CrossRef]
- Ahmed, K.A.; Caudell, J.J.; El-Haddad, G.; Berglund, A.E.; Welsh, E.A.; Yue, B.; Hoffe, S.E.; Naghavi, A.O.; Abuodeh, Y.A.; Frakes, J.M.; et al. Radiosensitivity Differences Between Liver Metastases Based on Primary Histology Suggest Implications for Clinical Outcomes After Stereotactic Body Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 1399–1404. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, Y.; Suzuki, M.; Yamaguchi, H.; Seto, I.; Machida, M.; Takagawa, Y.; Jingu, K.; Kikuchi, Y.; Murakami, M. Successful treatment with proton beam therapy for a solitary sternal metastasis of breast cancer: A case report. J. Med. Case Rep. 2022, 16, 111. [Google Scholar] [CrossRef]
- Johnson, A.; Depauw, N.; Zieminski, S.; Jimenez, R. Proton Radiotherapy for Patients with Oligometastatic Breast Cancer Involving the Sternum. Int. J. Part. Ther. 2022, 8, 66–71. [Google Scholar] [CrossRef]
- Hoyer, M.; Petersen, J.B. SP-0374: Protons for oligometastases? Radiother. Oncol. 2018, 127, S191. [Google Scholar] [CrossRef]
- Hanson, P.W.; Elaimy, A.L.; Lamoreaux, W.T.; Demakas, J.J.; Fairbanks, R.K.; Mackay, A.R.; Taylor, B.; Cooke, B.S.; Thumma, S.R.; Lee, C.M. A concise review of the efficacy of stereotactic radiosurgery in the management of melanoma and renal cell carcinoma brain metastases. World J. Surg. Oncol. 2012, 10, 176. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, B.; Hanssens, P.; Wolff, R.; Söderman, M.; Lindquist, C.; Beute, G. Thirty years’ experience with Gamma Knife surgery for metastases to the brain: Clinical article. J. Neurosurg. 2009, 111, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, A.K.; Flickinger, J.C.; Kondziolka, D.; Lunsford, L.D. Stereotactic radiosurgery for four or more intracranial metastases. Int. J. Radiat. Oncol. Biol. Phys. 2006, 64, 898–903. [Google Scholar] [CrossRef] [PubMed]
- Mazzola, R.; Jereczek-Fossa, B.A.; Franceschini, D.; Tubin, S.; Filippi, A.R.; Tolia, M.; Lancia, A.; Minniti, G.; Corradini, S.; Arcangeli, S.; et al. Oligometastasis and local ablation in the era of systemic targeted and immunotherapy. Radiat. Oncol. 2020, 15, 92. [Google Scholar] [CrossRef] [PubMed]
- Gomez, D.R.; Yang, T.J.; Tsai, C.J. Emerging Paradigm of Consolidative Thoracic Radiotherapy in Oligometastatic NSCLC. Semin. Radiat. Oncol. 2021, 31, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Marvaso, G.; Volpe, S.; Pepa, M.; Augugliaro, M.; Corrao, G.; Biffi, A.; Zaffaroni, M.; Bergamaschi, L.; La Fauci, F.M.; Mistretta, F.A.; et al. Oligorecurrent Prostate Cancer and Stereotactic Body Radiotherapy: Where Are We Now? A Systematic Review and Meta-analysis of Prospective Studies. Eur. Urol. Open Sci. 2021, 27, 19–28. [Google Scholar] [CrossRef]
- Aoyama, H.; Shirato, H.; Tago, M.; Nakagawa, K.; Toyoda, T.; Hatano, K.; Kenjyo, M.; Oya, N.; Hirota, S.; Shioura, H.; et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: A randomized controlled trial. JAMA 2006, 295, 2483–2491. [Google Scholar] [CrossRef]
- Yamamoto, M.; Serizawa, T.; Shuto, T.; Akabane, A.; Higuchi, Y.; Kawagishi, J.; Yamanaka, K.; Sato, Y.; Jokura, H.; Yomo, S.; et al. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): A multi-institutional prospective observational study. Lancet Oncol. 2014, 15, 387–395. [Google Scholar] [CrossRef]
- Alongi, F.; Arcangeli, S.; Filippi, A.R.; Ricardi, U.; Scorsetti, M. Review and Uses of Stereotactic Body Radiation Therapy for Oligometastases. Oncologist 2012, 17, 1100–1107. [Google Scholar] [CrossRef]
- Fourkal, E.; Li, J.S.; Xiong, W.; Nahum, A.; Ma, C.M. Intensity modulated radiation therapy using laser-accelerated protons: A Monte Carlo dosimetric study. Phys. Med. Biol. 2003, 48, 3977–4000. [Google Scholar] [CrossRef] [PubMed]
- Nomura, K.; Iwata, H.; Toshito, T.; Omachi, C.; Nagayoshi, J.; Nakajima, K.; Ogino, H.; Shibamoto, Y. Biological effects of passive scattering and spot scanning proton beams at the distal end of the spread-out Bragg peak in single cells and multicell spheroids. Int. J. Radiat. Biol. 2021, 97, 695–703. [Google Scholar] [CrossRef]
- Bortfeld, T.; Paganetti, H.; Kooy, H. MO-A-T-6B-01: Proton Beam Radiotherapy—The State of the Art. Med. Phys. 2005, 32, 2048–2049. [Google Scholar] [CrossRef]
- Lowe, M.; Gosling, A.; Nicholas, O.; Underwood, T.; Miles, E.; Chang, Y.-C.; Amos, R.; Burnet, N.; Clark, C.; Patel, I.; et al. Comparing Proton to Photon Radiotherapy Plans: UK Consensus Guidance for Reporting Under Uncertainty for Clinical Trials. Clin. Oncol. 2020, 32, 459–466. [Google Scholar] [CrossRef]
- Ortiz, R.; Belshi, R.; De Marzi, L.; Prezado, Y. Proton minibeam radiation therapy for treating metastases: A treatment plan study. Med. Phys. 2023, 50, 2463–2473. [Google Scholar] [CrossRef]
- Singh, R.; Valluri, A.; Didwania, P.; Lehrer, E.J.; Baliga, S.; Hiniker, S.; Braunstein, S.E.; Murphy, E.S.; Lazarev, S.; Tinkle, C.; et al. Efficacy and Safety of Stereotactic Body Radiation Therapy for Pediatric Malignancies: The LITE-SABR Systematic Review and Meta-Analysis. Adv. Radiat. Oncol. 2023, 8, 101123. [Google Scholar] [CrossRef]
- Beaumont, J.; Dawdall, L.; Hall, R.; Lowe, M.; Colaco, R. A comparison of proton vs. photon planning in patients treated with Stereotactic Body Radiotherapy (SBRT) for spine metastases. In Proceedings of the 60th Annual Conference of the Particle Therapy Cooperative Group, Miami, FL, USA, 27 June–2 July 2022; p. 83. [Google Scholar]
- Friedrich, T. Proton RBE dependence on dose in the setting of hypofractionation. Br. J. Radiol. 2020, 93, 20190291. [Google Scholar] [CrossRef]
- Carabe, A.; Moteabbed, M.; Depauw, N.; Schuemann, J.; Paganetti, H. Range uncertainty in proton therapy due to variable biological effectiveness. Phys. Med. Biol. 2012, 57, 1159–1172. [Google Scholar] [CrossRef] [PubMed]
- Paganetti, H.; Beltran, C.J.; Both, S.; Dong, L.; Flanz, J.B.; Furutani, K.M.; Grassberger, C.; Grosshans, D.R.; Knopf, A.-C.; Langendijk, J.A.; et al. Roadmap: Proton therapy physics and biology. Phys. Med. Biol. 2021, 66, 05RM01. [Google Scholar] [CrossRef]
- Volpe, S.; Piperno, G.; Colombo, F.; Biffi, A.; Comi, S.; Mastroleo, F.; Camarda, A.M.; Casbarra, A.; Cattani, F.; Corrao, G.; et al. Hypofractionated Proton Therapy for Non-Small Cell Lung Cancer: Ready for Prime Time? A Systematic Review and Meta-Analysis. SSRN Electron. J. 2022, 110, 102464. [Google Scholar] [CrossRef]
- MacKay, R.I. Image Guidance for Proton Therapy. Clin. Oncol. 2018, 30, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.I., Jr.; Ling, T.C.; Slater, J.D.; Yang, G.Y. Stereotactic body proton therapy for liver metastases. Trans. Cancer Res. 2012, 1, 271–275. [Google Scholar] [CrossRef]
- Chang, S.; Liu, G.; Zhao, L.; Zheng, W.; Yan, D.; Chen, P.; Li, X.; Yang, K.; Deraniyagala, R.; Stevens, C.; et al. Redefine the Role of Spot-Scanning Proton Beam Therapy for the Single Brain Metastasis Stereotactic Radiosurgery. Front. Oncol. 2022, 12, 804036. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Li, X.; Qin, A.; Zhou, J.; Yan, D.; Stevens, C.; Krauss, D.; Kabolizadeh, P. Have we reached proton beam therapy dosimetric limitations?—A novel robust, delivery-efficient and continuous spot-scanning proton arc (SPArc) therapy is to improve the dosimetric outcome in treating prostate cancer. Acta Oncol. 2018, 57, 435–437. [Google Scholar] [CrossRef]
- Prezado, Y.; Fois, G.R. Proton-minibeam radiation therapy: A proof of concept. Med. Phys. 2013, 40, 031712. [Google Scholar] [CrossRef] [PubMed]
- Prezado, Y.; Jouvion, G.; Hardy, D.; Patriarca, A.; Nauraye, C.; Bergs, J.; González, W.; Guardiola, C.; Juchaux, M.; Labiod, D.; et al. Proton minibeam radiation therapy spares normal rat brain: Long-Term Clinical, Radiological and Histopathological Analysis. Sci. Rep. 2017, 7, 14403. [Google Scholar] [CrossRef] [PubMed]
- Prezado, Y.; Jouvion, G.; Guardiola, C.; Gonzalez, W.; Juchaux, M.; Bergs, J.; Nauraye, C.; Labiod, D.; De Marzi, L.; Pouzoulet, F.; et al. Tumor Control in RG2 Glioma-Bearing Rats: A Comparison Between Proton Minibeam Therapy and Standard Proton Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2019, 104, 266–271. [Google Scholar] [CrossRef]
- Prezado, Y. Divide and conquer: Spatially fractionated radiation therapy. Expert Rev. Mol. Med. 2022, 24, e3. [Google Scholar] [CrossRef]
- Lamirault, C.; Doyère, V.; Juchaux, M.; Pouzoulet, F.; Labiod, D.; Dendale, R.; Patriarca, A.; Nauraye, C.; Le Dudal, M.; Jouvion, G.; et al. Short and long-term evaluation of the impact of proton minibeam radiation therapy on motor, emotional and cognitive functions. Sci. Rep. 2020, 10, 13511. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, B.; Lowe, M.; Traneus, E.; Krieger, M.; Schuemann, J. Treatment planning considerations for the development of FLASH proton therapy. Radiother. Oncol. 2022, 175, 222–230. [Google Scholar] [CrossRef]
- Vozenin, M.-C.; Bourhis, J.; Durante, M. Towards clinical translation of FLASH radiotherapy. Nat. Rev. Clin. Oncol. 2022, 19, 791–803. [Google Scholar] [CrossRef]
- Marcu, L.G.; Bezak, E.; Peukert, D.D.; Wilson, P. Translational Research in FLASH Radiotherapy—From Radiobiological Mechanisms to In Vivo Results. Biomedicines 2021, 9, 181. [Google Scholar] [CrossRef]
- Kacem, H.; Almeida, A.; Cherbuin, N.; Vozenin, M.-C. Understanding the FLASH effect to unravel the potential of ultra-high dose rate irradiation. Int. J. Radiat. Biol. 2022, 98, 506–516. [Google Scholar] [CrossRef]
- Cunningham, S.; McCauley, S.; Vairamani, K.; Speth, J.; Girdhani, S.; Abel, E.; Sharma, R.A.; Perentesis, J.P.; Wells, S.I.; Mascia, A.; et al. FLASH Proton Pencil Beam Scanning Irradiation Minimizes Radiation-Induced Leg Contracture and Skin Toxicity in Mice. Cancers 2021, 13, 1012. [Google Scholar] [CrossRef] [PubMed]
- Velalopoulou, A.; Karagounis, I.V.; Cramer, G.M.; Kim, M.M.; Skoufos, G.; Goia, D.; Hagan, S.; Verginadis, I.I.; Shoniyozov, K.; Chiango, J.; et al. FLASH Proton Radiotherapy Spares Normal Epithelial and Mesenchymal Tissues While Preserving Sarcoma Response. Cancer Res. 2021, 81, 4808–4821. [Google Scholar] [CrossRef] [PubMed]
- Chabi, S.; Van To, T.H.; Leavitt, R.; Poglio, S.; Jorge, P.G.; Jaccard, M.; Petersson, K.; Petit, B.; Roméo, P.-H.; Pflumio, F.; et al. Ultra-high-dose-rate FLASH and Conventional-Dose-Rate Irradiation Differentially Affect Human Acute Lymphoblastic Leukemia and Normal Hematopoiesis. Int. J. Radiat. Oncol. Biol. Phys. 2021, 109, 819–829. [Google Scholar] [CrossRef]
- MacKay, R.; Burnet, N.; Lowe, M.; Rothwell, B.; Kirkby, N.; Kirkby, K.; Hendry, J. FLASH radiotherapy: Considerations for multibeam and hypofractionation dose delivery. Radiother. Oncol. 2021, 164, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Chmura, S.J.; Winter, K.A.; Al-Hallaq, H.A.; Borges, V.F.; Jaskowiak, N.T.; Matuszak, M.; Milano, M.T.; Salama, J.K.; Woodward, W.A.; White, J.R. NRG-BR002: A phase IIR/III trial of standard of care therapy with or without stereotactic body radiotherapy (SBRT) and/or surgical ablation for newly oligometastatic breast cancer (NCT02364557). J. Clin. Oncol. 2019, 37, TPS1117. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaito, S.; Marvaso, G.; Ortiz, R.; Crellin, A.; Aznar, M.C.; Indelicato, D.J.; Pan, S.; Whitfield, G.; Alongi, F.; Jereczek-Fossa, B.A.; et al. Proton Beam Therapy in the Oligometastatic/Oligorecurrent Setting: Is There a Role? A Literature Review. Cancers 2023, 15, 2489. https://doi.org/10.3390/cancers15092489
Gaito S, Marvaso G, Ortiz R, Crellin A, Aznar MC, Indelicato DJ, Pan S, Whitfield G, Alongi F, Jereczek-Fossa BA, et al. Proton Beam Therapy in the Oligometastatic/Oligorecurrent Setting: Is There a Role? A Literature Review. Cancers. 2023; 15(9):2489. https://doi.org/10.3390/cancers15092489
Chicago/Turabian StyleGaito, Simona, Giulia Marvaso, Ramon Ortiz, Adrian Crellin, Marianne C. Aznar, Daniel J. Indelicato, Shermaine Pan, Gillian Whitfield, Filippo Alongi, Barbara Alicja Jereczek-Fossa, and et al. 2023. "Proton Beam Therapy in the Oligometastatic/Oligorecurrent Setting: Is There a Role? A Literature Review" Cancers 15, no. 9: 2489. https://doi.org/10.3390/cancers15092489
APA StyleGaito, S., Marvaso, G., Ortiz, R., Crellin, A., Aznar, M. C., Indelicato, D. J., Pan, S., Whitfield, G., Alongi, F., Jereczek-Fossa, B. A., Burnet, N., Li, M. P., Rothwell, B., Smith, E., & Colaco, R. J. (2023). Proton Beam Therapy in the Oligometastatic/Oligorecurrent Setting: Is There a Role? A Literature Review. Cancers, 15(9), 2489. https://doi.org/10.3390/cancers15092489






