Usefulness of New Neutrophil-Related Hematologic Parameters in Patients with Myelodysplastic Syndrome
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
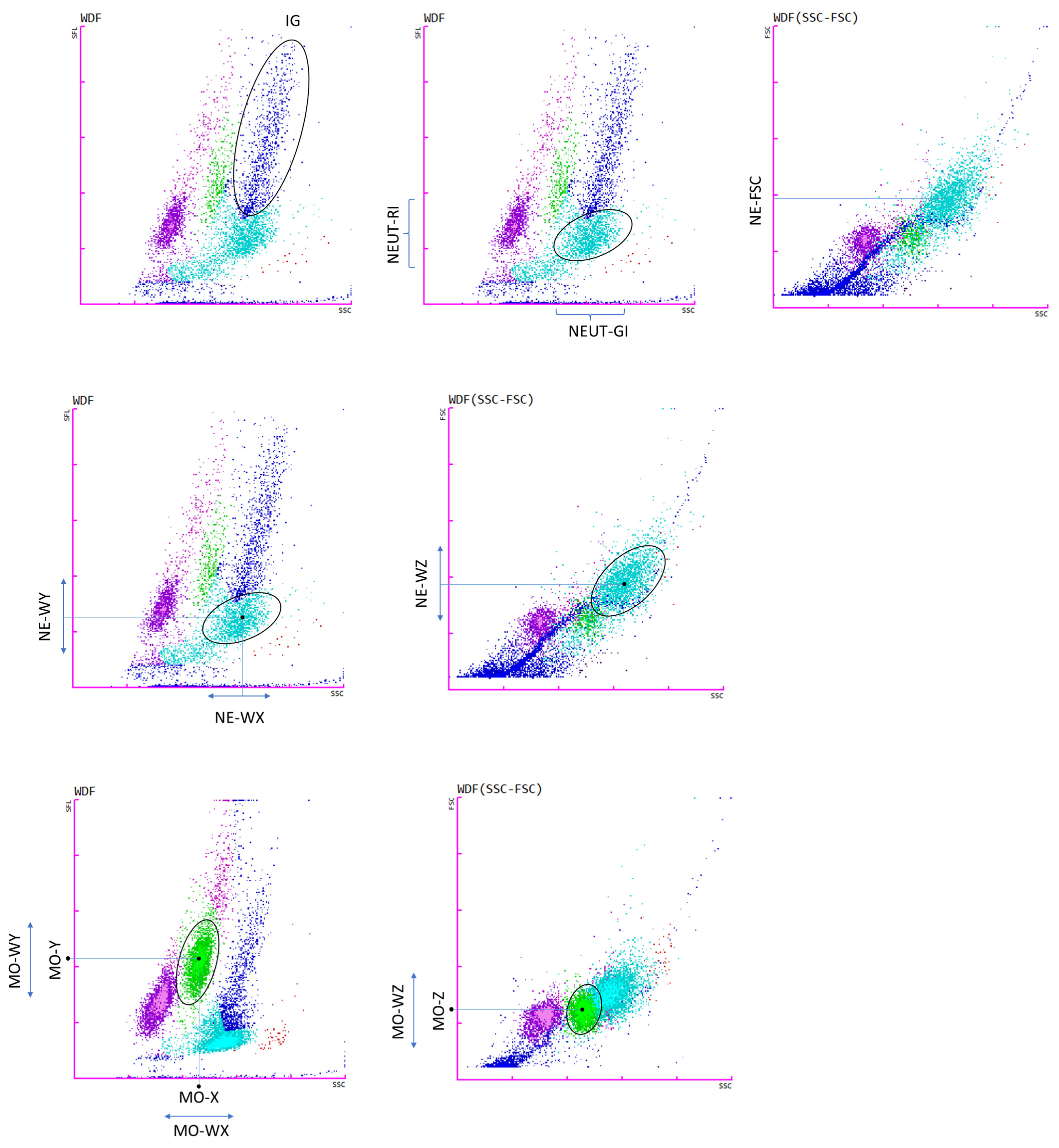
2.2. New Hematological Parameters Connected with Neutrophils and Monocytes
2.3. Statistical Analysis
3. Results
3.1. Cytogenetic Characteristics of Study Groups
3.2. Leukocytes Characteristic of MDS Patients with and without Cytogenetic Changes
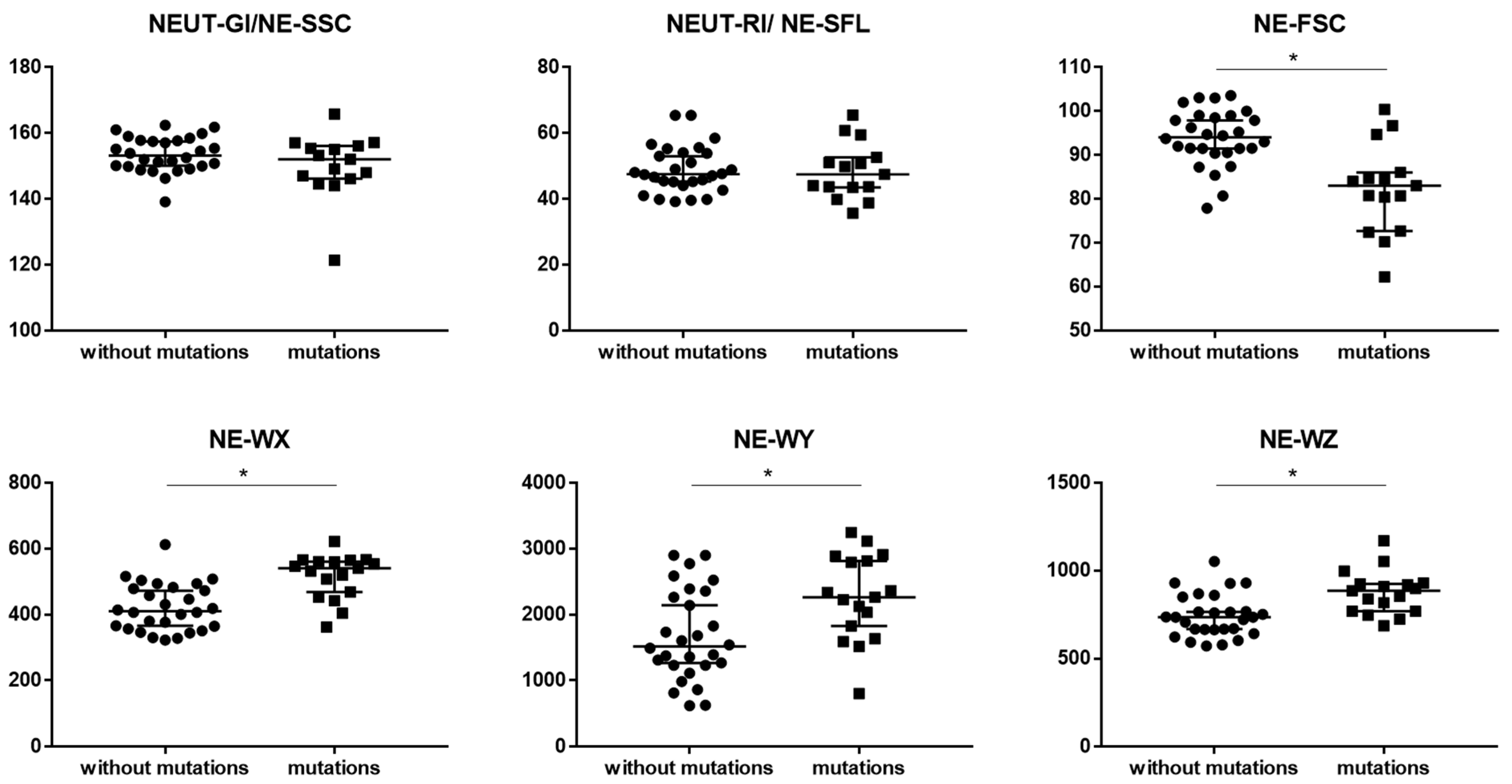
3.3. New Parameters Connected with Neutrophils
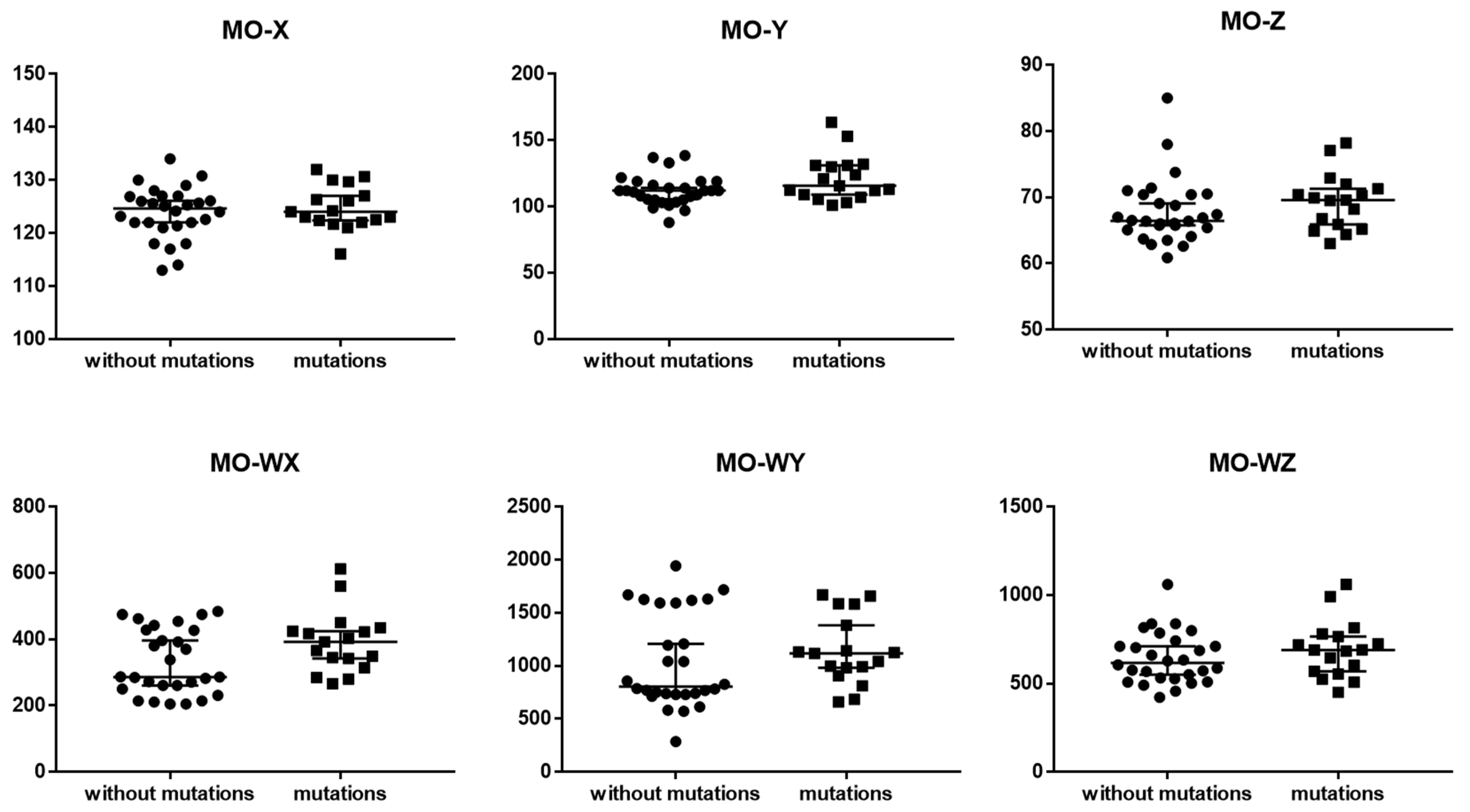
3.4. New Parameters Connected with Monocytes
4. Discussion
4.1. New Parameters Connected with Neutrophils
4.2. New Parameters Connected with Monocytes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| ID | Age | f/m | Chromosome Abnormalities | WBC [×103/µL] | RBC [×106/µL] | Hb [g/dL] | MCV [fL] | PLT [×103/µL] | Neutrophils [×103/µL] |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 63 | m | del(11q) | 4.84 | 3.26 | 12.3 | 110 | 62 | 2.09 |
| 2 | 72 | m | del(7q) and i(17q) | 2.75 | 2.37 | 7.3 | 93 | 45 | 0.44 |
| 3 | 70 | m | −7 | 3.82 | 4.18 | 14.0 | 97 | 129 | 1.86 |
| 4 | 72 | m | +19 and i(17q) | 8.24 | 3.23 | 11.2 | 102 | 194 | 5.69 |
| 5 | 82 | m | complex karyotype | 5.75 | 2.99 | 10.5 | 104 | 180 | 2.08 |
| 6 | 44 | m | del(11q) | 2.87 | 4.66 | 12.9 | 83 | 79 | 1.5 |
| 7 | 52 | f | t(3q) | 5.50 | 3.57 | 8.9 | 82 | 446 | 3.2 |
| 8 | 61 | m | complex karyotype | 6.87 | 3.07 | 10.7 | 102 | 232 | 4.9 |
| 9 | 81 | f | del(7q) and +8 | 3.48 | 2.31 | 7.1 | 90 | 53 | 1.67 |
| 10 | 75 | f | del(11q) | 8.06 | 3.39 | 10.4 | 89 | 245 | 5.12 |
| 11 | 64 | f | +8 | 1.95 | 2.86 | 10.1 | 106 | 291 | 0.46 |
| 12 | 57 | f | +8 | 5.80 | 2.45 | 9.0 | 107 | 411 | 2.9 |
| 13 | 64 | m | del(3q) | 7.87 | 2.99 | 9.5 | 102 | 273 | 5.42 |
| 14 | 88 | m | del(12p) | 6.00 | 2.23 | 7.1 | 95 | 186 | 2.16 |
| 15 | 75 | f | +19 and +8 | 6.80 | 2.68 | 8.6 | 99 | 13 | 4.35 |
| 16 | 89 | f | +8 | 4.43 | 2.74 | 8.1 | 93 | 60 | 1.72 |
| 17 | 92 | m | del(5q) and del(12p) | 4.32 | 2.54 | 8.6 | 100 | 280 | 2.14 |
References
- Tanaka, T.N.; Bejar, R. MDS overlap disorders and diagnostic boundaries. Blood 2019, 133, 1086–1095. [Google Scholar] [CrossRef] [PubMed]
- Tiu, R.V.; Visconte, V.; Traina, F.; Schwandt, A.; Maciejewski, J.P. Updates in cytogenetics and molecular markers in MDS. Curr. Hematol. Malig. Rep. 2011, 6, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Misawa, S.; Horiike, S. TP53 mutations in myelodysplastic syndrome. Leuk. Lymphoma 1996, 23, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.E.; Hou, H.A.; Tsai, C.H.; Wu, S.J.; Kuo, Y.Y.; Tseng, M.H.; Liu, M.C.; Liu, C.W.; Chou, W.C.; Chen, C.Y.; et al. Dynamics of DNMT3A mutation and prognostic relevance in patients with primary myelodysplastic syndrome. Clin. Epigenet. 2018, 10, 42. [Google Scholar] [CrossRef]
- Mangaonkar, A.A.; Gangat, N.; Al-Kali, A.; Elliott, M.A.; Begna, K.H.; Hanson, C.A.; Ketterling, R.P.; Wolanskyj-Spinner, A.P.; Hogan, W.J.; Litzow, M.R.; et al. Prognostic impact of ASXL1 mutations in patients with myelodysplastic syndromes and multilineage dysplasia with or without ring sideroblasts. Leuk. Res. 2018, 71, 60–62. [Google Scholar] [CrossRef]
- Zahid, M.F.; Malik, U.A.; Sohail, M.; Hassan, I.N.; Ali, S.; Shaukat, M.H.S. Cytogenetic Abnormalities in Myelodysplastic Syndromes: An Overview. Int. J. Hematol. Oncol. Stem Cell Res. 2017, 11, 231–239. [Google Scholar]
- Pitel, B.A.; Sharma, N.; Zepeda-Mendoza, C.; Smadbeck, J.B.; Pearce, K.E.; Cook, J.M.; Vasmatzis, G.; Sachs, Z.; Kanagal-Shamanna, R.; Viswanatha, D.; et al. Myeloid malignancies with 5q and 7q deletions are associated with extreme genomic complexity, biallelic TP53 variants, and very poor prognosis. Blood Cancer J. 2021, 11, 18. [Google Scholar] [CrossRef]
- Hosono, N. Genetic abnormalities and pathophysiology of MDS. Int. J. Clin. Oncol. 2019, 24, 885–892. [Google Scholar] [CrossRef]
- Yu, J.; Li, Y.; Li, T.; Li, Y.; Xing, H.; Sun, H.; Sun, L.; Wan, D.; Liu, Y.; Xie, X.; et al. Gene mutational analysis by NGS and its clinical significance in patients with myelodysplastic syndrome and acute myeloid leukemia. Exp. Hematol. Oncol. 2020, 9, 2. [Google Scholar] [CrossRef]
- Spaulding, T.P.; Stockton, S.S.; Savona, M.R. The evolving role of next generation sequencing in myelodysplastic syndromes. Br. J. Haematol 2020, 188, 224–239. [Google Scholar] [CrossRef]
- Sysmex, E.G. Novel Haematological Parameters for Rapidly Monitoring the Immune System Response; Norderstedt, Germany. Sysmex Eur. GmbH 2017, 27, 1–5. [Google Scholar]
- Schillinger, F.; Sourdeau, E.; Boubaya, M.; Baseggio, L.; Clauser, S.; Cornet, E.; Debord, C.; Defour, J.P.; Dubois, F.; Eveillard, M.; et al. A new approach for diagnosing chronic myelomonocytic leukemia using structural parameters of Sysmex XN(TM) analyzers in routine laboratory practice. Scand. J. Clin. Lab. Invest. 2018, 78, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Le Roux, G.; Vlad, A.; Eclache, V.; Malanquin, C.; Collon, J.F.; Gantier, M.; Schillinger, F.; Peltier, J.Y.; Savin, B.; Letestu, R.; et al. Routine diagnostic procedures of myelodysplastic syndromes: Value of a structural blood cell parameter (NEUT-X) determined by the Sysmex XE-2100. Int. J. Lab. Hematol. 2010, 32, e237–e243. [Google Scholar] [CrossRef] [PubMed]
- Kwiecien, I.; Rutkowska, E.; Kulik, K.; Klos, K.; Plewka, K.; Raniszewska, A.; Rzepecki, P.; Chcialowski, A. Neutrophil Maturation, Reactivity and Granularity Research Parameters to Characterize and Differentiate Convalescent Patients from Active SARS-CoV-2 Infection. Cells 2021, 10, 2332. [Google Scholar] [CrossRef]
- Buoro, S.; Manenti, B.; Seghezzi, M.; Dominoni, P.; Barbui, T.; Ghirardi, A.; Carobbio, A.; Marchesi, G.; Riva, I.; Nasi, A.; et al. Innovative haematological parameters for early diagnosis of sepsis in adult patients admitted in intensive care unit. J. Clin. Pathol. 2018, 71, 330–335. [Google Scholar] [CrossRef]
- Henriot, I.; Launay, E.; Boubaya, M.; Cremet, L.; Illiaquer, M.; Caillon, H.; Desjonqueres, A.; Gillet, B.; Bene, M.C.; Eveillard, M. New parameters on the hematology analyzer XN-10 (SysmexTM) allow to distinguish childhood bacterial and viral infections. Int. J. Lab. Hematol. 2017, 39, 14–20. [Google Scholar] [CrossRef]
- Zeeshan-Haider, R.; Urrechaga, E.; Uddin-Ujjan, I.; Sultan-Shamsi, T. Neutrophil Scattering Data Driven Pre-Microscopic Flagging of Acute Leukemic Cases. Rev. Investig. Clin. 2020, 72, 37–45. [Google Scholar] [CrossRef]
- Maenhout, T.M.; Marcelis, L. Immature granulocyte count in peripheral blood by the Sysmex haematology XN series compared to microscopic differentiation. J. Clin. Pathol. 2014, 67, 648–650. [Google Scholar] [CrossRef]
- Cornet, E.; Boubaya, M.; Troussard, X. Contribution of the new XN-1000 parameters NEUT-RI and NEUT-WY for managing patients with immature granulocytes. Int. J. Lab. Hematol. 2015, 37, e123–e126. [Google Scholar] [CrossRef]
- Di Luise, D.; Giannotta, J.A.; Ammirabile, M.; De Zordi, V.; Torricelli, S.; Bottalico, S.; Chiaretto, M.L.; Fattizzo, B.; Migliorini, A.C.; Ceriotti, F. Cell Population Data NE-WX, NE-FSC, LY-Y of Sysmex XN-9000 can provide additional information to differentiate macrocytic anaemia from myelodysplastic syndrome: A preliminary study. Int. J. Lab. Hematol. 2022, 44, e40–e43. [Google Scholar] [CrossRef]
- Swerdlow, S.H.; Campo, E.; Harris, N.L.; Jaffe, E.S. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, 4th ed.; International Agency for Research on Cancer (IARC) Publication: Lyon, France, 2017; pp. 97–120. [Google Scholar]
- Haase, D.; Germing, U.; Schanz, J.; Pfeilstocker, M.; Nosslinger, T.; Hildebrandt, B.; Kundgen, A.; Lubbert, M.; Kunzmann, R.; Giagounidis, A.A.; et al. New insights into the prognostic impact of the karyotype in MDS and correlation with subtypes: Evidence from a core dataset of 2124 patients. Blood 2007, 110, 4385–4395. [Google Scholar] [CrossRef] [PubMed]
- Rivers, A.; Slayton, W.B. Congenital cytopenias and bone marrow failure syndromes. Semin. Perinatol. 2009, 33, 20–28. [Google Scholar] [CrossRef]
- Lu, Q.; Li, Y.; Li, T.; Hou, T.; Zhao, Y.; Feng, S.; Yang, X.; Zhu, M.; Shen, Y. Evaluation of immature granulocyte parameters in myeloid neoplasms assayed by Sysmex XN hematology analyzer. J. Hematopathol. 2022, 15, 1–6. [Google Scholar] [CrossRef]
- Mishra, M.; Odyuo, B.; Webster, J. Immature granulocyte percentage in early chronic myeloid leukemia. J. Appl. Hematol. 2021, 12, 217–219. [Google Scholar] [CrossRef]
- Ansari-Lari, M.A.; Kickler, T.S.; Borowitz, M.J. Immature granulocyte measurement using the Sysmex XE-2100. Relationship to infection and sepsis. Am. J. Clin. Pathol. 2003, 120, 795–799. [Google Scholar] [CrossRef]
- Furundarena, J.R.; Araiz, M.; Uranga, M.; Sainz, M.R.; Agirre, A.; Trassorras, M.; Uresandi, N.; Montes, M.C.; Argoitia, N. The utility of the Sysmex XE-2100 analyzer’s NEUT-X and NEUT-Y parameters for detecting neutrophil dysplasia in myelodysplastic syndromes. Int. J. Lab. Hematol. 2010, 32, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Han, E.; Lee, H.K.; Kim, Y.; Han, K. Screening of myelodysplastic syndrome using cell population data obtained from an automatic hematology analyzer. Int. J. Lab. Hematol. 2021, 43, e54–e57. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Clauser, S.; Freynet, N.; Bardet, V. Automated Detection of Dysplasia: Data Mining from Our Hematology Analyzers. Diagnostics 2022, 12, 1556. [Google Scholar] [CrossRef]
- Geyer, J.T.; Verma, S.; Mathew, S.; Wang, Y.L.; Racchumi, J.; Espinal-Witter, R.; Subramaniyam, S.; Knowles, D.M.; Orazi, A. Bone marrow morphology predicts additional chromosomal abnormalities in patients with myelodysplastic syndrome with del(5q). Hum. Pathol. 2013, 44, 346–356. [Google Scholar] [CrossRef]
- Mangaonkar, A.A.; Lasho, T.L.; Finke, C.M.; Gangat, N.; Al-Kali, A.; Elliott, M.A.; Begna, K.H.; Alkhateeb, H.; Wolanskyj-Spinner, A.P.; Hanson, C.A.; et al. Prognostic interaction between bone marrow morphology and SF3B1 and ASXL1 mutations in myelodysplastic syndromes with ring sideroblasts. Blood Cancer J. 2018, 8, 18. [Google Scholar] [CrossRef]
- Pollyea, D.A.; Hedin, B.R.; O’Connor, B.P.; Alper, S. Monocyte function in patients with myelodysplastic syndrome. J. Leukoc. Biol. 2018, 104, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Chhabra, G.; Padhi, S.; Mohapatra, S.; Panigrahi, A.; Sable, M.N.; Das, P.K. Usefulness of Leucocyte Cell Population Data by Sysmex XN1000 Hematology Analyzer in Rapid Identification of Acute Leukemia. Indian J. Hematol. Blood Transfus. 2022, 38, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Urrechaga, E.; Boveda, O.; Aguirre, U. Improvement in detecting sepsis using leukocyte cell population data (CPD). Clin. Chem. Lab. Med. 2019, 57, 918–926. [Google Scholar] [CrossRef] [PubMed]

| Parameters | Parameters Description |
|---|---|
| IG (Immature Granulocytes) | The IG fraction includes promyelocytes, myelocytes and metamyelocytes (without blasts and band cells) |
| NE-SFL (NEUT-RI) (Neutrophil Reactivity Intensity) | Represents the mean value of fluorescence intensity and increases in proportion to the content of nucleic acids in the cell. This reflects metabolic activity. |
| NE-SSC (NEUT-GI) (Neutrophil Granularity Intensity) | Dependent on neutrophil complexity. Increases in the presence of cytoplasmic granulation or vacuoles. |
| NE-WX/MO-WX | Reflects the width of dispersion of cells population, with respect to cell side-scatter (SSC). |
| NE-WY/MO-WY | Represents the fluorescence distribution width of the cell population, with respect to cell fluorescence intensity (SFL). |
| NE-WZ/MO-WZ | Reflects the distribution width of the cell population, with respect to cell forward scatter (FSC). It is proportional to the width of dispersion of cell size. |
| NE-FSC | Neutrophil forward scatter mean intensity. Reflects neutrophil cell size. |
| MO-X MO-Y MO-Z | Depends on cell complexity, presence of granularity and vacuoles. Represents the mean value of fluorescence intensity. Indicates cell size, with respect to cell forward scatter (FSC). |
| All Study Grup | MDS Patients without Mutations | MDS Patients with Mutations | |
|---|---|---|---|
| n | 45 | 28 | 17 |
| Sex: f/m (n) | 23/22 | 16/12 | 7/10 |
| Age (mean ± SD years) | 66 ± 14 | ||
| Women (mean ± SD years) | 61 ± 14 | 67 ± 13 | 70 ± 13 |
| Men (mean ± SD years) | 59 ± 13 | 61 ± 16 | 71 ± 14 |
| Blood hematological parameters (mean ± SD years) | |||
| WBC [×103/µL] | 5.8 ± 4.3 | 6.1 ± 5.3 | 5.3 ± 1.9 |
| RBC [×106/µL] | 3.1 ± 4.3 | 3.2 ± 0.7 | 3.0 ± 0.7 |
| Hb [g/dL] | 10.0 ± 1.9 | 10.1 ± 1.9 | 9.8 ± 2.0 |
| MCV [fL] | 96.3 ± 8.8 | 95.6 ± 9.3 | 97.3 ± 8.1 |
| PLT [×103/µL] | 185 ± 124 | 184 ± 124 | 187 ± 128 |
| Neutrophils [×103/µL] | 3.3 ± 3.2 | 3.6 ± 3.9 | 2.8 ± 1.7 |
| MDS Patients without Mutations Median (Q1–Q3) | MDS Patients with Mutations Median (Q1–Q3) | * p < 0.05 Mann–Whitney (U Test) | |
|---|---|---|---|
| Neutrophils [103/µL] | 8.0 (5.1–13.0) | 19.3 (8.9–27.5) | * 0.016302 |
| Lymphocytes [103/µL] | 2.5 (1.9–3.5) | 4.3 (3.1–5.3) | * 0.006918 |
| Monocytes [103/µL] | 0.9 (0.4–1.6) | 3.3 (0.9–4.9) | * 0.005343 |
| Eosinophils [103/µL] | 0.3 (0.1–0.6) | 0.7 (0.2–1.1) | 0.066356 |
| Basophils [103/µL] | 0.2 (0.1–0.3) | 0.5 (0.2–0.9) | * 0.007126 |
| Immature Granulocytes [103/µL] | 2.3 (0.6–4.5) | 7.4 (2.9–9.7) | * 0.016302 |
| Neutrophils [%] | 65.3 (62.8–71.3) | 66.5 (62.2–73.9) | 0.561047 |
| Lymphocytes [%] | 20.9 (16.5–25.8) | 18.1 (11.3–20.7) | 0.158842 |
| Monocytes [%] | 7.1 (5.4–10.2) | 10.5 (7.0–14.2) | * 0.032669 |
| Eosinophils [%] | 1.8 (0.9–4.0) | 2.0 (1.1–2.4) | 0.920524 |
| Basophils [%] | 1.4 (1.0–1.9) | 1.7 (1.5–2.4) | 0.245649 |
| Immature Granulocytes [%] | 19.1 (8.8–21.9) | 23.1 (18.5–25.5) | 0.101854 |
| Blasts [%] | 1.2 (1.0–2.3) | 1.2 (1.0–1.7) | 0.793117 |
| MDS Patients without Mutations Median (Q1–Q3) | MDS Patients with Mutations Median (Q1–Q3) | * p < 0.05 Mann–Whitney (U Test) | |
|---|---|---|---|
| NEUT-GI (NE-SSC) | 153.2 (149.9–157.7) | 152.1 (146.2–156.2) | 0.174810 |
| NEUT-RI (NE-SFL) | 47.5 (44.6–54.0) | 47.5 (43.5–52.7) | 0.696185 |
| NE-FSC | 94.1 (91.0–98.7) | 83.0 (72.7–86.1) | * 0.000278 |
| NE-WX | 410.5 (361.0–481.0) | 541.0 (469.0–561.0) | * 0.000099 |
| NE-WY | 1517.0 (1230.0–2312.5) | 2266.0 (1828.0–2815.0) | * 0.008657 |
| NE-WZ | 736.0 (665.0–809.0) | 887.0 (769.0–925.0) | * 0.000988 |
| MO-X | 124.6 (121.7–126.9) | 124.0 (122.4–127.0) | 0.685158 |
| MO-Y | 112.0 (105.0–117.6) | 115.8 (109.0–131.2) | 0.085358 |
| MO-Z | 66.4 (65.2–70.4) | 69.6 (65.9–71.3) | 0.204324 |
| MO-WX | 286.5 (255.5–427.5) | 392.0 (342.0–424.0) | 0.089794 |
| MO-WY | 805.5 (734.5–1593) | 1118.0 (983.0–1383.0) | 0.269285 |
| MO-WZ | 617.0 (529.5–726.0) | 689.0 (569.0–765.0) | 0.371312 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwiecień, I.; Rutkowska, E.; Gawroński, K.; Kulik, K.; Dudzik, A.; Zakrzewska, A.; Raniszewska, A.; Sawicki, W.; Rzepecki, P. Usefulness of New Neutrophil-Related Hematologic Parameters in Patients with Myelodysplastic Syndrome. Cancers 2023, 15, 2488. https://doi.org/10.3390/cancers15092488
Kwiecień I, Rutkowska E, Gawroński K, Kulik K, Dudzik A, Zakrzewska A, Raniszewska A, Sawicki W, Rzepecki P. Usefulness of New Neutrophil-Related Hematologic Parameters in Patients with Myelodysplastic Syndrome. Cancers. 2023; 15(9):2488. https://doi.org/10.3390/cancers15092488
Chicago/Turabian StyleKwiecień, Iwona, Elżbieta Rutkowska, Krzysztof Gawroński, Katarzyna Kulik, Alicja Dudzik, Agata Zakrzewska, Agata Raniszewska, Waldemar Sawicki, and Piotr Rzepecki. 2023. "Usefulness of New Neutrophil-Related Hematologic Parameters in Patients with Myelodysplastic Syndrome" Cancers 15, no. 9: 2488. https://doi.org/10.3390/cancers15092488
APA StyleKwiecień, I., Rutkowska, E., Gawroński, K., Kulik, K., Dudzik, A., Zakrzewska, A., Raniszewska, A., Sawicki, W., & Rzepecki, P. (2023). Usefulness of New Neutrophil-Related Hematologic Parameters in Patients with Myelodysplastic Syndrome. Cancers, 15(9), 2488. https://doi.org/10.3390/cancers15092488







