A Nomogram Incorporating Neutrophil-to-Lymphocyte Ratio and Squamous Cell Carcinoma Antigen Predicts the Prognosis of Oral Cancers
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Collection of Study Data
2.3. Measurement of Serum Indices
2.4. Follow-Up and Study Endpoints
2.5. Statistical Analysis
3. Results
3.1. Characteristics of Included Patients
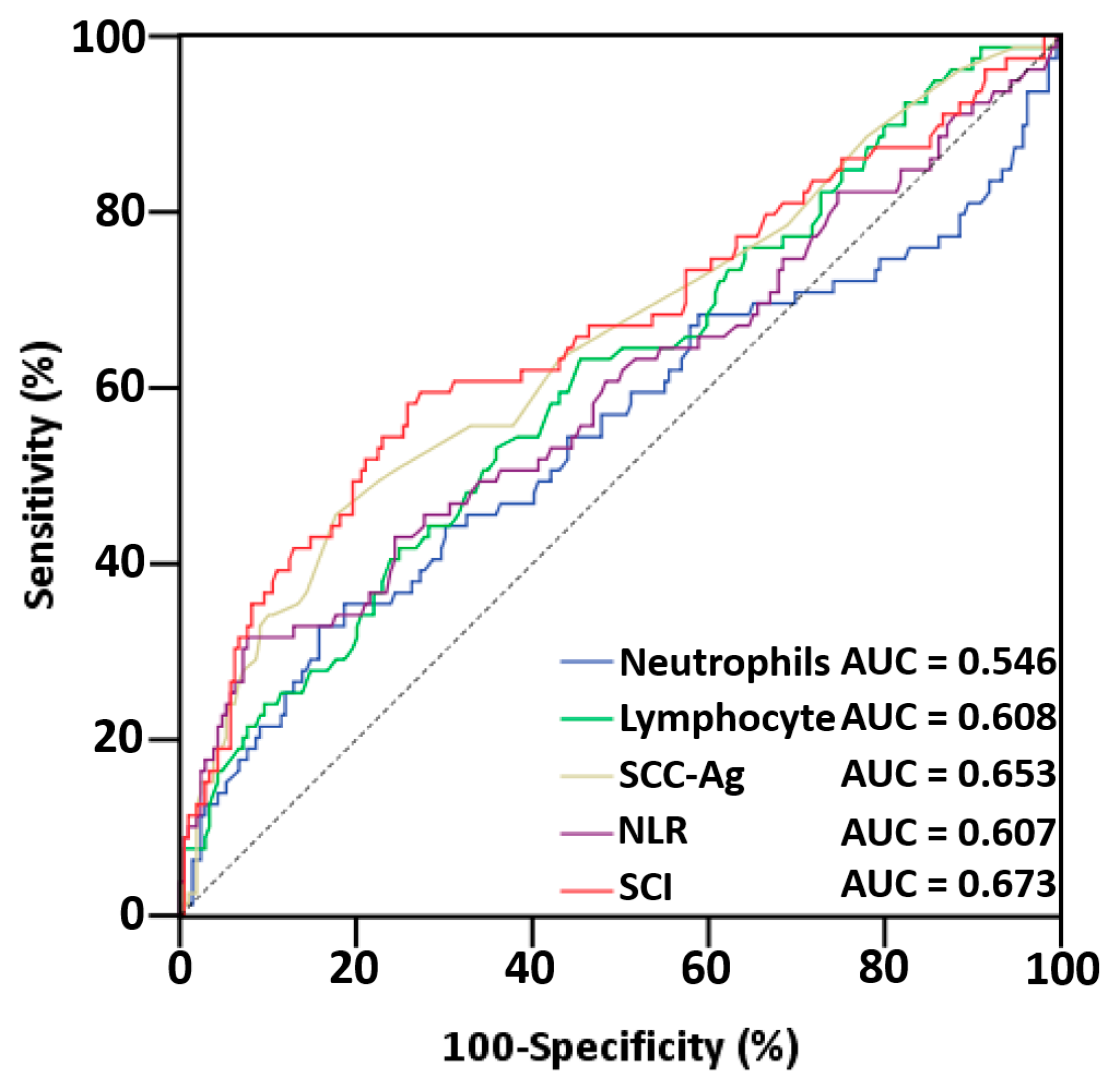
3.2. Analysis of ROC Curves and AUCs of Serum Indices
3.3. Associations between SCI and Clinicopathological Features
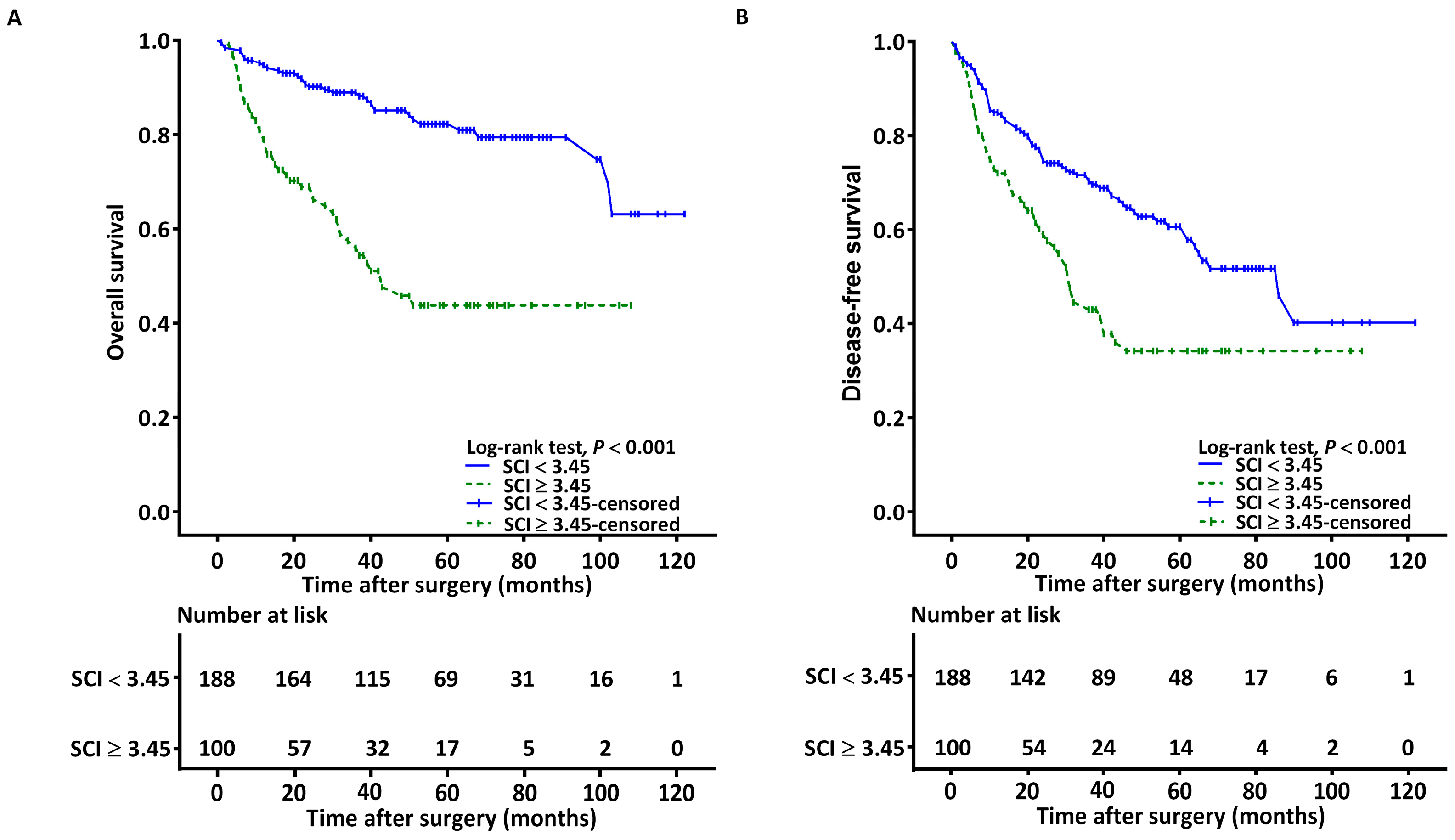
3.4. Associations between SCI and OS
3.5. Associations between SCI and DFS
3.6. Stratified Analysis
3.7. Predictive Nomogram Construction
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kao, S.Y.; Lim, E. An Overview of Detection and Screening of Oral Cancer in Taiwan. Chin. J. Dent. Res. 2015, 18, 7–12. [Google Scholar] [PubMed]
- Ettinger, K.S.; Ganry, L.; Fernandes, R.P. Oral Cavity Cancer. Oral Maxillofac. Surg. Clin. N. Am. 2019, 31, 13–29. [Google Scholar] [CrossRef] [PubMed]
- Warnakulasuriya, S. Living with Oral Cancer: Epidemiology with Particular Reference to Prevalence and Life-Style Changes That Influence Survival. Oral Oncol. 2010, 46, 407–410. [Google Scholar] [CrossRef] [PubMed]
- Jardim, J.F.; Francisco, A.L.; Gondak, R.; Damascena, A.; Kowalski, L.P. Prognostic Impact of Perineural Invasion and Lymphovascular Invasion in Advanced Stage Oral Squamous Cell Carcinoma. Int. J. Oral Maxillofac. Surg. 2015, 44, 23–28. [Google Scholar] [CrossRef] [PubMed]
- den Toom, I.J.; Janssen, L.M.; van Es, R.J.J.; Karagozoglu, K.H.; de Keizer, B.; van Weert, S.; Willems, S.M.; Bloemena, E.; Leemans, C.R.; de Bree, R. Depth of Invasion in Patients with Early Stage Oral Cancer Staged by Sentinel Node Biopsy. Head Neck 2019, 41, 2100–2106. [Google Scholar] [CrossRef] [PubMed]
- Varsha, B.K.; Radhika, M.B.; Makarla, S.; Kuriakose, M.A.; Kiran, G.S.; Padmalatha, G.V. Perineural Invasion in Oral Squamous Cell Carcinoma: Case Series and Review of Literature. J. Oral Maxillofac. Pathol. 2015, 19, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Wreesmann, V.B.; Katabi, N.; Palmer, F.L.; Montero, P.H.; Migliacci, J.C.; Gonen, M.; Carlson, D.; Ganly, I.; Shah, J.P.; Ghossein, R.; et al. Influence of Extracapsular Nodal Spread Extent on Prognosis of Oral Squamous Cell Carcinoma. Head Neck 2016, 38 (Suppl. S1), E1192–E1199. [Google Scholar] [CrossRef]
- Kato, H.; Torigoe, T. Radioimmunoassay for Tumor Antigen of Human Cervical Squamous Cell Carcinoma. Cancer 1977, 40, 1621–1628. [Google Scholar] [CrossRef]
- Chen, I.H.; Liao, C.T.; Wang, H.M.; Huang, J.J.; Kang, C.J.; Huang, S.F. Using Scc Antigen and Crp Levels as Prognostic Biomarkers in Recurrent Oral Cavity Squamous Cell Carcinoma. PLoS ONE 2014, 9, e103265. [Google Scholar] [CrossRef]
- Yin, N.; Liu, W. Clinical Value of Tumor Marker Index Based on Preoperative Cyfra 21-1 and Scc-Ag in the Evaluation of Prognosis and Treatment Effectiveness in Patients with Esophageal Squamous Cell Carcinoma. Onco Targets Ther. 2020, 13, 4135–4143. [Google Scholar] [CrossRef]
- Pectasides, D.; Bafaloucos, D.; Antoniou, F.; Gogou, L.; Economides, N.; Varthalitis, J.; Dimitriades, M.; Kosmidis, P.; Athanassiou, A. Tpa, Tati, Cea, Afp, Beta-Hcg, Psa, Scc, and Ca 19-9 for Monitoring Transitional Cell Carcinoma of the Bladder. Am. J. Clin. Oncol. 1996, 19, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Henkenberens, C.; Toklu, H.; Tamme, C.; Bruns, F. Clinical Value of Squamous Cell Carcinoma Antigen (Sccag) in Anal Cancer—A Single-Center Retrospective Analysis. Anticancer Res. 2016, 36, 3173–3177. [Google Scholar] [PubMed]
- Vassilakopoulos, T.; Troupis, T.; Sotiropoulou, C.; Zacharatos, P.; Katsaounou, P.; Parthenis, D.; Noussia, O.; Troupis, G.; Papiris, S.; Kittas, C.; et al. Diagnostic and Prognostic Significance of Squamous Cell Carcinoma Antigen in Non-Small Cell Lung Cancer. Lung Cancer 2001, 32, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Wang, W.; Wang, Y.; Liu, C.; Wang, P. The Role of Squamous Cell Carcinoma Antigen (Scc Ag) in Outcome Prediction after Concurrent Chemoradiotherapy and Treatment Decisions for Patients with Cervical Cancer. Radiat. Oncol. 2019, 14, 146. [Google Scholar] [CrossRef]
- Dante, D.E.P.; Young, C.K.; Chien, H.T.; Tsao, C.K.; Fok, C.C.; Fan, K.H.; Liao, C.T.; Wang, H.M.; Kang, C.J.; Chang, J.T.; et al. Prognostic Roles of Scc Antigen, Crp and Cyfra 21-1 in Oral Cavity Squamous Cell Carcinoma. Anticancer Res. 2019, 39, 2025–2033. [Google Scholar]
- Huang, S.F.; Wei, F.C.; Liao, C.T.; Wang, H.M.; Lin, C.Y.; Lo, S.; Huang, J.J.; Chen, I.H.; Kang, C.J.; Chien, H.T.; et al. Risk Stratification in Oral Cavity Squamous Cell Carcinoma by Preoperative Crp and Scc Antigen Levels. Ann. Surg. Oncol. 2012, 19, 3856–3864. [Google Scholar] [CrossRef]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef]
- Colotta, F.; Allavena, P.; Sica, A.; Garlanda, C.; Mantovani, A. Cancer-Related Inflammation, the Seventh Hallmark of Cancer: Links to Genetic Instability. Carcinogenesis 2009, 30, 1073–1081. [Google Scholar] [CrossRef]
- Brenner, D.R.; Scherer, D.; Muir, K.; Schildkraut, J.; Boffetta, P.; Spitz, M.R.; Le Marchand, L.; Chan, A.T.; Goode, E.L.; Ulrich, C.M.; et al. A Review of the Application of Inflammatory Biomarkers in Epidemiologic Cancer Research. Cancer Epidemiol. Biomarks Prev. 2014, 23, 1729–1751. [Google Scholar] [CrossRef]
- Nakashima, H.; Matsuoka, Y.; Yoshida, R.; Nagata, M.; Hirosue, A.; Kawahara, K.; Sakata, J.; Arita, H.; Hiraki, A.; Nakayama, H. Pre-Treatment Neutrophil to Lymphocyte Ratio Predicts the Chemoradiotherapy Outcome and Survival in Patients with Oral Squamous Cell Carcinoma: A Retrospective Study. BMC Cancer 2016, 16, 41. [Google Scholar] [CrossRef]
- Lin, C.Y.; Fan, K.H.; Lee, L.Y.; Hsueh, C.; Yang, L.Y.; Ng, S.H.; Wang, H.M.; Hsieh, C.H.; Lin, C.H.; Tsao, C.K.; et al. Precision Adjuvant Therapy Based on Detailed Pathologic Risk Factors for Resected Oral Cavity Squamous Cell Carcinoma: Long-Term Outcome Comparison of Cgmh and Nccn Guidelines. Int. J. Radiat. Oncol. Biol. Phys. 2020, 106, 916–925. [Google Scholar] [CrossRef] [PubMed]
- Brusselaers, N.; Lagergren, J. The Charlson Comorbidity Index in Registry-Based Research. Methods Inf. Med. 2017, 56, 401–406. [Google Scholar] [PubMed]
- Ko, C.A.; Fang, K.H.; Hsu, C.M.; Lee, Y.C.; Chang, G.H.; Huang, E.I.; Tsai, M.S.; Tsai, Y.T. The Preoperative C-Reactive Protein-Lymphocyte Ratio and the Prognosis of Oral Cavity Squamous Cell Carcinoma. Head Neck 2021, 43, 2740–2754. [Google Scholar] [CrossRef] [PubMed]
- Harrell, F.E., Jr.; Lee, K.L.; Mark, D.B. Multivariable Prognostic Models: Issues in Developing Models, Evaluating Assumptions and Adequacy, and Measuring and Reducing Errors. Stat. Med. 1996, 15, 361–387. [Google Scholar] [CrossRef]
- Su, Y.L.; Tsai, K.L.; Chiu, T.J.; Lin, Y.M.; Lee, K.C.; Lu, C.C.; Chen, H.H.; Wu, C.C.; Hsu, H.C. Development and Validation of a Novel Serum Prognostic Marker for Patients with Metastatic Colorectal Cancer on Regorafenib Treatment. Cancers 2021, 13, 5080. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jia, J.; Lin, L.; Guo, J.; Ye, X.; Zheng, X.; Chen, Y. Predictive Value of Hematological Markers of Systemic Inflammation for Managing Cervical Cancer. Oncotarget 2017, 8, 44824–44832. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H. Squamous Cell Carcinoma Antigen: Clinical Application and Research Status. Diagnostics 2022, 12, 1065. [Google Scholar] [CrossRef]
- Yasumatsu, R.; Nakashima, T.; Azuma, K.; Hirakawa, N.; Kuratomi, Y.; Tomita, K.; Cataltepe, S.; Silverman, G.A.; Clayman, G.L.; Komiyama, S. Scca1 Expression in T-Lymphocytes Peripheral to Cancer Cells Is Associated with the Elevation of Serum Scc Antigen in Squamous Cell Carcinoma of the Tongue. Cancer Lett. 2001, 167, 205–213. [Google Scholar] [CrossRef]
- Lin, W.H.; Chen, I.H.; Wei, F.C.; Huang, J.J.; Kang, C.J.; Hsieh, L.L.; Wang, H.M.; Huang, S.F. Clinical Significance of Preoperative Squamous Cell Carcinoma Antigen in Oral-Cavity Squamous Cell Carcinoma. Laryngoscope 2011, 121, 971–977. [Google Scholar] [CrossRef]
- Takeda, A.; Kajiya, A.; Iwasawa, A.; Nakamura, Y.; Hibino, T. Aberrant Expression of Serpin Squamous Cell Carcinoma Antigen 2 in Human Tumor Tissues and Cell Lines: Evidence of Protection from Tumor Necrosis Factor-Mediated Apoptosis. Biol. Chem. 2002, 383, 1231–1236. [Google Scholar] [CrossRef]
- Lee, S.; Kim, D.W.; Kwon, S.; Kim, H.J.; Cha, I.H.; Nam, W. Prognostic Value of Systemic Inflammatory Markers for Oral Cancer Patients Based on the 8th Edition of Ajcc Staging System. Sci. Rep. 2020, 10, 12111. [Google Scholar] [CrossRef]
- Yasumatsu, R.; Wakasaki, T.; Hashimoto, K.; Nakashima, K.; Manako, T.; Taura, M.; Matsuo, M.; Nakagawa, T. Monitoring the Neutrophil-to-Lymphocyte Ratio May Be Useful for Predicting the Anticancer Effect of Nivolumab in Recurrent or Metastatic Head and Neck Cancer. Head Neck 2019, 41, 2610–2618. [Google Scholar] [CrossRef] [PubMed]
- Sueoka, K.; Nawata, S.; Nakagawa, T.; Murakami, A.; Takeda, O.; Suminami, Y.; Kato, H.; Sugino, N. Tumor-Associated Serpin, Squamous Cell Carcinoma Antigen Stimulates Matrix Metalloproteinase-9 Production in Cervical Squamous Cell Carcinoma Cell Lines. Int. J. Oncol. 2005, 27, 1345–1353. [Google Scholar] [CrossRef] [PubMed]
- Moeckelmann, N.; Ebrahimi, A.; Tou, Y.K.; Gupta, R.; Low, T.H.; Ashford, B.; Ch’ng, S.; Palme, C.E.; Clark, J.R. Prognostic Implications of the 8th Edition American Joint Committee on Cancer (Ajcc) Staging System in Oral Cavity Squamous Cell Carcinoma. Oral Oncol. 2018, 85, 82–86. [Google Scholar] [CrossRef]
- Balachandran, V.P.; Gonen, M.; Smith, J.J.; DeMatteo, R.P. Nomograms in Oncology: More Than Meets the Eye. Lancet Oncol. 2015, 16, e173–e180. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | |
|---|---|
| Age (years) | |
| ≥65 | 98 (34.0%) |
| <65 | 190 (66.0%) |
| Sex | |
| Women | 26 (9.0%) |
| Men | 262 (91.0%) |
| Tumor location | |
| Tongue | 110 (38.2%) |
| Buccal | 96 (33.4%) |
| Gingiva | 41 (14.2%) |
| Retromolar trigone | 15 (5.2%) |
| Mouth floor | 11 (3.8%) |
| Lip | 10 (3.5%) |
| Hard palate | 5 (1.7%) |
| AJCC stage | |
| I | 61 (21.2%) |
| II | 39 (13.5%) |
| III | 40 (13.9%) |
| IV | 148 (51.4%) |
| T status | |
| T1 | 79 (27.5%) |
| T2 | 53 (18.4%) |
| T3 | 39 (13.5%) |
| T4 | 117 (40.6%) |
| N status | |
| N0 | 186 (64.6%) |
| N1 | 27 (9.4%) |
| N2 | 62 (21.5%) |
| N3 | 13 (4.5%) |
| PNI | 72 (25.0%) |
| ENE | 58 (20.1%) |
| LVI | 20 (6.9%) |
| Tumor differentiation | |
| W-D and M-D | 253 (87.8%) |
| P-D | 35 (12.2%) |
| Closest margin | |
| ≥5 mm | 210 (72.9%) |
| <5 mm | 78 (27.1%) |
| DOI ≥ 10 mm | 133 (46.2%) |
| Treatment modality | |
| Surgery only | 136 (47.3%) |
| Surgery + RT | 39 (13.5%) |
| Surgery + CRT | 113 (39.2%) |
| CCI | |
| 0 | 157 (54.5%) |
| 1 | 83 (28.8%) |
| ≥2 | 48 (16.7%) |
| Personal Habits | |
| Smoking | 241 (83.7%) |
| Alcohol drinking | 191 (66.3%) |
| Areca nut chewing | 233 (80.9%) |
| SCC-Ag (ng/mL), median (IQR) | 1.00 (0.70–1.70) |
| Neutrophil (×103/μL), median (IQR) | 5.01 (3.61–6.42) |
| Lymphocyte (×103/μL), median (IQR) | 2.01 (1.60–2.59) |
| SCI, median (IQR) | 2.45 (1.42–4.76) |
| Index | AUC | 95% CI | p | p a |
|---|---|---|---|---|
| Neutrophil | 0.546 | (0.464–0.628) | 0.228 | <0.001 |
| Lymphocyte | 0.608 | (0.534–0.682) | 0.005 | <0.001 |
| SCC-Ag | 0.653 | (0.578–0.728) | <0.001 | <0.001 |
| NLR | 0.607 | (0.528–0.687) | 0.005 | <0.001 |
| SCI | 0.673 | (0.593–0.749) | <0.001 | - |
| Variable | Number of Patients | ||
|---|---|---|---|
| SCI < 3.45, n = 188 | SCI ≥ 3.45, n =100 | p | |
| Sex | 0.191 a | ||
| Women | 20 (10.6%) | 6 (6.0%) | |
| Men | 168 (89.4%) | 94 (94.0%) | |
| Age | 0.194 a | ||
| <65 | 129 (68.6%) | 61 (61.0%) | |
| ≥65 | 59 (31.4%) | 39 (39.0%) | |
| AJCC stage | <0.001 a | ||
| I–II | 91 (48.4%) | 9 (9.0%) | |
| III–IV | 97 (51.6%) | 91 (91.0%) | |
| T status | <0.001 a | ||
| T1–T2 | 113 (60.1%) | 19 (19.0%) | |
| T3–T4 | 75 (39.9%) | 81 (81.0%) | |
| N status | |||
| N0 | 138 (73.4%) | 48 (48.0%) | <0.001 a |
| N1–N3 | 50 (26.6%) | 52 (52.0%) | |
| PNI | |||
| Absent | 152 (80.9%) | 64 (64.0%) | 0.002 a |
| Present | 36 (19.1%) | 36 (36.0%) | |
| LVI | |||
| Absent | 183 (97.3%) | 85 (85.0%) | <0.001 a |
| Present | 5 (2.7%) | 15 (15.0%) | |
| ENE | <0.001 a | ||
| Absent | 165 (87.8%) | 65 (65.0%) | |
| Present | 23 (12.2%) | 35 (35.0%) | |
| Tumor differentiation | 0.145 a | ||
| W-D/M-D | 169 (89.9%) | 84 (84.0%) | |
| P-D | 19 (10.1%) | 16 (16.0%) | |
| Closest margin | 0.562 a | ||
| ≥5 mm | 135 (71.8%) | 75 (75.0%) | |
| <5 mm | 53 (28.2%) | 25 (25.0%) | |
| DOI ≥ 10 mm | <0.001 a | ||
| No | 129 (68.6%) | 26 (26.0%) | |
| Yes | 59 (31.4%) | 74 (74.0%) | |
| Tumor location | 0.215 a | ||
| Tongue | 76 (40.4%) | 34 (34.0%) | |
| Buccal mucosa | 56 (29.8%) | 40 (40.0%) | |
| Other | 56 (29.8%) | 26 (26.0%) | |
| Personal habits | 0.808 a | ||
| No exposure | 20 (10.6%) | 13 (13.0%) | |
| One exposure | 10 (5.3%) | 6 (6.0%) | |
| Two or all exposure | 158 (84.0%) | 81 (81.0%) | |
| Treatment modality | <0.001 a | ||
| Surgery | 111 (59.0%) | 25 (25.0%) | |
| Surgery + RT | 26 (13.8%) | 13 (13.0%) | |
| Surgery + CRT | 51 (27.1%) | 62 (62.0%) | |
| CCI | 0.029 a | ||
| 0 | 111 (59.0%) | 46 (46.0%) | |
| 1 | 53 (28.2%) | 30 (30.0%) | |
| ≥2 | 24 (12.8%) | 24 (24.0%) | |
| Survival in months, median (IQR) | 49.00 (30.25–70.75) | 25.00 (13.00–48.00) | <0.001 b |
| Variable | Univariable Analysis (OS) | Univariable Analysis (DFS) | ||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Sex | ||||
| Women | Reference | Reference | ||
| Men | 1.584 (0.640–3.921) | 0.320 | 1.316 (0.640–3.921) | 0.320 |
| Age (years) | ||||
| <65 | Reference | Reference | ||
| ≥65 | 0.760 (0.468–1.236) | 0.269 | 0.684 (0.465–1.006) | 0.053 |
| AJCC stage | ||||
| I | Reference | Reference | ||
| II | 1.287 (0.392–4.219) | 0.677 | 0.649 (0.305–1.379) | 0.261 |
| III | 1.010 (0.284–3.585) | 0.988 | 0.938 (0.469–1.876) | 0.857 |
| IV | 5.831 (2.250–13.493) | <0.001 | 2.120 (1.313–3.422) | 0.002 |
| Presence of PNI | ||||
| No | Reference | Reference | ||
| Yes | 2.294 (1.453–3.622) | <0.001 | 1.336 (0.905–1.973) | 0.144 |
| Presence of LVI | ||||
| No | Reference | Reference | ||
| Yes | 3.707 (1.943–7.071) | <0.001 | 1.882 (1.010–3.505) | 0.046 |
| Tumor differentiation | ||||
| W-D/M-D | Reference | Reference | ||
| P-D | 3.260 (1.935–5.492) | <0.001 | 2.224 (1.410–3.508) | 0.001 |
| Treatment modality | ||||
| Surgery | Reference | Reference | ||
| Surgery + RT | 1.400 (0.625–3.135) | 0.414 | 0.854 (0.466–1.565) | 0.609 |
| Surgery + CRT | 3.300 (2.004–5.434) | <0.001 | 1.633 (1.131–2.357) | 0.009 |
| Tumor location | ||||
| Tongue | Reference | Reference | ||
| Buccal mucosa | 1.319 (0.776–2.240) | 0.306 | 0.854 (0.466–1.565) | 0.609 |
| Other sites | 1.171 (0.672–2.040) | 0.577 | 1.633 (0.531–2.357) | 0.439 |
| Closest margin | ||||
| ≥ 5 mm | Reference | Reference | ||
| < 5 mm | 1.325 (0.826–2.124) | 0.243 | 1.278 (0.881–1.854) | 0.196 |
| Personal habits | ||||
| No exposure | Reference | Reference | ||
| One exposure | 2.213 (0.713–6.867) | 0.169 | 2.220 (0.855–5.764) | 0.101 |
| Two or more exposure | 1.540 (0.668–3.551) | 0.311 | 1.919 (0.972–3.791) | 0.060 |
| CCI | ||||
| 0 | Reference | Reference | ||
| 1 | 1.057 (0.615–1.817) | 0.841 | 0.682 (0.441–1.053) | 0.084 |
| ≥2 | 1.916 (1.121–3.273) | 0.017 | 1.058 (0.672–1.667) | 0.808 |
| SCC-Ag | ||||
| <1.65 | Reference | Reference | ||
| ≥1.65 | 3.521 (2.250–5.512) | <0.001 | 2.006 (1.390–2.893) | <0.001 |
| SCI | ||||
| <3.45 | Reference | Reference | ||
| ≥3.45 | 3.803 (2.419–5.980) | <0.001 | 1.900 (1.335–2.703) | <0.001 |
| NLR | ||||
| <4.51 | Reference | Reference | ||
| ≥4.51 | 4.465 (2.762–7.219) | <0.001 | 2.710 (1.783–4.118) | <0.001 |
| Variable | SCC-Ag and NLR Model | SCI Model | ||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Overall Survival | ||||
| AJCC stage | ||||
| I | Reference | Reference | ||
| II | ||||
| III | ||||
| IV | 3.702 (2.028–6.758) | <0.001 | 3.935 (2.148–7.209) | <0.001 |
| Tumor differentiation | ||||
| W-D/M-D | Reference | Reference | ||
| P-D | 2.976 (1.738–5.095) | <0.001 | 2.855 (1.677–4.861) | <0.001 |
| CCI | ||||
| 0 | Reference | Reference | ||
| 1 | ||||
| ≥2 | 1.265 (1.147–1.689) | 0.021 | 1.309 (1.183–1.744) | 0.016 |
| SCC-Ag | ||||
| <1.65 | Reference | |||
| ≥1.65 | 1.905 (1.164–3.118) | 0.011 | ||
| SCI | ||||
| <3.45 | Reference | |||
| ≥3.45 | 2.378 (1.356–3.735) | 0.002 | ||
| NLR | ||||
| <4.51 | Reference | |||
| ≥4.51 | 2.611 (1.580–4.315) | <0.001 | ||
| Disease-free survival | ||||
| AJCC stage | ||||
| I | Reference | Reference | ||
| II | ||||
| III | ||||
| IV | 2.326 (1.436–3.768) | <0.001 | 1.961 (1.320–2.913) | <0.001 |
| Tumor differentiation | ||||
| W-D/M-D | Reference | Reference | ||
| P-D | 2.238 (1.403–3.570) | <0.001 | 2.061 (1.302–3.262) | 0.002 |
| SCC-Ag | ||||
| <1.65 | Reference | |||
| ≥1.65 | 1.546 (1.041–2.295) | 0.031 | ||
| SCI | ||||
| <3.45 | Reference | |||
| ≥3.45 | 2.219 (1.437–3.425) | <0.001 | ||
| NLR | ||||
| <4.51 | Reference | |||
| ≥4.51 | 2.051 (1.361–2.867) | 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, Y.-T.; Lai, C.-H.; Chang, G.-H.; Hsu, C.-M.; Tsai, M.-S.; Liao, C.-T.; Kang, C.-J.; Tsai, Y.-H.; Lee, Y.-C.; Huang, E.I.; et al. A Nomogram Incorporating Neutrophil-to-Lymphocyte Ratio and Squamous Cell Carcinoma Antigen Predicts the Prognosis of Oral Cancers. Cancers 2023, 15, 2492. https://doi.org/10.3390/cancers15092492
Tsai Y-T, Lai C-H, Chang G-H, Hsu C-M, Tsai M-S, Liao C-T, Kang C-J, Tsai Y-H, Lee Y-C, Huang EI, et al. A Nomogram Incorporating Neutrophil-to-Lymphocyte Ratio and Squamous Cell Carcinoma Antigen Predicts the Prognosis of Oral Cancers. Cancers. 2023; 15(9):2492. https://doi.org/10.3390/cancers15092492
Chicago/Turabian StyleTsai, Yao-Te, Chia-Hsuan Lai, Geng-He Chang, Cheng-Ming Hsu, Ming-Shao Tsai, Chun-Ta Liao, Chung-Jan Kang, Yuan-Hsiung Tsai, Yi-Chan Lee, Ethan I. Huang, and et al. 2023. "A Nomogram Incorporating Neutrophil-to-Lymphocyte Ratio and Squamous Cell Carcinoma Antigen Predicts the Prognosis of Oral Cancers" Cancers 15, no. 9: 2492. https://doi.org/10.3390/cancers15092492
APA StyleTsai, Y.-T., Lai, C.-H., Chang, G.-H., Hsu, C.-M., Tsai, M.-S., Liao, C.-T., Kang, C.-J., Tsai, Y.-H., Lee, Y.-C., Huang, E. I., Tsai, M.-H., & Fang, K.-H. (2023). A Nomogram Incorporating Neutrophil-to-Lymphocyte Ratio and Squamous Cell Carcinoma Antigen Predicts the Prognosis of Oral Cancers. Cancers, 15(9), 2492. https://doi.org/10.3390/cancers15092492








