Demographics and Clinicopathologic Profile of Pulmonary Sarcomatoid Carcinoma with Survival Analysis and Genomic Landscape
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Demographic Characteristics and Tumor Characteristics: Grading, Staging, and Size
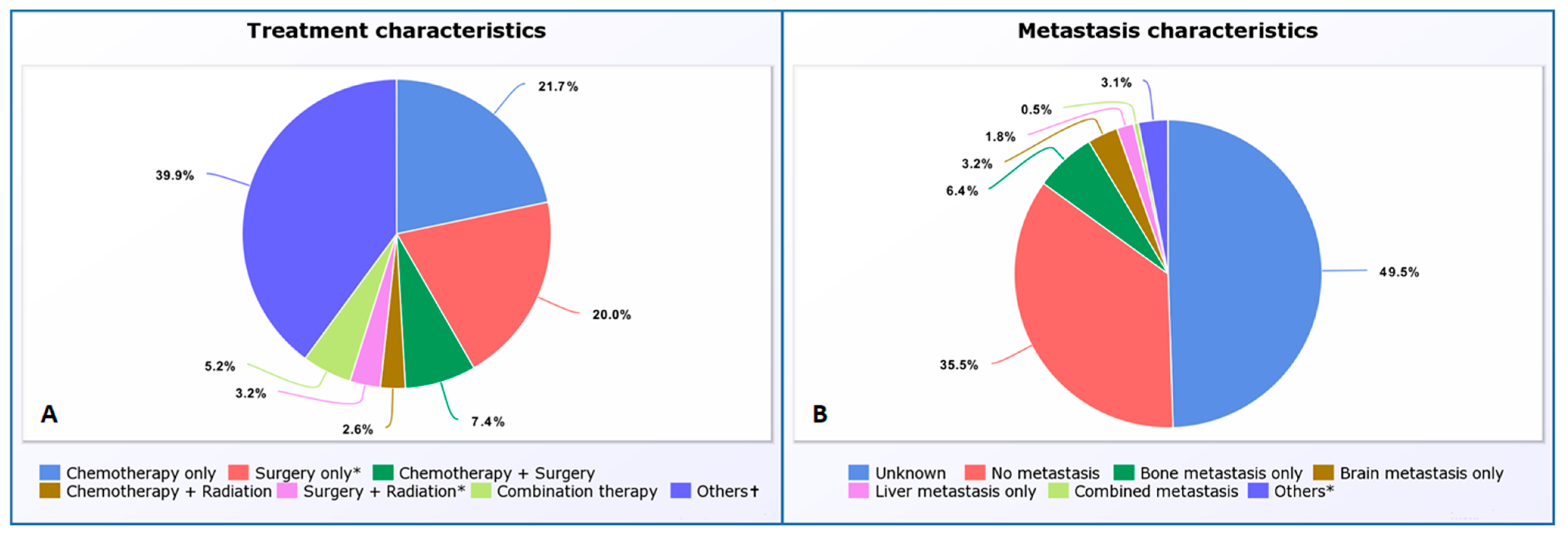
3.2. Metastatic and Lymph Node Status at Diagnosis and PSC Treatment Characteristics
4. Outcomes and Survival Analysis
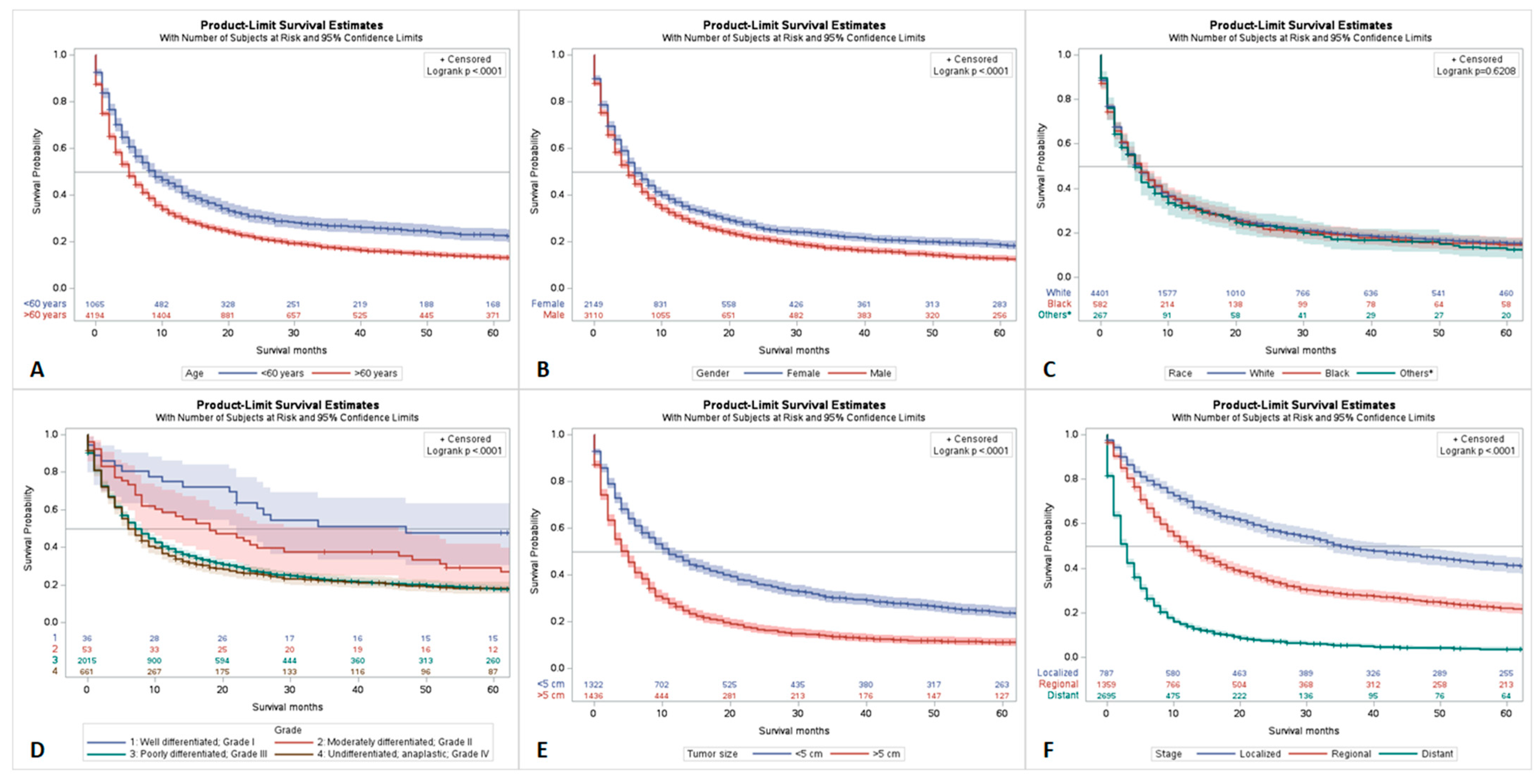
4.1. Survival Analysis by Age, Race, Gender, Tumor Grade, Stage, and Size
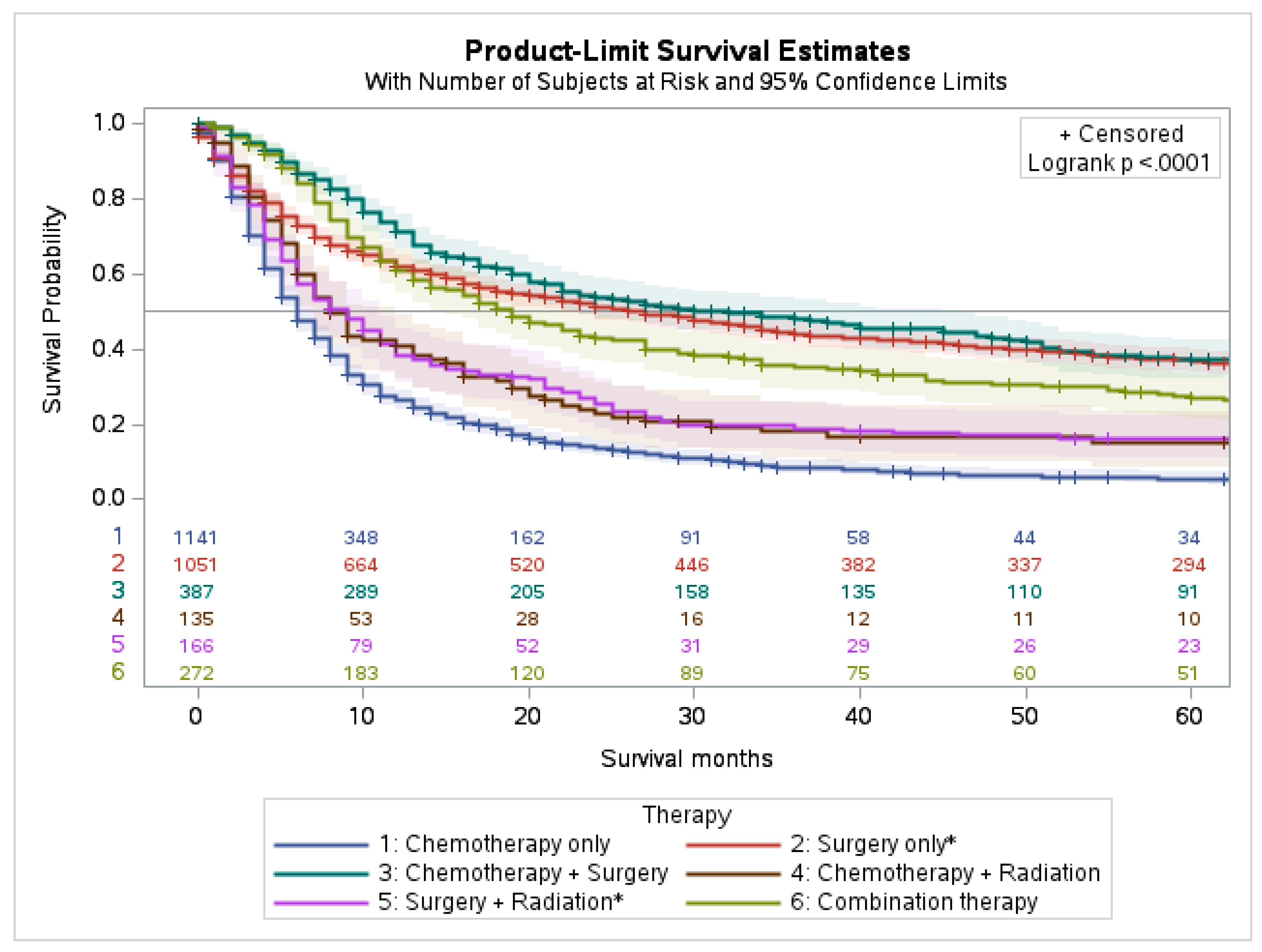
4.2. Survival Characteristics by Treatment Modality
4.3. Multivariable Analysis
4.4. Genetic Mutations in PSC
5. Discussion
Genetic Profiling and Emerging Therapeutics
6. Limitations
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PSC | Pulmonary sarcomatoid carcinoma |
| NSCLC | Non-small cell lung carcinoma |
| SEER | Surveillance, Epidemiology, and End Results |
| COSMIC | Catalogue Of Somatic Mutations in Cancer |
| EMA | Cytokeratins, epithelial membrane antigen |
| CEA | Carcinoembryonic antigen |
| NIH | National Cancer Institute |
References
- Thiery, J.P. Epithelial-mesenchymal transitions in tumour progression. Nat. Rev. Cancer 2002, 2, 442–454. [Google Scholar] [CrossRef] [PubMed]
- Travis, W.D. Sarcomatoid neoplasms of the lung and pleura. Arch. Pathol. Lab. Med. 2010, 134, 1645–1658. [Google Scholar] [CrossRef] [PubMed]
- Baldovini, C.; Rossi, G.; Ciarrocchi, A. Approaches to Tumor Classification in Pulmonary Sarcomatoid Carcinoma. Lung Cancer 2019, 10, 131–149. [Google Scholar] [CrossRef] [PubMed]
- Riudavets, M.; Garcia de Herreros, M.; Besse, B.; Mezquita, L. Radon and Lung Cancer: Current Trends and Future Perspectives. Cancers 2022, 14, 3142. [Google Scholar] [CrossRef] [PubMed]
- Pelosi, G.; Sonzogni, A.M.; De Pas, T.; Galetta, D.; Veronesi, G.; Spaggiari, L.; Manzotti, M.; Fumagalli, C.; Bresaola, E.; Nappi, O.; et al. Review article: Pulmonary sarcomatoid carcinomas: A practical overview. Int. J. Surg. Pathol. 2010, 18, 103–120. [Google Scholar] [CrossRef]
- Travis, W.D.; Brambilla, E.; Nicholson, A.G.; Yatabe, Y.; Austin, J.H.; Beasley, M.B.; Chirieac, L.R.; Dacic, S.; Duhig, E.; Flieder, D.B.; et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J. Thorac. Oncol. 2015, 10, 1243–1260. [Google Scholar] [CrossRef]
- Roesel, C.; Terjung, S.; Weinreich, G.; Hager, T.; Chalvatzoulis, E.; Metzenmacher, M.; Welter, S. Sarcomatoid carcinoma of the lung: A rare histological subtype of non-small cell lung cancer with a poor prognosis even at earlier tumour stages. Interact. Cardiovasc. Thorac. Surg. 2017, 24, 407–413. [Google Scholar] [CrossRef]
- Travis, W.D.; Brambilla, E.; Burke, A.P.; Marx, A.; Nicholson, A.G. Introduction to The 2015 World Health Organization Classification of Tumors of the Lung, Pleura, Thymus, and Heart. J. Thorac. Oncol. 2015, 10, 1240–1242. [Google Scholar] [CrossRef]
- Pang, A.; Carbini, M.; Moreira, A.L.; Maki, R.G. Carcinosarcomas and Related Cancers: Tumors Caught in the Act of Epithelial-Mesenchymal Transition. J. Clin. Oncol. 2018, 36, 210–216. [Google Scholar] [CrossRef]
- Yendamuri, S.; Caty, L.; Pine, M.; Adem, S.; Bogner, P.; Miller, A.; Demmy, T.L.; Groman, A.; Reid, M. Outcomes of sarcomatoid carcinoma of the lung: A Surveillance, Epidemiology, and End Results Database analysis. Surgery 2012, 152, 397–402. [Google Scholar] [CrossRef]
- Li, X.; Wu, D.; Liu, H.; Chen, J. Pulmonary sarcomatoid carcinoma: Progress, treatment and expectations. Ther. Adv. Med. Oncol. 2020, 12, 1758835920950207. [Google Scholar] [CrossRef] [PubMed]
- Gang, J.; Yan, Q.; Xiang, S.; Zheng, L.; Zhao, L. Clinicopathological characteristics and prognostic factors of pulmonary sarcomatoid carcinoma: A large population analysis. Ann. Transl. Med. 2021, 9, 121. [Google Scholar] [CrossRef] [PubMed]
- Ung, M.; Rouquette, I.; Filleron, T.; Taillandy, K.; Brouchet, L.; Bennouna, J.; Delord, J.-P.; Milia, J.; Mazières, J. Characteristics and Clinical Outcomes of Sarcomatoid Carcinoma of the Lung. Clin. Lung Cancer 2016, 17, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Xing, L.; Yuan, Y. A clinical analysis of 114 cases of sarcomatoid carcinoma of the lung. Clin. Exp. Med. 2018, 18, 555–562. [Google Scholar] [CrossRef]
- Lin, Y.; Yang, H.; Cai, Q.; Wang, D.; Rao, H.; Lin, S.; Long, H.; Fu, J.; Zhang, L.; Lin, P.; et al. Characteristics and Prognostic Analysis of 69 Patients With Pulmonary Sarcomatoid Carcinoma. Am. J. Clin. Oncol. 2016, 39, 215–222. [Google Scholar] [CrossRef]
- Chaft, J.E.; Sima, C.S.; Ginsberg, M.S.; Huang, J.; Kris, M.G.; Travis, W.D.; Azzoli, C.G. Clinical outcomes with perioperative chemotherapy in sarcomatoid carcinomas of the lung. J. Thorac. Oncol. 2012, 7, 1400–1405. [Google Scholar] [CrossRef]
- Yang, Z.; Xu, J.; Li, L.; Li, R.; Wang, Y.; Tian, Y.; Guo, W.; Wang, Z.; Tan, F.; Ying, J.; et al. Integrated molecular characterization reveals potential therapeutic strategies for pulmonary sarcomatoid carcinoma. Nat. Commun. 2020, 11, 4878. [Google Scholar] [CrossRef]
- Schrock, A.B.; Li, S.D.; Frampton, G.M.; Suh, J.; Braun, E.; Mehra, R.; Buck, S.C.; Bufill, J.A.; Peled, N.; Karim, N.A.; et al. Pulmonary Sarcomatoid Carcinomas Commonly Harbor Either Potentially Targetable Genomic Alterations or High Tumor Mutational Burden as Observed by Comprehensive Genomic Profiling. J. Thorac. Oncol. 2017, 12, 932–942. [Google Scholar] [CrossRef]
- Zhou, F.; Huang, Y.; Cai, W.; Li, J.; Su, C.; Ren, S.; Wu, C.; Zhou, C. The genomic and immunologic profiles of pure pulmonary sarcomatoid carcinoma in Chinese patients. Lung Cancer 2021, 153, 66–72. [Google Scholar] [CrossRef]
- Lococo, F.; Gandolfi, G.; Rossi, A.; Pinto, C.; Rapicetta, C.; Cavazza, A.; Cesario, A.; Galeone, C.; Paci, M.; Ciarrocchi, A. Deep Sequencing Analysis Reveals That KRAS Mutation Is a Marker of Poor Prognosis in Patients with Pulmonary Sarcomatoid Carcinoma. J. Thorac. Oncol. 2016, 11, 1282–1292. [Google Scholar] [CrossRef]
- Mehrad, M.; Roy, S.; LaFramboise, W.A.; Petrosko, P.; Miller, C.; Incharoen, P.; Dacic, S. KRAS mutation is predictive of outcome in patients with pulmonary sarcomatoid carcinoma. Histopathology 2018, 73, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.-Y.; Zhong, W.-Z.; Zhang, X.-C.; Su, J.; Xie, Z.; Liu, S.-Y.; Tu, H.-Y.; Chen, H.-J.; Sun, Y.-L.; Zhou, Q.; et al. Potential Predictive Value of TP53 and KRAS Mutation Status for Response to PD-1 Blockade Immunotherapy in Lung Adenocarcinoma. Clin. Cancer Res. 2017, 23, 3012–3024. [Google Scholar] [CrossRef] [PubMed]
- Sequist, L.V.; Yang, J.C.-H.; Yamamoto, N.; Obyrne, K.; Hirsh, V.; Mok, T.; Geater, S.L.; Orlov, S.; Tsai, C.-M.; Boyer, M.; et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J. Clin. Oncol. 2013, 31, 3327–3334. [Google Scholar] [CrossRef] [PubMed]
- Cataldo, V.D.; Gibbons, D.L.; Pérez-Soler, R.; Quintás-Cardama, A. Treatment of non-small-cell lung cancer with erlotinib or gefitinib. N. Engl. J. Med. 2011, 364, 947–955. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, S.S.; Vansteenkiste, J.; Planchard, D.; Cho, B.C.; Gray, J.E.; Ohe, Y.; Zhou, C.; Reungwetwattana, T.; Cheng, Y.; Chewaskulyong, B.; et al. Overall Survival with Osimertinib in Untreated, EGFR-Mutated Advanced NSCLC. N. Engl. J. Med. 2020, 382, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Fang, J.; Li, X.; Cao, L.; Zhou, J.; Guo, Q.; Liang, Z.; Cheng, Y.; Jiang, L.; Yang, N.; et al. Once-daily savolitinib in Chinese patients with pulmonary sarcomatoid carcinomas and other non-small-cell lung cancers harbouring MET exon 14 skipping alterations: A multicentre, single-arm, open-label, phase 2 study. Lancet Respir. Med. 2021, 9, 1154–1164. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Jia, Y.; Stoopler, M.B.; Shen, Y.; Cheng, H.; Chen, J.; Mansukhani, M.; Koul, S.; Halmos, B.; Borczuk, A.C. Next-Generation Sequencing of Pulmonary Sarcomatoid Carcinoma Reveals High Frequency of Actionable MET Gene Mutations. J. Clin. Oncol. 2016, 34, 794–802. [Google Scholar] [CrossRef]
- Heist, R.S.; Seto, T.; Han, J.-Y.; Reguart, N.; Garon, E.B.; Groen, H.J.M.; Tan, D.S.W.; Hida, T.; De Jonge, M.; Orlov, S.V.; et al. CMET-22. Capmatinib (INC280) in Metδex14-Mutated Advanced Non-Small Cell Lung Cancer (NSCLC): Efficacy Data from the Phase 2 Geometry Mono-1 Study. Neuro-Oncology 2019, 21 (Suppl. S6), vi56. [Google Scholar] [CrossRef]
- Gong, C.; Xiong, H.; Qin, K.; Wang, J.; Cheng, Y.; Zhao, J.; Zhang, J. MET alterations in advanced pulmonary sarcomatoid carcinoma. Front. Oncol. 2022, 12, 1017026. [Google Scholar] [CrossRef]
- Planchard, D.; Besse, B.; Groen, H.J.; Souquet, P.J.; Quoix, E.; Baik, C.S.; Barlesi, F.; Kim, T.M.; Mazieres, J.; Novello, S.; et al. Dabrafenib plus trametinib in patients with previously treated BRAF(V600E)-mutant metastatic non-small cell lung cancer: An open-label, multicentre phase 2 trial. Lancet Oncol. 2016, 17, 984–993. [Google Scholar] [CrossRef]
- Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-dabrafenib-combination-trametinib-unresectable-or-metastatic-solid (accessed on 1 January 2023.).
- Mayer, I.A.; Abramson, V.G.; Formisano, L.; Balko, J.M.; Estrada, M.V.; Sanders, M.E.; Juric, D.; Solit, D.; Berger, M.F.; Won, H.H.; et al. A Phase Ib Study of Alpelisib (BYL719), a PI3Kα-Specific Inhibitor, with Letrozole in ER+/HER2- Metastatic Breast Cancer. Clin. Cancer Res. 2017, 23, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Patnaik, A.; Appleman, L.J.; Tolcher, A.W.; Papadopoulos, K.P.; Beeram, M.; Rasco, D.W.; Weiss, G.J.; Sachdev, J.C.; Chadha, M.; Fulk, M.; et al. First-in-human phase I study of copanlisib (BAY 80-6946), an intravenous pan-class I phosphatidylinositol 3-kinase inhibitor, in patients with advanced solid tumors and non-Hodgkin’s lymphomas. Ann. Oncol. 2016, 27, 1928–1940. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Lackner, M.R.; Oudard, S.; Escudier, B.; Ralph, C.; Brown, J.E.; Hawkins, R.E.; Castellano, D.; Rini, B.I.; Staehler, M.D.; et al. Randomized Open-Label Phase II Trial of Apitolisib (GDC-0980), a Novel Inhibitor of the PI3K/Mammalian Target of Rapamycin Pathway, Versus Everolimus in Patients with Metastatic Renal Cell Carcinoma. J. Clin. Oncol. 2016, 34, 1660–1668. [Google Scholar] [CrossRef]
- Hyman, D.M.; Smyth, L.M.; Donoghue, M.T.; Westin, S.N.; Bedard, P.L.; Dean, E.J.; Bando, H.; El-Khoueiry, A.B.; Pérez-Fidalgo, J.A.; Mita, A.; et al. AKT Inhibition in Solid Tumors with AKT1 Mutations. J. Clin. Oncol. 2017, 35, 2251–2259. [Google Scholar] [CrossRef] [PubMed]
- Fallet, V.; Saffroy, R.; Girard, N.; Mazieres, J.; Lantuejoul, S.; Vieira, T.; Rouquette, I.; Thivolet-Bejui, F.; Ung, M.; Poulot, V.; et al. High-throughput somatic mutation profiling in pulmonary sarcomatoid carcinomas using the LungCarta™ Panel: Exploring therapeutic targets. Ann. Oncol. 2015, 26, 1748–1753. [Google Scholar] [CrossRef]
- Nakagomi, T.; Goto, T.; Hirotsu, Y.; Shikata, D.; Yokoyama, Y.; Higuchi, R.; Amemiya, K.; Okimoto, K.; Oyama, T.; Mochizuki, H.; et al. New therapeutic targets for pulmonary sarcomatoid carcinomas based on their genomic and phylogenetic profiles. Oncotarget 2018, 9, 10635–10649. [Google Scholar] [CrossRef]
- Pelosi, G.; Scarpa, A.; Manzotti, M.; Veronesi, G.; Spaggiari, L.; Fraggetta, F.; Nappi, O.; Benini, E.; Pasini, F.; Antonello, D.; et al. K-ras gene mutational analysis supports a monoclonal origin of biphasic pleomorphic carcinoma of the lung. Mod. Pathol. 2004, 17, 538–546. [Google Scholar] [CrossRef]
- Byers, L.A.; Diao, L.; Wang, J.; Saintigny, P.; Girard, L.; Peyton, M.; Shen, L.; Fan, Y.; Giri, U.; Tumula, P.K.; et al. An epithelial-mesenchymal transition gene signature predicts resistance to EGFR and PI3K inhibitors and identifies Axl as a therapeutic target for overcoming EGFR inhibitor resistance. Clin. Cancer Res. 2013, 19, 279–290. [Google Scholar] [CrossRef]
- Howlader, N.; Forjaz, G.; Mooradian, M.J.; Meza, R.; Kong, C.Y.; Cronin, K.A.; Mariotto, A.B.; Lowy, D.R.; Feuer, E.J. The Effect of Advances in Lung-Cancer Treatment on Population Mortality. N. Engl. J. Med. 2020, 383, 640–649. [Google Scholar] [CrossRef]
- Hellmann, M.D.; Ciuleanu, T.-E.; Pluzanski, A.; Lee, J.S.; Otterson, G.A.; Audigier-Valette, C.; Minenza, E.; Linardou, H.; Burgers, S.; Salman, P.; et al. Nivolumab plus Ipilimumab in Lung Cancer with a High Tumor Mutational Burden. N. Engl. J. Med. 2018, 378, 2093–2104. [Google Scholar] [CrossRef]
- Salati, M.; Baldessari, C.; Calabrese, F.; Rossi, G.; Pettorelli, E.; Grizzi, G.; Dominici, M.; Barbieri, F. Nivolumab-Induced Impressive Response of Refractory Pulmonary Sarcomatoid Carcinoma with Brain Metastasis. Case Rep. Oncol. 2018, 11, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Kotlowska, M.P.; Rueda, A.G.; Olmedo, M.E.; Benito, A.; Roldán, A.S.; Méndez, M.A.F.; Gorospe, L.; Palacios, J.; López, P.G. Efficacy of immunotherapy in sarcomatoid lung cancer, a case report and literature review. Respir. Med. Case Rep. 2019, 26, 310–314. [Google Scholar] [CrossRef]
- Skoulidis, F.; Goldberg, M.E.; Greenawalt, D.M.; Hellmann, M.D.; Awad, M.M.; Gainor, J.F.; Schrock, A.B.; Hartmaier, R.J.; Trabucco, S.E.; Gay, L.; et al. STK11/LKB1 Mutations and PD-1 Inhibitor Resistance in KRAS-Mutant Lung Adenocarcinoma. Cancer Discov. 2018, 8, 822–835. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lin, W.; Yang, Z.; Li, R.; Gao, Y.; He, J. Multimodality Treatment of Pulmonary Sarcomatoid Carcinoma: A Review of Current State of Art. J. Oncol. 2022, 2022, 8541157. [Google Scholar] [CrossRef] [PubMed]
- Domblides, C.; Leroy, K.; Monnet, I.; Mazières, J.; Barlesi, F.; Gounant, V.; Baldacci, S.; Mennecier, B.; Toffart, A.-C.; Audigier-Valette, C.; et al. Efficacy of Immune Checkpoint Inhibitors in Lung Sarcomatoid Carcinoma. J. Thorac. Oncol. 2020, 15, 860–866. [Google Scholar] [CrossRef]
- Kim, M.; Keam, B.; Ock, C.Y.; Kim, S.H.; Kim, Y.J.; Lim, S.M.; Kim, J.S.; Kim, T.M.; Hong, S.H.; Ahn, M.S.; et al. Phase II study of durvalumab and tremelimumab in pulmonary sarcomatoid carcinoma: KCSG-LU16-07. Thorac. Cancer 2020, 11, 3482–3489. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Yang, X.; Hao, J.; Guo, C.; Pu, Q.; Liu, L. Clinicopathological features and prognostic analysis of metastatic pulmonary sarcomatoid carcinoma: A SEER analysis. J. Thorac. Dis. 2021, 13, 893–905. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Yang, Q.; Xu, Z.; Luo, B.; Li, F.; Yu, Y.; Sun, J. Survival Analysis and Prediction Model for Pulmonary Sarcomatoid Carcinoma Based on SEER Database. Front. Oncol. 2021, 11, 630885. [Google Scholar] [CrossRef]
| Variable (n = 5259) | Frequency (%) | |
|---|---|---|
| Age (years) | 1–9 | 5 (0.1%) |
| 10–19 | 2 (0.0%) | |
| 20–29 | 13 (0.2%) | |
| 30–39 | 35 (0.7%) | |
| 40–49 | 255 (4.8%) | |
| 50–59 | 755 (14.4%) | |
| 60–69 | 1374 (26.1%) | |
| 70–79 | 1693 (32.2%) | |
| 80+ | 1127 (21.4%) | |
| Gender | Female | 2149 (40.9%) |
| Male | 3110 (59.1%) | |
| Race | Unknown | 9 (0.2%) |
| White | 4401 (83.7%) | |
| Black | 582 (11.1%) | |
| Asian or Pacific Islander | 242 (4.6%) | |
| American Indian or Alaska Native | 25 (0.5%) | |
| Grade (n = 5259) | Frequency (%) | |
| Unknown | 2494 (47.4%) | |
| Known | 2765 (52.6%) | |
| Grade where known (n = 2765) | ||
| Well differentiated—Grade I | 36 (1.3%) | |
| Moderately differentiated—Grade II | 53 (1.9%) | |
| Poorly differentiated—Grade III | 2015 (72.9%) | |
| Undifferentiated/Anaplastic—Grade IV | 661 (23.9%) | |
| Variable (n = 5259) | Frequency (%) | |
| Stage | Unknown | 418 (7.9%) |
| Known | 4841 (92.1%) | |
| Stage where known (n = 4841) | ||
| Localized | 787 (16.2%) | |
| Regional | 1359 (28.1%) | |
| Distant | 2695 (55.7%) | |
| Size | Unknown | 2489 (47.3%) |
| Known | 2770 (52.7%) | |
| Size where known (n = 2770) | ||
| In mm or No tumor found | 12 (0.4%) | |
| 1–3 cm | 616 (22.2%) | |
| 3.1–5 cm | 706 (25.5%) | |
| 5.1–7 cm | 601 (21.7%) | |
| ≥7.1 cm | 835 (30.2%) | |
| Variables | Multivariate Analysis; Hazard Ratio (p-Value) | |
|---|---|---|
| Age | >60 years | 1.442 (0.001) |
| Gender | Male | 1.151 (0.001) |
| Stage | Distant | 2.934 (0.001) |
| Size | >5 cm | 1.391 (0.001) |
| Bone metastasis | Yes | 1.169 (0.038) |
| Brain metastasis | Yes | 1.313 (0.002) |
| Liver metastasis | Yes | 1.767 (0.001) |
| Chemotherapy | Yes | 0.505 (0.001) |
| Surgery | Yes | 0.382 (0.001) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ullah, A.; Ahmed, A.; Yasinzai, A.Q.K.; Lee, K.T.; Khan, I.; Asif, B.; Khan, I.; Tareen, B.; Kakar, K.; Andam, G.; et al. Demographics and Clinicopathologic Profile of Pulmonary Sarcomatoid Carcinoma with Survival Analysis and Genomic Landscape. Cancers 2023, 15, 2469. https://doi.org/10.3390/cancers15092469
Ullah A, Ahmed A, Yasinzai AQK, Lee KT, Khan I, Asif B, Khan I, Tareen B, Kakar K, Andam G, et al. Demographics and Clinicopathologic Profile of Pulmonary Sarcomatoid Carcinoma with Survival Analysis and Genomic Landscape. Cancers. 2023; 15(9):2469. https://doi.org/10.3390/cancers15092469
Chicago/Turabian StyleUllah, Asad, Asim Ahmed, Abdul Qahar Khan Yasinzai, Kue Tylor Lee, Israr Khan, Bina Asif, Imran Khan, Bisma Tareen, Kaleemullah Kakar, Gul Andam, and et al. 2023. "Demographics and Clinicopathologic Profile of Pulmonary Sarcomatoid Carcinoma with Survival Analysis and Genomic Landscape" Cancers 15, no. 9: 2469. https://doi.org/10.3390/cancers15092469
APA StyleUllah, A., Ahmed, A., Yasinzai, A. Q. K., Lee, K. T., Khan, I., Asif, B., Khan, I., Tareen, B., Kakar, K., Andam, G., Heneidi, S., Khan, J., Khan, H., Karki, N. R., Del Rivero, J., & Karim, N. A. (2023). Demographics and Clinicopathologic Profile of Pulmonary Sarcomatoid Carcinoma with Survival Analysis and Genomic Landscape. Cancers, 15(9), 2469. https://doi.org/10.3390/cancers15092469










