The Role of Surgery in Primary Chest Wall Tumors: Over 20 Years’ Experience in Resection and Reconstruction
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Preoperative Assessments
2.2. Collected Data
2.3. Statistical Analysis
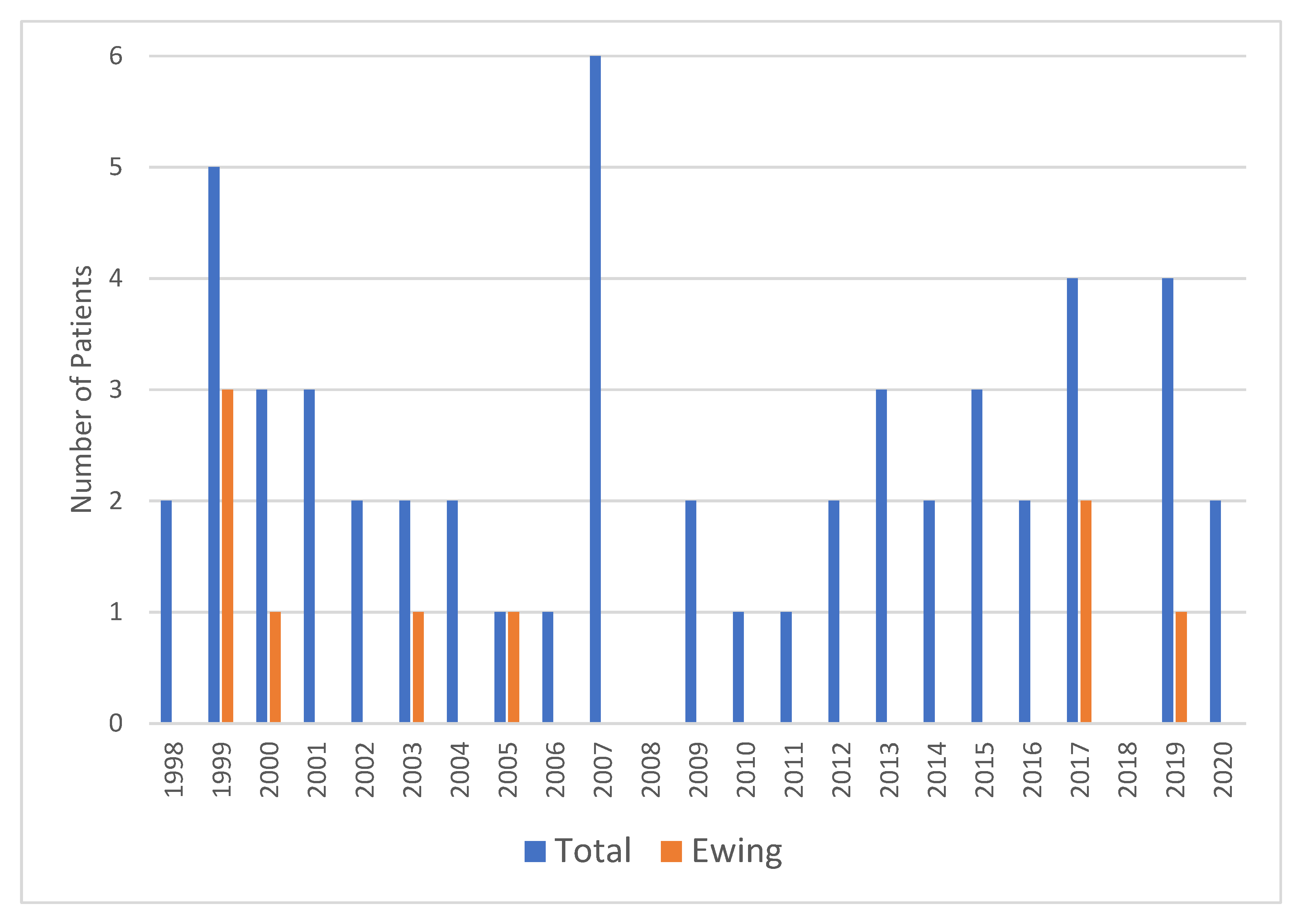
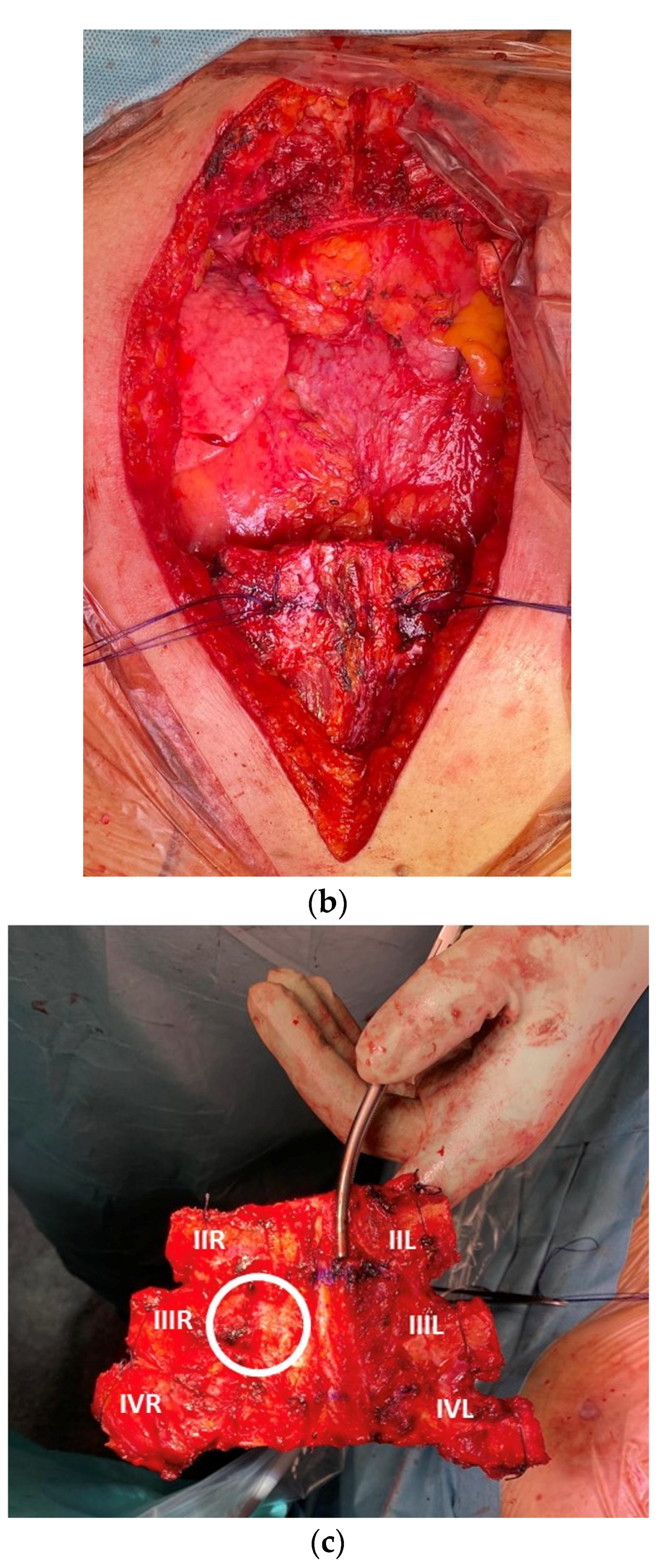
3. Results
4. Discussion
Strength and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Incarbone, M.; Pastorino, U. Surgical Treatment of Chest Wall Tumors. World J. Surg. 2001, 25, 218–230. [Google Scholar] [CrossRef]
- Shah, A.A.; D’Amico, T.A. Primary Chest Wall Tumors. J. Am. Coll. Surg. 2010, 210, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.E.; Keshavjee, S. Primary Chest Wall Tumors. Thorac. Surg. Clin. 2010, 20, 495–507. [Google Scholar] [CrossRef]
- King, R.M.; Pairolero, P.C.; Trastek, V.F.; Piehler, J.M.; Payne, W.S.; Bernatz, P.E. Primary Chest Wall Tumors: Factors Affecting Survival. Ann. Thorac. Surg. 1986, 41, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Adjuvant chemotherapy for localised resectable soft-tissue sarcoma of adults: Meta-analysis of individual data. Sarcoma Meta-analysis Collaboration. Lancet 1997, 350, 1647–1654. [CrossRef]
- Pervaiz, N.; Colterjohn, N.; Farrokhyar, F.; Tozer, R.; Figueredo, A.; Ghert, M. A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft-tissue sarcoma. Cancer 2008, 113, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Zagars, G.K.; Ballo, M.T.; Pisters, P.W.T.; Pollock, R.E.; Patel, S.R.; Benjamin, R.S.; Evans, H.L. Prognostic factors for patients with localized soft-tissue sarcoma treated with conservation surgery and radiation therapy: An analysis of 1225 patients. Cancer 2003, 97, 2530–2543. [Google Scholar] [CrossRef]
- Khanfir, K.; Alzieu, L.; Terrier, P.; Le Péchoux, C.; Bonvalot, S.; Vanel, D.; Le Cesne, A. Does adjuvant radiation therapy increase loco-regional control after optimal resection of soft-tissue sarcoma of the extremities? Eur. J. Cancer 2003, 39, 1872–1880. [Google Scholar] [CrossRef]
- Agha, R.; Abdall-Razak, A.; Crossley, E.; Dowlut, N.; Iosifidis, C.; Mathew, G.; Beamishaj; Bashashati, M.; Millham, F.H.; Orgill, D.P.; et al. STROCSS 2019 Guideline: Strengthening the reporting of cohort studies in surgery. Int. J. Surg. 2019, 72, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Clavien, P.A.; Barkun, J.; de Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; de Santibañes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The Clavien-Dindo classification of surgical complications: Five-year experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef]
- Cipriano, C.; Griffin, A.M.; Ferguson, P.C.; Wunder, J.S. Developing an Evidence-based Followup Schedule for Bone Sarcomas Based on Local Recurrence and Metastatic Progression. Clin. Orthop. Relat. Res. 2017, 475, 830–838. [Google Scholar] [CrossRef]
- Chiang, C.-J.; Fong, Y.-C.; Hsu, H.-C. Extraskeletal mesenchymal chondrosarcoma. J. Chin. Med. Assoc. 2003, 66, 307–310. [Google Scholar] [PubMed]
- Ciriaco, P.; Casiraghi, M.; Negri, G.; Gioia, G.; Carretta, A.; Melloni, G.; Zannini, P. Early Surgical Repair of Isolated Traumatic Sternal Fractures Using a Cervical Plate System. J. Trauma 2009, 66, 462–464. [Google Scholar] [CrossRef] [PubMed]
- Dell’Amore, A.; Kalab, M.; Miller, A.S., 3rd; Dolci, G.; Liparulo, V.; Beigee, F.S.; Rosso, L.; Ferrigno, P.; Pangoni, A.; Schiavon, M.; et al. Indications and Results of Sternal Allograft Transplantation: Learning from a Worldwide Experience. Ann. Thorac. Surg. 2020, 112, 238–247. [Google Scholar] [CrossRef]
- Billè, A.; Okiror, L.; Karenovics, W.; Routledge, T. Experience with titanium devices for rib fixation and coverage of chest wall defects. Interact. Cardiovasc. Thorac. Surg. 2012, 15, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Petrella, F.; Casiraghi, M.; Mariolo, A.V.; Diotti, C.; Spaggiari, L. Rigid prosthesis removal following chest wall resection and reconstruction for cancer. Shanghai Chest 2018, 2, 64. [Google Scholar] [CrossRef]
- Petrella, F.; Iacono, G.L.; Casiraghi, M.; Gherzi, L.; Prisciandaro, E.; Garusi, C.; Spaggiari, L. Chest wall resection and reconstruction by composite prosthesis for locally recurrent breast carcinoma. J. Thorac. Dis. 2020, 12, 39–41. [Google Scholar] [CrossRef] [PubMed]
- Loi, M.; Mazzella, A.; Desideri, I.; Fournel, L.; Hamelin, E.C.; Icard, P.; Bobbio, A.; Alifano, M. Chest wall resection and reconstruction for lung cancer: Surgical techniques and example of integrated multimodality approach. J. Thorac. Dis. 2020, 12, 22–30. [Google Scholar] [CrossRef]
- Rahman, A.R.M.A.; Rahouma, M.; Gaafar, R.; Bahaa, S.; Loay, I.; Kamel, M.; Abdelbaki, H.; Yahia, M. Contributing factors to the outcome of primary malignant chest wall tumors. J. Thorac. Dis. 2017, 9, 5184–5193. [Google Scholar] [CrossRef]
- Hazel, K.; Weyant, M.J. Chest Wall Resection and Reconstruction: Management of Complications. Thorac. Surg. Clin. 2015, 25, 517–521. [Google Scholar] [CrossRef]
- Mariolo, A.V.; Casiraghi, M.; Galetta, D.; Spaggiari, L. Robotic Hybrid Approach for an Anterior Pancoast Tumor in a Severely Obese Patient. Ann. Thorac. Surg. 2018, 106, e115–e116. [Google Scholar] [CrossRef] [PubMed]
- Hennon, M.W.; Dexter, E.U.; Huang, M.; Kane, J.; Nwogu, C.; Picone, A.; Yendamuri, S.; Demmy, T.L. Does Thoracoscopic Surgery Decrease the Morbidity of Combined Lung and Chest Wall Resection? Ann. Thorac. Surg. 2015, 99, 1929–1935. [Google Scholar] [CrossRef] [PubMed]
- Shewale, J.B.; Mitchell, K.G.; Nelson, D.B.; Conley, A.P.; Rice, D.C.; Antonoff, M.B.; Hofstetter, W.L.; Walsh, G.L.; Swisher, S.G.; Roth, J.A.; et al. Predictors of survival after resection of primary sarcomas of the chest wall-A large, single-institution series. J. Surg. Oncol. 2018, 118, 518–524. [Google Scholar] [CrossRef] [PubMed]









| Variable | Alive (No. = 37) | Deceased (No. = 16) | Log-Rank Test (p-Value) | |
|---|---|---|---|---|
| Sex | M = 25 F = 28 | 18 (72) 19 (68) | 7 (28) 9 (32) | 0.49 |
| Age | <46 = 27 ≥46 = 26 | 19 (70) 18 (69) | 8 (30) 8 (31) | 0.58 |
| Preoperative diagnosis | Yes = 25 No = 28 | 10 (40) 27 (96) | 15 (60) 1 (4) | 0.08 |
| Radicality | R0 = 48 R1 = 5 | 33 (69) 4 (80) | 15 (31) 1 (20) | 0.52 |
| Grading | 0–1 = 18 >1 = 35 | 15 (83) 22 (63) | 3 (17) 13 (37) | 0.11 |
| Dimension | <65 mm = 31 ≥65 mm = 22 | 25 (81) 12 (55) | 6 (19) 10 (45) | 0.04 |
| Resected ribs | ≤2 = 33 ≥20 | 27 (82) 10 (50) | 6 (8) 10 (50) | 0.016 |
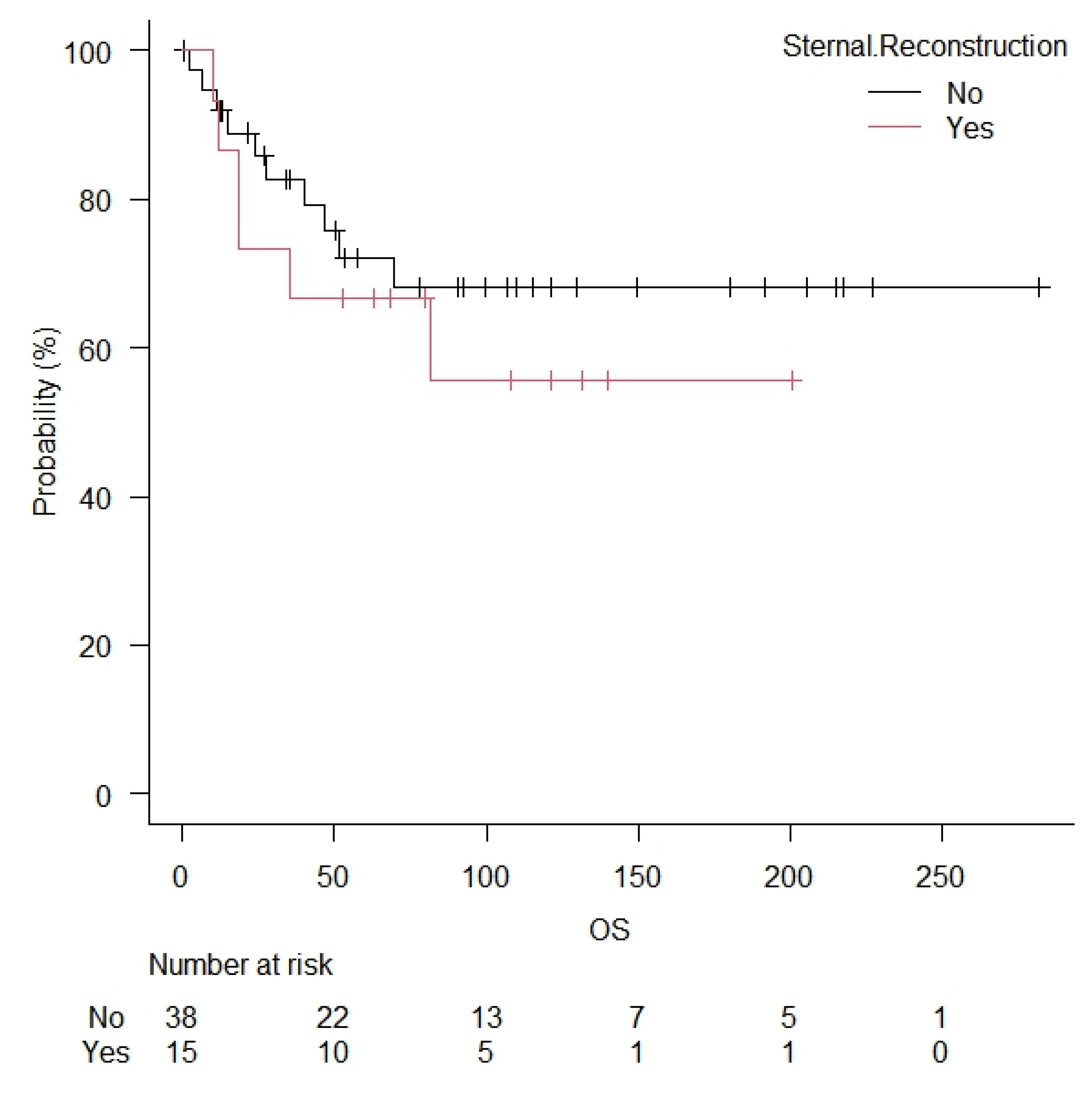
| Sternal resection | Yes = 38 No = 15 | 28 (74) 9 (60) | 10 (26) 6 (40) | 0.26 |
| Reconstructive prosthesis | Methyl-methacrylate = 32 Other = 21 | 19 (59) 18 (86) | 13 (41) 3 (14) | 0.058 |
| Muscular flap reconstruction | Yes = 29 No = 24 | 21 (72) 16 (67) | 8 (28) 6 (33) | 0.44 |
| Histology | Sarcoma = 51 Other = 2 | 35 (69) 2 (100) | 16 (31) 0 | 0.23 |
| Neoadjuvant treatment | None = 37 Chemotherapy = 15 Radiotherapy = 1 | 30 (81) 6 (40) 1 (100) | 7 (19) 9 (60) 0 | 0.036 |
| Adjuvant treatments | None = 25 Chemotherapy = 6 Radiotherapy = 10 Chemo-radiotherapy = 12 | 21 (84) 2 (33) 10 (100) 4 (33) | 4 (16) 4 (67) 0 8 (67) | 0.014 |
| ICU stay | Yes= 38 No = 15 | 30 (79) 7 (47) | 8 (21) 8 (53) | 0.038 |
| Hospital length of stay | <7 = 31 ≥7 = 22 | 24 (77) 13 (59) | 7 (23) 9 (41) | 0.23 |
| 30 days morbidity | Yes = 35 No = 18 | 28 (80) 9 (50) | 7 (20) 9 (50) | 0.023 |
| Variable | Recurrence (No. = 30) | No Recurrence (No. = 23) | Log-Rank Test (p-Value) | |
|---|---|---|---|---|
| Sex | M = 25 F = 28 | 13 (52) 17 (60) | 12 (48) 11 (40) | 0.36 |
| Age | <46 = 27 ≥46 = 26 | 15 (55) 15 (58) | 12 (45) 11 (42) | 0.55 |
| Preoperative diagnosis | Yes = 41 No = 12 | 21 (51) 9 (75) | 20 (49) 3 (25) | 0.13 |
| Radicality | R0 = 48 R1 = 5 | 27 (56) 3 (60) | 21 (44) 2 (40) | 0.63 |
| Grading | 0–1 = 18 >1 = 35 | 14 (78) 16 (46) | 4 (22) 19 (54) | 0.023 |
| Dimension | <65 mm = 31 ≥65 mm = 22 | 21 (68) 9 (41) | 10 (32) 13 (59) | 0.045 |
| Resected ribs | ≤2 = 33 ≥20 | 23 (70) 7 (35) | 10 (30) 13 (65) | 0.015 |
| Sternal resection | Yes = 38 No = 15 | 22 (58) 8 (53) | 16 (42) 7 (47) | 0.48 |
| Reconstructive prosthesis | Methyl-methacrylate = 32 Other = 21 | 16 (50) 14 (67) | 16 (50) 7 (33) | 0.19 |
| Muscular flap reconstruction | Yes = 29 No = 24 | 18 (62) 12 (50) | 11 (38) 12 (50) | 0.27 |
| Histology | Sarcoma = 51 Other = 2 | 26 (51) 1 (50) | 26 (49) 1 (50) | 0.23 |
| Neoadjuvant treatment | None = 37 Chemotherapy = 15 Radiotherapy = 1 | 24 (65) 5 (33) 1 (100) | 13 (35) 10 (67) 0 | 0.058 |
| Adjuvant treatments | None = 25 Chemotherapy = 6 Radiotherapy = 10 Chemo-radiotherapy = 12 | 19 (76) 1 (17) 7 (70) 3 (25) | 6 (24) 5 (83) 3 (30) 9 (75) | 0.026 |
| ICU stay | Yes = 38 No = 15 | 23 (61) 7 (47) | 15 (39) 8 (53) | 0.28 |
| Hospital length of stay | <7 = 31 ≥7 = 22 | 18 (58) 12 (55) | 13 (42) 10 (45) | 0.51 |
| 30 days morbidity | Yes = 35 No = 18 | 20 (57) 10 (56) | 15 (43) 8 (44) | 0.57 |
| Outcomes | HR | 95% CI | p-Value |
|---|---|---|---|
| Dimension ≥ 65 mm | 1.03 | 0.081–1.48 | 0.13 |
| Ribs > 2 | 1.58 | 1.31–2.48 | 0.045 |
| Neoadjuvant treatments | 1.03 | 0.12–1.38 | 0.64 |
| Adjuvant treatments | 1.64 | 1.37–2.18 | 0.047 |
| ICU stay | 1.32 | 0.24–1.68 | 0.46 |
| Postoperative complications | 1.66 | 0.23–1.45 | 0.057 |
| Outcomes | HR | 95% CI | p-Value |
|---|---|---|---|
| Grading > 1 | 1.65 | 0.17–1.97 | 0.53 |
| Dimension ≥ 65 mm | 1.34 | 0.34–1.68 | 0.13 |
| Ribs > 2 | 1.58 | 1.23–2.13 | 0.048 |
| Adjuvant treatments | 2.32 | 1.23–2.67 | 0.046 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lo Iacono, G.; Mazzella, A.; Mohamed, S.; Petrella, F.; Sedda, G.; Casiraghi, M.; Girelli, L.; Bertolaccini, L.; Spaggiari, L. The Role of Surgery in Primary Chest Wall Tumors: Over 20 Years’ Experience in Resection and Reconstruction. Cancers 2023, 15, 2153. https://doi.org/10.3390/cancers15072153
Lo Iacono G, Mazzella A, Mohamed S, Petrella F, Sedda G, Casiraghi M, Girelli L, Bertolaccini L, Spaggiari L. The Role of Surgery in Primary Chest Wall Tumors: Over 20 Years’ Experience in Resection and Reconstruction. Cancers. 2023; 15(7):2153. https://doi.org/10.3390/cancers15072153
Chicago/Turabian StyleLo Iacono, Giorgio, Antonio Mazzella, Shehab Mohamed, Francesco Petrella, Giulia Sedda, Monica Casiraghi, Lara Girelli, Luca Bertolaccini, and Lorenzo Spaggiari. 2023. "The Role of Surgery in Primary Chest Wall Tumors: Over 20 Years’ Experience in Resection and Reconstruction" Cancers 15, no. 7: 2153. https://doi.org/10.3390/cancers15072153
APA StyleLo Iacono, G., Mazzella, A., Mohamed, S., Petrella, F., Sedda, G., Casiraghi, M., Girelli, L., Bertolaccini, L., & Spaggiari, L. (2023). The Role of Surgery in Primary Chest Wall Tumors: Over 20 Years’ Experience in Resection and Reconstruction. Cancers, 15(7), 2153. https://doi.org/10.3390/cancers15072153