Pro-Gastrin-Releasing Peptide as a Biomarker in Lung Neuroendocrine Neoplasm
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Strategy
2.2. Cohorts
2.3. Blood for ProGRP and CgA Measurement
2.4. Histological Diagnosis
2.5. Biomarker Measurement
2.5.1. ProGRP Measurement
2.5.2. CgA Measurement
2.6. Radiological Evaluation of LNEN Disease
2.7. Statistical Analysis
3. Results
3.1. Comparisons between ProGRP and CgA as a Diagnostic Test
3.2. Relationship to LNEN Detection
CgA and ProGRP for Disease Detection
3.3. Diagnostic Accuracy of the ProGRP vs. CgA Assays
4. Discussion
5. Conclusions and Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.X.; Yuan, J.; Gao, Z.M.; Zhang, Z.G. LncRNA TUC338 promotes invasion of lung cancer by activating MAPK pathway. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 443–449. [Google Scholar] [CrossRef]
- Mao, Y.; Yang, D.; He, J.; Krasna, M.J. Epidemiology of Lung Cancer. Surg. Oncol. Clin. N. Am. 2016, 25, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Bade, B.C.; Dela Cruz, C.S. Lung Cancer 2020: Epidemiology, Etiology, and Prevention. Clin. Chest Med. 2020, 41, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef]
- WHO Classification of Tumours Editorial Board. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart, 4th ed.; Travis, W.D., Brambilla, E., Burke, A.P., Marx, A., Nicholson, A.G., Eds.; World Health Organization: Lyon, France, 2015; ISBN 978-928-322-436-5.
- Travis, W.D.; Brambilla, E.; Nicholson, A.G.; Yatabe, Y.; Austin, J.H.M.; Beasley, M.B.; Chirieac, L.R.; Dacic, S.; Duhig, E.; Flieder, D.B.; et al. The 2015 World Health Organization classification of lung tumors: Impact of genetic, clinical and radiologic advances since the 2004 classification. J. Thorac. Oncol. 2015, 10, 1243–1260. [Google Scholar] [CrossRef]
- Wang, L.; Wang, D.; Zheng, G.; Yang, Y.; Du, L.; Dong, Z.; Zhang, X.; Wang, C. Clinical Evaluation and Therapeutic Monitoring Value of Serum Tumor Markers in Lung Cancer. Int. J. Biol. Markers 2016, 31, 80–87. [Google Scholar] [CrossRef]
- Lu, Q.Q.; Li, J.; Li, Y.H.; Liu, H.; Xie, X.B. On detection of chromogranin A, synaptophysin, neuronspecific enolase and progastrin-releasing peptide in small cell lung cancer. Zhonghua Yu Fang Yi Xue Za Zhi 2022, 56, 1017–1022. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Li, Y.; Wang, L.; Zhou, Y.; Shen, Y.; Xu, F.; Chen, Y. Selective application of neuroendocrine markers in the diagnosis and treatment of small cell lung cancer. Clin. Chim. Acta 2020, 509, 295–303. [Google Scholar] [CrossRef]
- McDonald, T.J.; Jornvall, H.; Nilsson, G.; Vagne, M.; Ghatei, M.; Bloom, S.R.; Mutt, V. Characterization of a gastrin-releasing peptide from porcine non-antral gastric tissue. Biochem. Biophys. Res. Commun. 1979, 90, 227–233. [Google Scholar] [CrossRef]
- Fang, L.; Huang, Z.; Lin, Y.; Fu, J.; Liang, X.; Liu, F. Clinical Application of Pro-Gastrin-Releasing Peptide. Clin. Lab. 2018, 64, 1259–1268. [Google Scholar] [CrossRef]
- Wojcik, E.; Kulpa, J.K. Pro-gastrin-releasing peptide (ProGRP) as a biomarker in small-cell lung cancer diagnosis, monitoring, and evaluation of treatment response. Lung Cancer 2017, 8, 231–240. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, Y.; Li, J.; Hao, X.; Hu, X. Utility of NSE, ProGRP and LDH in Diagnosis and Treatment in Patients with Small Cell Lung Cancer. Zhongguo Fei Ai Za Zhi 2016, 19, 590–594. [Google Scholar] [CrossRef] [PubMed]
- Molina, R.; Auge, J.M.; Filella, X.; Vinalos, N.; Alicarte, J.; Domingo, J.M.; Ballesta, A.M. Pro-gastrin-releasing peptide (proGRP) in patients with benign and malignant diseases: Comparison with CEA, SCC, CYFRA 21-1 and NSE in patients with lung cancer. Anticancer Res. 2005, 25, 1773–1778. [Google Scholar] [PubMed]
- Korse, C.M.; Taal, B.G.; Bonfrer, J.M.G.; Vincent, A.; van Velthuysen, M.L.; Baas, P. An elevated progastrin-releasing peptide level in patients with well-differentiated neuroendocrine tumours indicates a primary tumour in the lung and predicts a shorter survival. Ann. Oncol. 2011, 22, 2625–2630. [Google Scholar] [CrossRef] [PubMed]
- Tutar, N.; Yetkin, N.A.; Yazıcı, C.; Önal, Ö.; Kontaş, O.; Keleştemur, F. Clinical significance of progastrin-releasing peptide, neuron-specific enolase, chromogranin a, and squamous cell cancer antigen in pulmonary neuroendocrine tumors. Turk. J. Med. Sci. 2019, 49, 774–781. [Google Scholar] [CrossRef]
- Yamamuro, T.; Inoue, K.; Nagai, Y.; Azuma, D.; Yamamoto, A.; Hara, K.; Kohara, M.; Iwata, T.; Nakatsuka, S.; Morii, E.; et al. A case of ectopic ACTH syndrome due to DDAVP-sensitive but V1b receptor-negative bronchial typical carcinoid with lymphatic metastasis and plasma ProGRP elevation. Endocr. J. 2018, 65, 1161–1169. [Google Scholar] [CrossRef]
- Hashimoto, H.; Ozeki, Y.; Kameda, K.; Taguchi, S. Typical Carcinoid of the Lung with Abnormal Elevation of Serum Pro-gastrin-releasing Peptide (ProGRP). Kyobu Geka 2018, 71, 593–596. [Google Scholar]
- Tarukawa, T.; Adachi, K. Atypical Pulmonary Carcinoid Tumor with Elevated Serum ProGRP; Report of a Case. Kyobu Geka 2018, 71, 236–239. [Google Scholar]
- Taira, N.; Kawabata, T.; Ichi, T.; Kushi, K.; Yohena, T.; Kawasaki, H.; Ishikawa, K.; Kato, S. Utility of the serum ProGRP level for follow-up of pulmonary carcinoid tumors. Am. J. Case Rep. 2014, 15, 337–339. [Google Scholar] [CrossRef]
- Kos-Kudła, B.; Foltyn, W.; Malczewska, A.; Bednarczuk, T.; Bolanowski, M.; Borowska, M.; Chmielik, E.; Ćwikła, J.B.; Gisterek, I.; Handkiewicz-Junak, D.; et al. Update of the diagnostic and therapeutic guidelines for gastro-entero-pancreatic neuroendocrine neoplasms (recommended by the Polish Network of Neuroendocrine Tumours) [Aktualizacja zaleceń ogólnych dotyczących postępowania diagnostyczno-terapeutycznego w nowotworach neuroendokrynnych układu pokarmowego (rekomendowane przez Polską Sieć Guzów Neuroendokrynnych)]. Endokrynol. Pol. 2022, 73, 387–454. [Google Scholar] [CrossRef]
- Rosiek, V.; Wójcik-Giertuga, M.; Kos-Kudła, B. Serum tumor markers for detection of bone metastases in patients with lung neuroendocrine neoplasms. Cancer Treat Res. Commun. 2022, 31, 100533. [Google Scholar] [CrossRef]
- Zweig, M.H.; Campbell, G. Receiver-operating characteristic (ROC) plots: A fundamental evaluation tool in clinical medicine. Clin. Chem. 1993, 39, 561–577. [Google Scholar] [CrossRef]
- Hanley, J.A.; McNeil, B.J. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology 1983, 148, 839–843. [Google Scholar] [CrossRef] [PubMed]
- Caplin, M.E.; Baudin, E.; Ferolla, P.; Filosso, P.; Garcia-Yuste, M.; Lim, E.; Oberg, K.; Pelosi, G.; Perren, A.; Rossi, R.E.; et al. ENETS consensus conference participants. Pulmonary neuroendocrine (carcinoid) tumors: European Neuroendocrine Tumor Society expert consensus and recommendations for best practice for typical and atypical pulmonary carcinoids. Ann. Oncol. 2015, 26, 1604–1620. [Google Scholar] [CrossRef] [PubMed]
- Savu, C.; Melinte, A.; Diaconu, C.; Stiru, O.; Gherghiceanu, F.; Tudorica, Ș.D.O.; Dumitrașcu, O.C.; Bratu, A.; Balescu, I.; Bacalbasa, N. Lung neuroendocrine tumors: A systematic literature review (Review). Exp. Ther. Med. 2022, 23, 176. [Google Scholar] [CrossRef] [PubMed]
- Malczewska, A.; Oberg, K.; Kos-Kudla, B. NETest is superior to chromogranin A in neuroendocrine neoplasia: A prospective ENETS CoE analysis. Endocr. Connect 2021, 10, 110–123. [Google Scholar] [CrossRef]
- Filosso, P.L.; Öberg, K.; Malczewska, A.; Lewczuk, A.; Roffinella, M.; Aslanian, H.; Bodei, L. Molecular identification of bronchopulmonary neuroendocrine tumours and neuroendocrine genotype in lung neoplasia using the NETest liquid biopsy. Eur. J. Cardiothorac. Surg. 2022, 57, 1195–1202. [Google Scholar] [CrossRef]
- Matar, S.; Malczewska, A.; Oberg, K.; Bodei, L.; Aslanian, H.; Lewczuk-Myślicka, A.; Filosso, P.L.; Suarez, A.L.; Kolasińska-Ćwikła, A.; Roffinella, M.; et al. Blood Chromogranin A Is Not Effective as a Biomarker for Diagnosis or Management of Bronchopulmonary Neuroendocrine Tumors/Neoplasms. Neuroendocrinology 2020, 110, 185–197. [Google Scholar] [CrossRef]
- Dam, G.; Grønbæk, H.; Sorbye, H.; Thiis Evensen, E.; Paulsson, B.; Sundin, A.; Jensen, C.; Ebbesen, D.; Knigge, U.; Tiensuu Janson, E. Prospective Study of Chromogranin A as a Predictor of Progression in Patients with Pancreatic, Small-Intestinal, and Unknown Primary Neuroendocrine Tumors. Neuroendocrinology 2020, 110, 217–224. [Google Scholar] [CrossRef]
- Lyubimova, N.V.; Churikova, T.K.; Kushlinskii, N.E. Chromogranin as a Biochemical Marker of Neuroendocrine Tumors. Bull. Exp. Biol. Med. 2016, 160, 702–704. [Google Scholar] [CrossRef]
- Giovanella, L.; Fontana, M.; Keller, F.; Campenni’, A.; Ceriani, L.; Paone, G. Circulating pro-gastrin releasing peptide (ProGRP) in patients with medullary thyroid carcinoma. Clin. Chem. Lab. Med. 2021, 59, 1569–1573. [Google Scholar] [CrossRef] [PubMed]
- Oleinikov, K.A.; Grozinsky-Glasberg, S.A.; Gross, D.J.A.; Nechushtan, H.A.; Peretz, T.A.; Maimon, O.A.; Nisman, B.A. ProGRP Is an Effective Marker for Disease Monitoring in Lung Carcinoids with Non-Informative Chromogranin A: Lessons from Clinical Practice. Endocr. Abstr. 2019, 63, GP3. [Google Scholar] [CrossRef]
- Endo, T.; Hasegawa, T.; Tezuka, Y.; Kanai, Y.; Otani, S.; Yamamoto, S.; Tetsuka, K.; Sato, Y.; Endo, S.; Sohara, Y. Atypical pulmonary carcinoid tumor with an abnormal elevation of serum gastrin-releasing peptide precursor: Report of a case. Kyobu Geka 2008, 61, 993–995. [Google Scholar] [PubMed]
- Li, J.; Chen, Y.; Wang, X.; Wang, C.; Xiao, M. The value of combined detection of CEA, CYFRA21-1, SCC-Ag, and pro-GRP in the differential diagnosis of lung cancer. Transl. Cancer Res. 2021, 10, 1900–1906. [Google Scholar] [CrossRef]
- Barchiesi, V.; Simeon, V.; Sandomenico, C.; Cantile, M.; Cerasuolo, D.; Chiodini, P.; Morabito, A.; Cavalcanti, E. Circulating Progastrin-Releasing Peptide in the Diagnosis of Small Cell Lung Cancer (SCLC) and in Therapeutic Monitoring. J. Circ. Biomark. 2021, 10, 9–13. [Google Scholar] [CrossRef]
- Nisman, B.; Olejnikov, K.; Neuchushtan, H.; Atlan, K.; Peled, N.; Gross, D.; Peretz, T.; Meirovitz, A.; Grozinsky—Glasberg, S. Plasma Progastrin-Releasing Peptide and Chromogranin A Assays for Diagnosing and Monitoring Lung Well-Differentiated Neuroendocrine Tumors: A Brief Report. J. Thorac. Oncol. 2023, 18, 369–376. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Q.; Wang, Y.; Xu, C. Evaluating the diagnostic and prognostic value of serum TuM2-PK, NSE, and ProGRP in small cell lung cancer. J. Clin. Lab. Anal. 2023, 37, e24865. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions, and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content. |
| Variable | Category | LNEN Patients (n = 290) | Controls (n = 54) |
|---|---|---|---|
| ProGRP [N: 46 pg/mL] | Mean:Median | 171.78:136.40 | 8.30:6.50 |
| CgA [N: <100 ug/L] | Mean:Median | 156.97:43.43 | 70.34:67.50 |
| Age (years) | Mean:Median | 58.74:62.36 | 39.84:38.00 |
| Gender | Male:Female | 80:210 | 18:36 |
| Functional status | NF | 270 (93.1%) | N/A |
| F (CS) | 19 (6.6%) | ||
| F (CD) | 1 (0.3%) | ||
| Disease status | SD | 201 (69.3%) | N/A |
| PD | 89 (30.7%) | ||
| TNM stage | Localized | 201 (69.3%) | N/A |
| Regional metastatic | 28 (9.7%) | ||
| Distant metastatic | 61 (21.0%) | ||
| Histology | TC | 192 (66.2%) | N/A |
| AC | 74 (25.5%) | ||
| LCNEC | 22 (7.6%) | ||
| DIPNECH | 2 (0.7%) |
| Variable | Groups | Median | IR | p |
|---|---|---|---|---|
| CgA [N: <100 µg/L] | Controls | 67.50 | 40.00–98.00 | <0.001 |
| LNEN | 43.43 | 27.03–81.87 | ||
| ProGRP [N: <46 pg/mL] | Controls | 6.50 | 5.50–8.00 | <0.0001 |
| LNEN | 136.40 | 93.90–204.50 |
| Essay | Histological LNEN Confirmation (n = 290) | |||
|---|---|---|---|---|
| Total | True Positive | False Negative | Accuracy | |
| ProGRP | 290 | 275 | 15 | 95% (275/290) |
| CgA | 290 | 46 | 242 | 29% (46/290) |
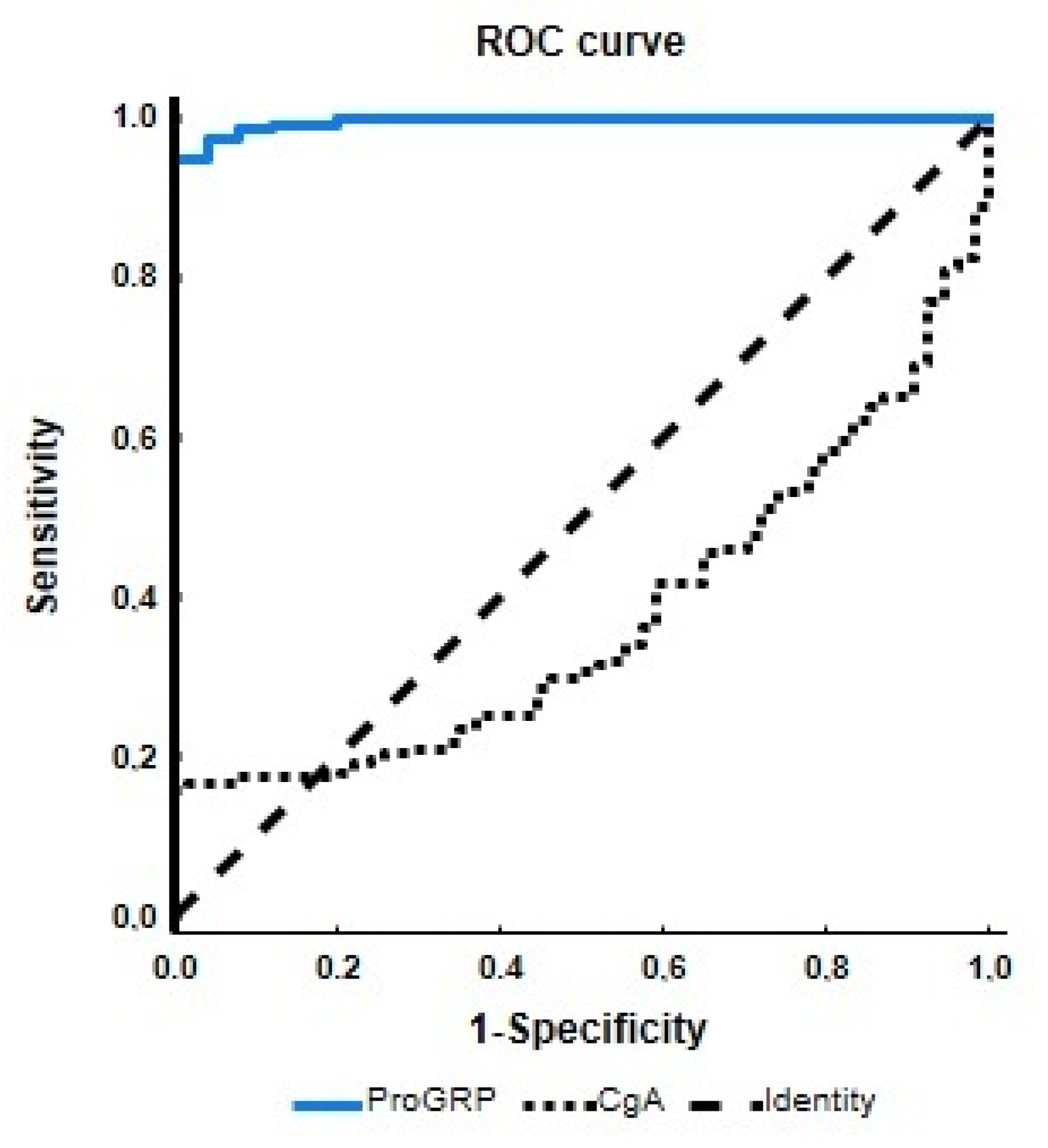
| Variable | AUC (95% CI) | SE | Z Score | p | Youden J Index (%) | Cut-Off Value | Sensitivity (%) | Specificity (%) | Accuracy (%) |
|---|---|---|---|---|---|---|---|---|---|
| ProGRP | 0.995 (0.99–1.00) | 0.003 | 177.30 | <0.001 | 94.80 | 32.30 pg/mL | 95 | 100 | 95 |
| CgA | 0.375 (0.31–0.44) | 0.035 | −3.61 | <0.001 | 15.97 | 128.25 ug/L | 16 | 100 | 29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosiek, V.; Kogut, A.; Kos-Kudła, B. Pro-Gastrin-Releasing Peptide as a Biomarker in Lung Neuroendocrine Neoplasm. Cancers 2023, 15, 3282. https://doi.org/10.3390/cancers15133282
Rosiek V, Kogut A, Kos-Kudła B. Pro-Gastrin-Releasing Peptide as a Biomarker in Lung Neuroendocrine Neoplasm. Cancers. 2023; 15(13):3282. https://doi.org/10.3390/cancers15133282
Chicago/Turabian StyleRosiek, Violetta, Angelika Kogut, and Beata Kos-Kudła. 2023. "Pro-Gastrin-Releasing Peptide as a Biomarker in Lung Neuroendocrine Neoplasm" Cancers 15, no. 13: 3282. https://doi.org/10.3390/cancers15133282
APA StyleRosiek, V., Kogut, A., & Kos-Kudła, B. (2023). Pro-Gastrin-Releasing Peptide as a Biomarker in Lung Neuroendocrine Neoplasm. Cancers, 15(13), 3282. https://doi.org/10.3390/cancers15133282






