Metronomic Chemotherapy: Anti-Tumor Pathways and Combination with Immune Checkpoint Inhibitors
Abstract
Simple Summary
Abstract
1. Introduction
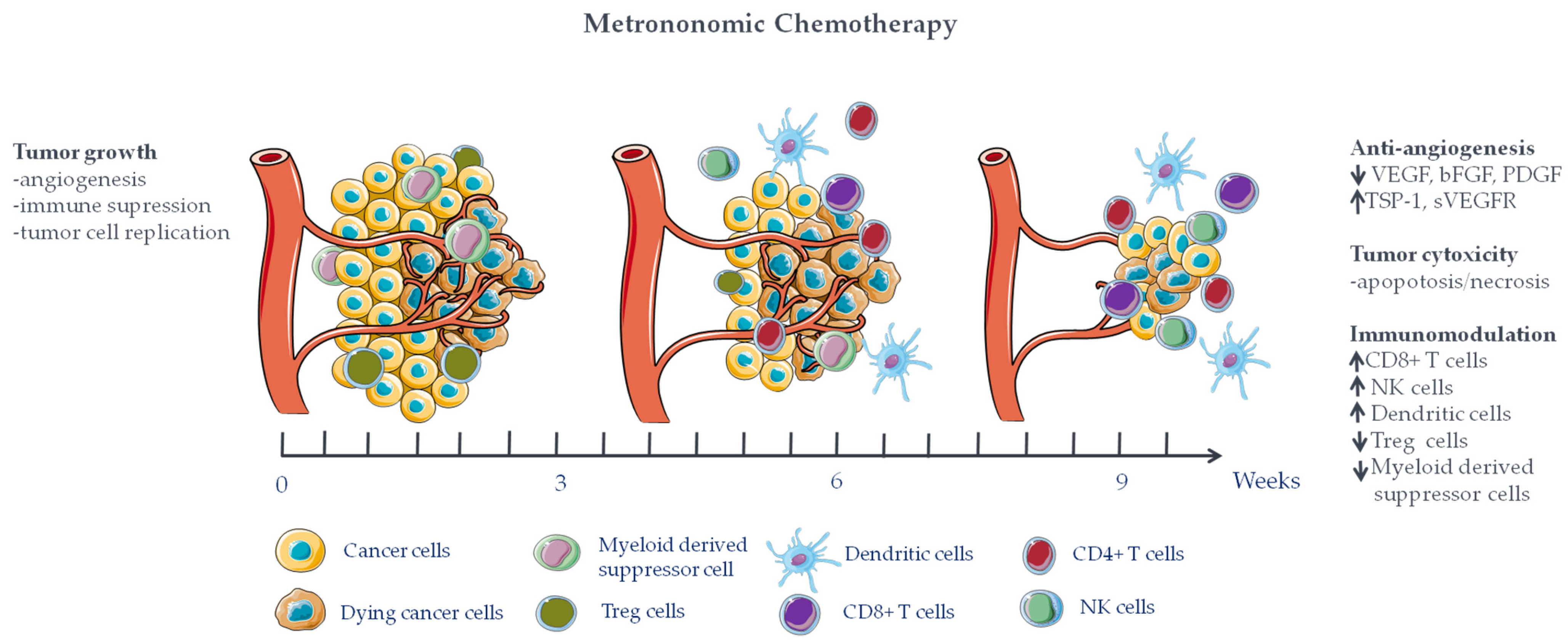
2. Metronomic Chemotherapy Mechanisms of Action
2.1. Anti-Angiogenic Effect
2.2. Immunomodulating Effects of mCT
2.3. mCT Directly Targets Tumor (Stem) Cells
2.4. mCT Mechanism of Actions Depend on Dose and Time-Exposure
3. Synergistic Role of mCT with Immune Checkpoint Inhibitors
| Refs. | Treatment Drugs mCT + ICI | Tumor Type | Immunologic Outcome | Efficacy |
|---|---|---|---|---|
| [106] | Cyclophosphamide + anti-PD-1 | Triple negative breast cancer | No increase of PD-1 or CD4+/CD8+ T cell | Not reported |
| [47] | Cisplatin/docetaxel + anti-PD-1 | Squamous cell lung carcinoma | Increased macrophage Increased TIL (CD45+, CD3+, CD8+) | Not reported |
| [108] | Carboplatin + anti-PD-1 | Lung cancer | Increased PD-1 expression Increased CD8+ T cell infiltration | Reduced tumor growth No adverse effect (mice body weight) |
| [101] | Gemcitabine + anti-PD-L1 | Squamous cell lung carcinoma | Increased M1-like macrophage Increased CD8+ T cell infiltration Decreased M2-like macrophage | Complete inhibition of tumor growth |
| [109] | Multipeptide vaccine + XX+ anti-PD-1 | Melanoma | Decreased Treg Increased INF-γ | Complete tumor inhibition |
| [110] | Gemcitabine + anti-PD-1 | Lung cancer | Increased CD8+ T cell Decreased IL-10 Decreased Treg | Milder leucopenia Highest antitumor efficacy |
4. Conclusions, Remarks, and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gasparini, G. Metronomic Scheduling: The Future of Chemotherapy? Lancet Oncol. 2001, 2, 733–740. [Google Scholar] [CrossRef]
- André, N.; Banavali, S.; Snihur, Y.; Pasquier, E. Has the Time Come for Metronomics in Low-Income and Middle-Income Countries? Lancet Oncol. 2013, 14, e239–e248. [Google Scholar] [CrossRef]
- Cazzaniga, M.E.; Cordani, N.; Capici, S.; Cogliati, V.; Riva, F.; Cerrito, M.G. Metronomic Chemotherapy. Cancers 2021, 13, 2236. [Google Scholar] [CrossRef] [PubMed]
- Browder, T.; Butterfield, C.E.; Kräling, B.M.; Shi, B.; Marshall, B.; O’Reilly, M.S.; Folkman, J. Antiangiogenic Scheduling of Chemotherapy Improves Efficacy against Experimental Drug-Resistant Cancer. Cancer Res. 2000, 60, 1878–1886. [Google Scholar]
- Hanahan, D.; Bergers, G.; Bergsland, E. Less Is More, Regularly: Metronomic Dosing of Cytotoxic Drugs Can Target Tumor Angiogenesis in Mice. J. Clin. Investig. 2000, 105, 1045–1047. [Google Scholar] [CrossRef]
- Man, S.; Bocci, G.; Francia, G.; Green, S.K.; Jothy, S.; Hanahan, D.; Bohlen, P.; Hicklin, D.J.; Bergers, G.; Kerbel, R.S. Antitumor Effects in Mice of Low-Dose (Metronomic) Cyclophosphamide Administered Continuously through the Drinking Water. Cancer Res. 2002, 62, 2731–2735. [Google Scholar]
- Kareva, I. A Combination of Immune Checkpoint Inhibition with Metronomic Chemotherapy as a Way of Targeting Therapy-Resistant Cancer Cells. Int. J. Mol. Sci. 2017, 18, 2134. [Google Scholar] [CrossRef] [PubMed]
- Varayathu, H.; Sarathy, V.; Thomas, B.E.; Mufti, S.S.; Naik, R. Combination Strategies to Augment Immune Check Point Inhibitors Efficacy—Implications for Translational Research. Front. Oncol. 2021, 11, 559161. [Google Scholar] [CrossRef] [PubMed]
- Bondarenko, M.; Le Grand, M.; Shaked, Y.; Raviv, Z.; Chapuisat, G.; Carrère, C.; Montero, M.-P.; Rossi, M.; Pasquier, E.; Carré, M.; et al. Metronomic Chemotherapy Modulates Clonal Interactions to Prevent Drug Resistance in Non-Small Cell Lung Cancer. Cancers 2021, 13, 2239. [Google Scholar] [CrossRef]
- Bocci, G.; Pelliccia, S.; Orlandi, P.; Caridi, M.; Banchi, M.; Musuraca, G.; Di Napoli, A.; Bianchi, M.P.; Patti, C.; Anticoli-Borza, P.; et al. Remarkable Remission Rate and Long-Term Efficacy of Upfront Metronomic Chemotherapy in Elderly and Frail Patients, with Diffuse Large B-Cell Lymphoma. J. Clin. Med. 2022, 11, 7162. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Ullah, N.; Nawaz, T.; Aziz, T. Molecular Mechanisms of Sanguinarine in Cancer Prevention and Treatment. Anticancer Agents Med. Chem. 2023, 23, 765–778. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Leong, S.W.; Wang, J.; Wu, Q.; Ghauri, M.A.; Sarwar, A.; Su, Q.; Zhang, Y. Cephalomannine Inhibits Hypoxia-Induced Cellular Function via the Suppression of APEX1/HIF-1α Interaction in Lung Cancer. Cell Death Dis. 2021, 12, 490. [Google Scholar] [CrossRef] [PubMed]
- Su, N.-W.; Wu, S.-H.; Chi, C.-W.; Liu, C.-J.; Tsai, T.-H.; Chen, Y.-J. Metronomic Cordycepin Therapy Prolongs Survival of Oral Cancer-Bearing Mice and Inhibits Epithelial-Mesenchymal Transition. Molecules 2017, 22, 629. [Google Scholar] [CrossRef] [PubMed]
- Nishida, N.; Yano, H.; Nishida, T.; Kamura, T.; Kojiro, M. Angiogenesis in Cancer. Vasc. Health Risk Manag. 2006, 2, 213–219. [Google Scholar] [CrossRef]
- Bergers, G.; Hanahan, D. Modes of Resistance to Anti-Angiogenic Therapy. Nat. Rev. Cancer 2008, 8, 592–603. [Google Scholar] [CrossRef]
- Miller, K.D.; Sweeney, C.J.; Sledge, G.W. Redefining the Target: Chemotherapeutics as Antiangiogenics. J. Clin. Oncol. 2001, 19, 1195–1206. [Google Scholar] [CrossRef]
- Bocci, G.; Nicolaou, K.C.; Kerbel, R.S. Protracted Low-Dose Effects on Human Endothelial Cell Proliferation and Survival in Vitro Reveal a Selective Antiangiogenic Window for Various Chemotherapeutic Drugs. Cancer Res. 2002, 62, 6938–6943. [Google Scholar]
- Klement, G.; Huang, P.; Mayer, B.; Green, S.K.; Man, S.; Bohlen, P.; Hicklin, D.; Kerbel, R.S. Differences in Therapeutic Indexes of Combination Metronomic Chemotherapy and an Anti-VEGFR-2 Antibody in Multidrug-Resistant Human Breast Cancer Xenografts. Clin. Cancer Res. 2002, 8, 221–232. [Google Scholar]
- Wang, J.; Lou, P.; Lesniewski, R.; Henkin, J. Paclitaxel at Ultra Low Concentrations Inhibits Angiogenesis without Affecting Cellular Microtubule Assembly. Anticancer Drugs 2003, 14, 13–19. [Google Scholar] [CrossRef]
- Lawler, J. Thrombospondin-1 as an Endogenous Inhibitor of Angiogenesis and Tumor Growth. J. Cell Mol. Med. 2002, 6, 1–12. [Google Scholar] [CrossRef]
- Beerepoot, L.V.; Mehra, N.; Vermaat, J.S.P.; Zonnenberg, B.A.; Gebbink, M.F.G.B.; Voest, E.E. Increased Levels of Viable Circulating Endothelial Cells Are an Indicator of Progressive Disease in Cancer Patients. Ann. Oncol. 2004, 15, 139–145. [Google Scholar] [CrossRef]
- Shaked, Y.; Bertolini, F.; Emmenegger, U.; Lee, C.R.; Kerbel, R.S. On the Origin and Nature of Elevated Levels of Circulating Endothelial Cells after Treatment with a Vascular Disrupting Agent. J. Clin. Oncol. 2006, 24, 4040. [Google Scholar] [CrossRef]
- Kerbel, R.S.; Benezra, R.; Lyden, D.C.; Hattori, K.; Heissig, B.; Nolan, D.J.; Mittal, V.; Shaked, Y.; Dias, S.; Bertolini, F.; et al. Endothelial Progenitor Cells Are Cellular Hubs Essential for Neoangiogenesis of Certain Aggressive Adenocarcinomas and Metastatic Transition but Not Adenomas. Proc. Natl. Acad. Sci. USA 2008, 105, E54. [Google Scholar] [CrossRef]
- Schito, L.; Rey, S.; Xu, P.; Man, S.; Cruz-Muñoz, W.; Kerbel, R.S. Metronomic Chemotherapy Offsets HIFα Induction upon Maximum-Tolerated Dose in Metastatic Cancers. EMBO Mol. Med. 2020, 12, e11416. [Google Scholar] [CrossRef] [PubMed]
- Albertsson, P.; Lennernäs, B.; Norrby, K. On Metronomic Chemotherapy: Modulation of Angiogenesis Mediated by VEGE-A. Acta Oncol. 2006, 45, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Schito, L.; Semenza, G.L. Hypoxia-Inducible Factors: Master Regulators of Cancer Progression. Trends Cancer 2016, 2, 758–770. [Google Scholar] [CrossRef]
- Merritt, W.M.; Danes, C.G.; Shahzad, M.M.K.; Lin, Y.G.; Kamat, A.A.; Han, L.Y.; Spannuth, W.A.; Nick, A.M.; Mangala, L.S.; Stone, R.L.; et al. Anti-Angiogenic Properties of Metronomic Topotecan in Ovarian Carcinoma. Cancer Biol. Ther. 2009, 8, 1596–1603. [Google Scholar] [CrossRef]
- Natale, G.; Bocci, G. Does Metronomic Chemotherapy Induce Tumor Angiogenic Dormancy? A Review of Available Preclinical and Clinical Data. Cancer Lett. 2018, 432, 28–37. [Google Scholar] [CrossRef]
- Aguirre-Ghiso, J.A. Models, Mechanisms and Clinical Evidence for Cancer Dormancy. Nat. Rev. Cancer 2007, 7, 834–846. [Google Scholar] [CrossRef]
- Endo, H.; Inoue, M. Dormancy in Cancer. Cancer Sci. 2019, 110, 474–480. [Google Scholar] [CrossRef]
- Udagawa, T. Tumor Dormancy of Primary and Secondary Cancers. APMIS 2008, 116, 615–628. [Google Scholar] [CrossRef]
- Moserle, L.; Amadori, A.; Indraccolo, S. The Angiogenic Switch: Implications in the Regulation of Tumor Dormancy. Curr. Mol. Med. 2009, 9, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Humeau, J.; Buqué, A.; Zitvogel, L.; Kroemer, G. Immunostimulation with Chemotherapy in the Era of Immune Checkpoint Inhibitors. Nat. Rev. Clin. Oncol. 2020, 17, 725–741. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular Mechanisms of Cell Death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef]
- Bedognetti, D.; Cesano, A.; Marincola, F.M.; Wang, E. The Biology of Immune-Active Cancers and Their Regulatory Mechanisms. Cancer Treat Res. 2020, 180, 149–172. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Vitale, I.; Warren, S.; Adjemian, S.; Agostinis, P.; Martinez, A.B.; Chan, T.A.; Coukos, G.; Demaria, S.; Deutsch, E.; et al. Consensus Guidelines for the Definition, Detection and Interpretation of Immunogenic Cell Death. J. Immunother. Cancer 2020, 8, e000337. [Google Scholar] [CrossRef]
- Garg, A.D.; Galluzzi, L.; Apetoh, L.; Baert, T.; Birge, R.B.; Bravo-San Pedro, J.M.; Breckpot, K.; Brough, D.; Chaurio, R.; Cirone, M.; et al. Molecular and Translational Classifications of DAMPs in Immunogenic Cell Death. Front. Immunol. 2015, 6, 588. [Google Scholar] [CrossRef]
- Oresta, B.; Pozzi, C.; Braga, D.; Hurle, R.; Lazzeri, M.; Colombo, P.; Frego, N.; Erreni, M.; Faccani, C.; Elefante, G.; et al. Mitochondrial Metabolic Reprogramming Controls the Induction of Immunogenic Cell Death and Efficacy of Chemotherapy in Bladder Cancer. Sci. Transl. Med. 2021, 13, eaba6110. [Google Scholar] [CrossRef]
- Nars, M.S.; Kaneno, R. Immunomodulatory Effects of Low Dose Chemotherapy and Perspectives of Its Combination with Immunotherapy. Int. J. Cancer 2013, 132, 2471–2478. [Google Scholar] [CrossRef]
- Obeid, M.; Tesniere, A.; Ghiringhelli, F.; Fimia, G.M.; Apetoh, L.; Perfettini, J.-L.; Castedo, M.; Mignot, G.; Panaretakis, T.; Casares, N.; et al. Calreticulin Exposure Dictates the Immunogenicity of Cancer Cell Death. Nat. Med. 2007, 13, 54–61. [Google Scholar] [CrossRef]
- Schiavoni, G.; Sistigu, A.; Valentini, M.; Mattei, F.; Sestili, P.; Spadaro, F.; Sanchez, M.; Lorenzi, S.; D’Urso, M.T.; Belardelli, F.; et al. Cyclophosphamide Synergizes with Type I Interferons through Systemic Dendritic Cell Reactivation and Induction of Immunogenic Tumor Apoptosis. Cancer Res. 2011, 71, 768–778. [Google Scholar] [CrossRef] [PubMed]
- Melief, C.J.M. Cancer Immunotherapy by Dendritic Cells. Immunity 2008, 29, 372–383. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.U.; Maharjan, R.; Pangeni, R.; Jha, S.K.; Lee, N.K.; Kweon, S.; Lee, H.K.; Chang, K.-Y.; Choi, Y.K.; Park, J.W.; et al. Modulating Tumor Immunity by Metronomic Dosing of Oxaliplatin Incorporated in Multiple Oral Nanoemulsion. J. Control. Release 2020, 322, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, R.; Assudani, D.; Nagaraj, S.; Hunter, T.; Cho, H.-I.; Antonia, S.; Altiok, S.; Celis, E.; Gabrilovich, D.I. Chemotherapy Enhances Tumor Cell Susceptibility to CTL-Mediated Killing during Cancer Immunotherapy in Mice. J. Clin. Investig. 2010, 120, 1111–1124. [Google Scholar] [CrossRef] [PubMed]
- Okimoto, T.; Kotani, H.; Iida, Y.; Koyanagi, A.; Tanino, R.; Tsubata, Y.; Isobe, T.; Harada, M. Pemetrexed Sensitizes Human Lung Cancer Cells to Cytotoxic Immune Cells. Cancer Sci. 2020, 111, 1910–1920. [Google Scholar] [CrossRef]
- Miyashita, T.; Miki, K.; Kamigaki, T.; Makino, I.; Nakagawara, H.; Tajima, H.; Takamura, H.; Kitagawa, H.; Fushida, S.; Ahmed, A.K.; et al. Low-Dose Gemcitabine Induces Major Histocompatibility Complex Class I-Related Chain A/B Expression and Enhances an Antitumor Innate Immune Response in Pancreatic Cancer. Clin. Exp. Med. 2017, 17, 19–31. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Li, J.; Yin, W.; Song, Z.; Zhang, Z.; Yi, T.; Tang, J.; Wu, D.; Lu, Y.; Wang, Z.; et al. Low-Dose Paclitaxel Enhances the Anti-Tumor Efficacy of GM-CSF Surface-Modified Whole-Tumor-Cell Vaccine in Mouse Model of Prostate Cancer. Cancer Immunol. Immunother. 2011, 60, 715–730. [Google Scholar] [CrossRef]
- Shurin, G.V.; Tourkova, I.L.; Kaneno, R.; Shurin, M.R. Chemotherapeutic Agents in Noncytotoxic Concentrations Increase Antigen Presentation by Dendritic Cells via an IL-12-Dependent Mechanism. J. Immunol. 2009, 183, 137–144. [Google Scholar] [CrossRef]
- Pinzon-Charry, A.; Schmidt, C.W.; López, J.A. The Key Role of CD40 Ligand in Overcoming Tumor-Induced Dendritic Cell Dysfunction. Breast Cancer Res. 2006, 8, 402. [Google Scholar] [CrossRef]
- Tanaka, H.; Matsushima, H.; Mizumoto, N.; Takashima, A. Classification of Chemotherapeutic Agents Based on Their Differential in Vitro Effects on Dendritic Cells. Cancer Res. 2009, 69, 6978–6986. [Google Scholar] [CrossRef]
- Cazzaniga, M.E.; Camerini, A.; Addeo, R.; Nolè, F.; Munzone, E.; Collovà, E.; Del Conte, A.; Mencoboni, M.; Papaldo, P.; Pasini, F.; et al. Metronomic Oral Vinorelbine in Advanced Breast Cancer and Non-Small-Cell Lung Cancer: Current Status and Future Development. Future Oncol. 2016, 12, 373–387. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.; Dong, T.; Yi, T.; Hu, J.; Zhang, Z.; Lin, S.; Niu, W. Impact of 5-Fu/Oxaliplatin on Mouse Dendritic Cells and Synergetic Effect with a Colon Cancer Vaccine. Chin. J. Cancer Res. 2018, 30, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Kaneno, R.; Shurin, G.V.; Tourkova, I.L.; Shurin, M.R. Chemomodulation of Human Dendritic Cell Function by Antineoplastic Agents in Low Noncytotoxic Concentrations. J. Transl. Med. 2009, 7, 58. [Google Scholar] [CrossRef] [PubMed]
- Schaer, D.A.; Geeganage, S.; Amaladas, N.; Lu, Z.H.; Rasmussen, E.R.; Sonyi, A.; Chin, D.; Capen, A.; Li, Y.; Meyer, C.M.; et al. The Folate Pathway Inhibitor Pemetrexed Pleiotropically Enhances Effects of Cancer Immunotherapy. Clin. Cancer Res. 2019, 25, 7175–7188. [Google Scholar] [CrossRef] [PubMed]
- Moschella, F.; Proietti, E.; Capone, I.; Belardelli, F. Combination Strategies for Enhancing the Efficacy of Immunotherapy in Cancer Patients. Ann. N. Y. Acad. Sci. 2010, 1194, 169–178. [Google Scholar] [CrossRef]
- Liu, J.-Y.; Wu, Y.; Zhang, X.-S.; Yang, J.-L.; Li, H.-L.; Mao, Y.-Q.; Wang, Y.; Cheng, X.; Li, Y.-Q.; Xia, J.-C.; et al. Single Administration of Low Dose Cyclophosphamide Augments the Antitumor Effect of Dendritic Cell Vaccine. Cancer Immunol. Immunother. 2007, 56, 1597–1604. [Google Scholar] [CrossRef]
- Wu, J.; Waxman, D.J. Metronomic Cyclophosphamide Eradicates Large Implanted GL261 Gliomas by Activating Antitumor Cd8+ T-Cell Responses and Immune Memory. Oncoimmunology 2015, 4, e1005521. [Google Scholar] [CrossRef] [PubMed]
- Doloff, J.C.; Waxman, D.J. VEGF Receptor Inhibitors Block the Ability of Metronomically Dosed Cyclophosphamide to Activate Innate Immunity-Induced Tumor Regression. Cancer Res. 2012, 72, 1103–1115. [Google Scholar] [CrossRef]
- Michels, T.; Shurin, G.V.; Naiditch, H.; Sevko, A.; Umansky, V.; Shurin, M.R. Paclitaxel Promotes Differentiation of Myeloid-Derived Suppressor Cells into Dendritic Cells in Vitro in a TLR4-Independent Manner. J. Immunotoxicol. 2012, 9, 292–300. [Google Scholar] [CrossRef]
- Suzuki, E.; Kapoor, V.; Jassar, A.S.; Kaiser, L.R.; Albelda, S.M. Gemcitabine Selectively Eliminates Splenic Gr-1+/CD11b+ Myeloid Suppressor Cells in Tumor-Bearing Animals and Enhances Antitumor Immune Activity. Clin. Cancer Res. 2005, 11, 6713–6721. [Google Scholar] [CrossRef] [PubMed]
- Tongu, M.; Harashima, N.; Monma, H.; Inao, T.; Yamada, T.; Kawauchi, H.; Harada, M. Metronomic Chemotherapy with Low-Dose Cyclophosphamide plus Gemcitabine Can Induce Anti-Tumor T Cell Immunity in Vivo. Cancer Immunol. Immunother. 2013, 62, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Peereboom, D.M.; Alban, T.J.; Grabowski, M.M.; Alvarado, A.G.; Otvos, B.; Bayik, D.; Roversi, G.; McGraw, M.; Huang, P.; Mohammadi, A.M.; et al. Metronomic Capecitabine as an Immune Modulator in Glioblastoma Patients Reduces Myeloid-Derived Suppressor Cells. JCI Insight 2019, 4, e130748. [Google Scholar] [CrossRef] [PubMed]
- Lutsiak, M.E.C.; Semnani, R.T.; De Pascalis, R.; Kashmiri, S.V.S.; Schlom, J.; Sabzevari, H. Inhibition of CD4(+)25+ T Regulatory Cell Function Implicated in Enhanced Immune Response by Low-Dose Cyclophosphamide. Blood 2005, 105, 2862–2868. [Google Scholar] [CrossRef]
- Laheurte, C.; Thiery-Vuillemin, A.; Calcagno, F.; Legros, A.; Simonin, H.; Boullerot, L.; Jacquin, M.; Nguyen, T.; Mouillet, G.; Borg, C.; et al. Metronomic Cyclophosphamide Induces Regulatory T Cells Depletion and PSA-Specific T Cells Reactivation in Patients with Biochemical Recurrent Prostate Cancer. Int. J. Cancer 2020, 147, 1199–1205. [Google Scholar] [CrossRef]
- Zhong, H.; Lai, Y.; Zhang, R.; Daoud, A.; Feng, Q.; Zhou, J.; Shang, J. Low Dose Cyclophosphamide Modulates Tumor Microenvironment by TGF-β Signaling Pathway. Int. J. Mol. Sci. 2020, 21, 957. [Google Scholar] [CrossRef] [PubMed]
- Marie, J.C.; Letterio, J.J.; Gavin, M.; Rudensky, A.Y. TGF-Beta1 Maintains Suppressor Function and Foxp3 Expression in CD4+CD25+ Regulatory T Cells. J. Exp. Med. 2005, 201, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Noordam, L.; Kaijen, M.E.H.; Bezemer, K.; Cornelissen, R.; Maat, L.A.P.W.M.; Hoogsteden, H.C.; Aerts, J.G.J.V.; Hendriks, R.W.; Hegmans, J.P.J.J.; Vroman, H. Low-Dose Cyclophosphamide Depletes Circulating Naïve and Activated Regulatory T Cells in Malignant Pleural Mesothelioma Patients Synergistically Treated with Dendritic Cell-Based Immunotherapy. Oncoimmunology 2018, 7, e1474318. [Google Scholar] [CrossRef]
- Ghiringhelli, F.; Menard, C.; Puig, P.E.; Ladoire, S.; Roux, S.; Martin, F.; Solary, E.; Le Cesne, A.; Zitvogel, L.; Chauffert, B. Metronomic Cyclophosphamide Regimen Selectively Depletes CD4+CD25+ Regulatory T Cells and Restores T and NK Effector Functions in End Stage Cancer Patients. Cancer Immunol. Immunother. 2007, 56, 641–648. [Google Scholar] [CrossRef]
- Banissi, C.; Ghiringhelli, F.; Chen, L.; Carpentier, A.F. Treg Depletion with a Low-Dose Metronomic Temozolomide Regimen in a Rat Glioma Model. Cancer Immunol. Immunother. 2009, 58, 1627–1634. [Google Scholar] [CrossRef]
- Shevchenko, I.; Karakhanova, S.; Soltek, S.; Link, J.; Bayry, J.; Werner, J.; Umansky, V.; Bazhin, A.V. Low-Dose Gemcitabine Depletes Regulatory T Cells and Improves Survival in the Orthotopic Panc02 Model of Pancreatic Cancer. Int. J. Cancer 2013, 133, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.-C.; Zhang, X.; Zhong, Z.-Y.; Li, Y.; Feng, L.; Eng, S.; Myles, D.R.; Johnson, R.; Wu, N.; Yin, Y.I.; et al. Therapeutic Effect against Human Xenograft Tumors in Nude Mice by the Third Generation Microtubule Stabilizing Epothilones. Proc. Natl. Acad. Sci. USA 2008, 105, 13157–13162. [Google Scholar] [CrossRef] [PubMed]
- Orlandi, P.; Di Desidero, T.; Salvia, G.; Muscatello, B.; Francia, G.; Bocci, G. Metronomic Vinorelbine Is Directly Active on Non Small Cell Lung Cancer Cells and Sensitizes the EGFRL858R/T790M Cells to Reversible EGFR Tyrosine Kinase Inhibitors. Biochem. Pharmacol. 2018, 152, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Vives, M.; Ginestà, M.M.; Gracova, K.; Graupera, M.; Casanovas, O.; Capellà, G.; Serrano, T.; Laquente, B.; Viñals, F. Metronomic Chemotherapy Following the Maximum Tolerated Dose Is an Effective Anti-Tumour Therapy Affecting Angiogenesis, Tumour Dissemination and Cancer Stem Cells. Int. J. Cancer 2013, 133, 2464–2472. [Google Scholar] [CrossRef]
- Chan, T.-S.; Hsu, C.-C.; Pai, V.C.; Liao, W.-Y.; Huang, S.-S.; Tan, K.-T.; Yen, C.-J.; Hsu, S.-C.; Chen, W.-Y.; Shan, Y.-S.; et al. Metronomic Chemotherapy Prevents Therapy-Induced Stromal Activation and Induction of Tumor-Initiating Cells. J. Exp. Med. 2016, 213, 2967–2988. [Google Scholar] [CrossRef] [PubMed]
- Bruni, E.; Reichle, A.; Scimeca, M.; Bonanno, E.; Ghibelli, L. Lowering Etoposide Doses Shifts Cell Demise From Caspase-Dependent to Differentiation and Caspase-3-Independent Apoptosis via DNA Damage Response, Inducing AML Culture Extinction. Front. Pharmacol. 2018, 9, 1307. [Google Scholar] [CrossRef] [PubMed]
- Taschner-Mandl, S.; Schwarz, M.; Blaha, J.; Kauer, M.; Kromp, F.; Frank, N.; Rifatbegovic, F.; Weiss, T.; Ladenstein, R.; Hohenegger, M.; et al. Metronomic Topotecan Impedes Tumor Growth of MYCN-Amplified Neuroblastoma Cells in Vitro and in Vivo by Therapy Induced Senescence. Oncotarget 2016, 7, 3571–3586. [Google Scholar] [CrossRef]
- Chakrabarty, A.; Chakraborty, S.; Bhattacharya, R.; Chowdhury, G. Senescence-Induced Chemoresistance in Triple Negative Breast Cancer and Evolution-Based Treatment Strategies. Front. Oncol. 2021, 11, 674354. [Google Scholar] [CrossRef]
- Cortes, C.L.; Veiga, S.R.; Almacellas, E.; Hernández-Losa, J.; Ferreres, J.C.; Kozma, S.C.; Ambrosio, S.; Thomas, G.; Tauler, A. Effect of Low Doses of Actinomycin D on Neuroblastoma Cell Lines. Mol. Cancer 2016, 15, 1. [Google Scholar] [CrossRef]
- Schwarze, S.R.; Fu, V.X.; Desotelle, J.A.; Kenowski, M.L.; Jarrard, D.F. The Identification of Senescence-Specific Genes during the Induction of Senescence in Prostate Cancer Cells. Neoplasia 2005, 7, 816–823. [Google Scholar] [CrossRef]
- Fares, C.M.; Van Allen, E.M.; Drake, C.G.; Allison, J.P.; Hu-Lieskovan, S. Mechanisms of Resistance to Immune Checkpoint Blockade: Why Does Checkpoint Inhibitor Immunotherapy Not Work for All Patients? Am. Soc. Clin. Oncol. Educ. Book 2019, 39, 147–164. [Google Scholar] [CrossRef]
- André, N.; Pasquier, E. Response to “Intermittent Androgen Blockade Should Be Regarded as Standard Therapy in Prostate Cancer”. Nat. Clin. Pract. Oncol. 2009, 6, E1. [Google Scholar] [CrossRef] [PubMed]
- Pasquier, E.; Kavallaris, M.; André, N. Metronomic Chemotherapy: New Rationale for New Directions. Nat. Rev. Clin. Oncol. 2010, 7, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Ferdous, T.; Harada, K.; Kin, T.; Harada, T.; Ueyama, Y. Efficacy of Schedule-Dependent Metronomic S-1 Chemotherapy in Human Oral Squamous Cell Carcinoma Cells. Int. J. Oncol. 2013, 43, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Waxman, D.J. Immunogenic Chemotherapy: Dose and Schedule Dependence and Combination with Immunotherapy. Cancer Lett. 2018, 419, 210–221. [Google Scholar] [CrossRef] [PubMed]
- Gerrits, C.J.; de Jonge, M.J.; Schellens, J.H.; Stoter, G.; Verweij, J. Topoisomerase I Inhibitors: The Relevance of Prolonged Exposure for Present Clinical Development. Br. J. Cancer 1997, 76, 952–962. [Google Scholar] [CrossRef]
- Chen, C.-S.; Doloff, J.C.; Waxman, D.J. Intermittent Metronomic Drug Schedule Is Essential for Activating Antitumor Innate Immunity and Tumor Xenograft Regression. Neoplasia 2014, 16, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Parra, K.; Valenzuela, P.; Lerma, N.; Gallegos, A.; Reza, L.C.; Rodriguez, G.; Emmenegger, U.; Di Desidero, T.; Bocci, G.; Felder, M.S.; et al. Impact of CTLA-4 Blockade in Conjunction with Metronomic Chemotherapy on Preclinical Breast Cancer Growth. Br. J. Cancer 2017, 116, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Kerbel, R.S.; Kamen, B.A. The Anti-Angiogenic Basis of Metronomic Chemotherapy. Nat. Rev. Cancer 2004, 4, 423–436. [Google Scholar] [CrossRef]
- Scharovsky, O.G.; Mainetti, L.E.; Rozados, V.R. Metronomic Chemotherapy: Changing the Paradigm That More Is Better. Curr. Oncol. 2009, 16, 7–15. [Google Scholar] [CrossRef]
- Shaked, Y.; Emmenegger, U.; Man, S.; Cervi, D.; Bertolini, F.; Ben-David, Y.; Kerbel, R.S. Optimal Biologic Dose of Metronomic Chemotherapy Regimens Is Associated with Maximum Antiangiogenic Activity. Blood 2005, 106, 3058–3061. [Google Scholar] [CrossRef]
- Zang, Y.; Lee, J.J.; Yuan, Y. Adaptive Designs for Identifying Optimal Biological Dose for Molecularly Targeted Agents. Clin. Trials 2014, 11, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, A.; Patil, V.M. Determining an Optimum Biological Dose of A Metronomic Chemotherapy. J. Data Sci. 2022, 15, 77–94. [Google Scholar] [CrossRef]
- Fraisse, J.; Dinart, D.; Tosi, D.; Bellera, C.; Mollevi, C. Optimal Biological Dose: A Systematic Review in Cancer Phase I Clinical Trials. BMC Cancer 2021, 21, 60. [Google Scholar] [CrossRef] [PubMed]
- Benzekry, S.; Pasquier, E.; Barbolosi, D.; Lacarelle, B.; Barlési, F.; André, N.; Ciccolini, J. Metronomic Reloaded: Theoretical Models Bringing Chemotherapy into the Era of Precision Medicine. Semin. Cancer Biol. 2015, 35, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Faivre, C.; Barbolosi, D.; Pasquier, E.; André, N. A Mathematical Model for the Administration of Temozolomide: Comparative Analysis of Conventional and Metronomic Chemotherapy Regimens. Cancer Chemother. Pharmacol. 2013, 71, 1013–1019. [Google Scholar] [CrossRef] [PubMed]
- Barbolosi, D.; Ciccolini, J.; Meille, C.; Elharrar, X.; Faivre, C.; Lacarelle, B.; André, N.; Barlesi, F. Metronomics Chemotherapy: Time for Computational Decision Support. Cancer Chemother. Pharmacol. 2014, 74, 647–652. [Google Scholar] [CrossRef]
- Braakhuis, B.J.; Ruiz van Haperen, V.W.; Boven, E.; Veerman, G.; Peters, G.J. Schedule-Dependent Antitumor Effect of Gemcitabine in in Vivo Model System. Semin. Oncol. 1995, 22, 42–46. [Google Scholar]
- Ciccolini, J.; Pasquier, E.; Lombard, A.; Giacometti, S.; Faivre, C.; Fanciullino, R.; Serdjebi, C.; Barbolosi, D.; Andre, N. Abstract 4506: Computational-Driven Metronomics: Application to Gemcitabine in Neuroblastoma-Bearing Mice. Cancer Res. 2015, 75, 4506. [Google Scholar] [CrossRef]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef]
- Taube, J.M.; Young, G.D.; McMiller, T.L.; Chen, S.; Salas, J.T.; Pritchard, T.S.; Xu, H.; Meeker, A.K.; Fan, J.; Cheadle, C.; et al. Differential Expression of Immune-Regulatory Genes Associated with PD-L1 Display in Melanoma: Implications for PD-1 Pathway Blockade. Clin. Cancer Res. 2015, 21, 3969–3976. [Google Scholar] [CrossRef]
- Sen, T.; Della Corte, C.M.; Milutinovic, S.; Cardnell, R.J.; Diao, L.; Ramkumar, K.; Gay, C.M.; Stewart, C.A.; Fan, Y.; Shen, L.; et al. Combination Treatment of the Oral CHK1 Inhibitor, SRA737, and Low-Dose Gemcitabine Enhances the Effect of Programmed Death Ligand 1 Blockade by Modulating the Immune Microenvironment in SCLC. J. Thorac. Oncol. 2019, 14, 2152–2163. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.R.; Tykodi, S.S.; Chow, L.Q.M.; Hwu, W.-J.; Topalian, S.L.; Hwu, P.; Drake, C.G.; Camacho, L.H.; Kauh, J.; Odunsi, K.; et al. Safety and Activity of Anti-PD-L1 Antibody in Patients with Advanced Cancer. N. Engl. J. Med. 2012, 366, 2455–2465. [Google Scholar] [CrossRef] [PubMed]
- Cerullo, V.; Diaconu, I.; Kangasniemi, L.; Rajecki, M.; Escutenaire, S.; Koski, A.; Romano, V.; Rouvinen, N.; Tuuminen, T.; Laasonen, L.; et al. Immunological Effects of Low-Dose Cyclophosphamide in Cancer Patients Treated with Oncolytic Adenovirus. Mol. Ther. 2011, 19, 1737–1746. [Google Scholar] [CrossRef] [PubMed]
- Ellebaek, E.; Engell-Noerregaard, L.; Iversen, T.Z.; Froesig, T.M.; Munir, S.; Hadrup, S.R.; Andersen, M.H.; Svane, I.M. Metastatic Melanoma Patients Treated with Dendritic Cell Vaccination, Interleukin-2 and Metronomic Cyclophosphamide: Results from a Phase II Trial. Cancer Immunol. Immunother. 2012, 61, 1791–1804. [Google Scholar] [CrossRef] [PubMed]
- Liikanen, I.; Ahtiainen, L.; Hirvinen, M.L.M.; Bramante, S.; Cerullo, V.; Nokisalmi, P.; Hemminki, O.; Diaconu, I.; Pesonen, S.; Koski, A.; et al. Oncolytic Adenovirus with Temozolomide Induces Autophagy and Antitumor Immune Responses in Cancer Patients. Mol. Ther. 2013, 21, 1212–1223. [Google Scholar] [CrossRef]
- Khan, K.A.; Ponce de Léon, J.L.; Benguigui, M.; Xu, P.; Chow, A.; Cruz-Muñoz, W.; Man, S.; Shaked, Y.; Kerbel, R.S. Immunostimulatory and Anti-Tumor Metronomic Cyclophosphamide Regimens Assessed in Primary Orthotopic and Metastatic Murine Breast Cancer. NPJ Breast Cancer 2020, 6, 29. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Du, Y.; Wang, Z.; Wang, X.; Duan, J.; Wan, R.; Xu, J.; Zhang, P.; Wang, D.; Tian, Y.; et al. Upfront Dose-Reduced Chemotherapy Synergizes with Immunotherapy to Optimize Chemoimmunotherapy in Squamous Cell Lung Carcinoma. J. Immunother. Cancer 2020, 8, e000807. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Xu, Q.; Huang, L.; Jin, J.; Zuo, X.; Zhang, Q.; Ye, L.; Zhu, S.; Zhan, P.; Ren, J.; et al. Low-Dose Carboplatin Reprograms Tumor Immune Microenvironment through STING Signaling Pathway and Synergizes with PD-1 Inhibitors in Lung Cancer. Cancer Lett. 2021, 500, 163–171. [Google Scholar] [CrossRef]
- Petrizzo, A.; Mauriello, A.; Luciano, A.; Rea, D.; Barbieri, A.; Arra, C.; Maiolino, P.; Tornesello, M.; Gigantino, V.; Botti, G.; et al. Inhibition of Tumor Growth by Cancer Vaccine Combined with Metronomic Chemotherapy and Anti-PD-1 in a Pre-Clinical Setting. Oncotarget 2018, 9, 3576–3589. [Google Scholar] [CrossRef]
- Skavatsou, E.; Semitekolou, M.; Morianos, I.; Karampelas, T.; Lougiakis, N.; Xanthou, G.; Tamvakopoulos, C. Immunotherapy Combined with Metronomic Dosing: An Effective Approach for the Treatment of NSCLC. Cancers 2021, 13, 1901. [Google Scholar] [CrossRef]
- Kareva, I.; Waxman, D.J.; Lakka Klement, G. Metronomic Chemotherapy: An Attractive Alternative to Maximum Tolerated Dose Therapy That Can Activate Anti-Tumor Immunity and Minimize Therapeutic Resistance. Cancer Lett. 2015, 358, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Zahreddine, H.; Borden, K.L.B. Mechanisms and Insights into Drug Resistance in Cancer. Front. Pharmacol. 2013, 4, 28. [Google Scholar] [CrossRef] [PubMed]
- Voorwerk, L.; Slagter, M.; Horlings, H.M.; Sikorska, K.; van de Vijver, K.K.; de Maaker, M.; Nederlof, I.; Kluin, R.J.C.; Warren, S.; Ong, S.; et al. Immune Induction Strategies in Metastatic Triple-Negative Breast Cancer to Enhance the Sensitivity to PD-1 Blockade: The TONIC Trial. Nat. Med. 2019, 25, 920–928. [Google Scholar] [CrossRef] [PubMed]
| mCT | ICI | Study Title | Status | Phase | Clinical Trial n° |
|---|---|---|---|---|---|
| Cyclophosphamide | Ipilimumab | A Phase I Clinical Trial of Combined Cryotherapy and Intra-tumoral Immunotherapy with Autologous Immature Dendritic Cells in Men with Castration-Resistant Prostatic Cancer and Metastases to Lymph Nodes and/or Bone Pre- or Post-Chemotherapy | Completed | I | NCT02423928 |
| Cyclophosphamide | Nivolumab/Ipilimumab | Autologous Dendritic Cells and Metronomic Cyclophosphamide in Combination with Checkpoint Blockade for Relapsed High-Grade Gliomas in Children and Adolescents | Recruiting | I | NCT03879512 |
| Vinblastine Cyclophosphamide Capecitabine | Nivolumab | Nivolumab in Combination with Metronomic Chemotherapy in Pediatrics Refractory/Relapsing Solid Tumors | Recruiting | I and II | NCT03585465 |
| Gemcitabine Doxorubicin Docetaxel | Nivolumab | GALLANT: Metronomic Gemcitabine, Doxorubicin, Docetaxel, and Nivolumab for Advanced Sarcoma | Recruiting | II | NCT04535713 |
| Temozolomide | Nivolumab | Temozolomide + Nivolumab in MGMT Methylated Oesophagogastric Cancer (ELEVATE) | Recruiting | II | NCT04984733 |
| Temozolomide | Nivolumab + Ipilimumab | A Longitudinal Assessment of Tumor Evolution in Patients with Brain Cancer. | Recruiting | I | NCT03425292 |
| Cyclophosphamide | Pembrolizumab | Phase 2 Study of an Immune Therapy, DPX-Survivac with Low Dose Cyclophosphamide Administered with Pembrolizumab in Patients with Persistent or Recurrent/Refractory Diffuse Large B-Cell Lymphoma (DLBCL) | Active, not recruiting | II | NCT03349450 |
| Vinorelbine | Atezolizumab | VinMetAtezo Study: Trial to Evaluate Safety and Efficacy of Vinorelbine with Metronomic Administration in Combination with Atezolizumab as Second-line Treatment for Patients with Stage IV NSCLC | Completed | II | NCT03801304 |
| Decitabine | PD-1 Antibody (SHR-1210) | Combined Chemotherapy and PD-1 Antibody (SHR-1210) with or without Low dose Decitabine Priming for Relapsed or Refractory Primary Mediastinal Large B-cell Lymphoma (rrPMBCL): Two Stage, Phase I/II Trial | Unknown | I and II | NCT03346642 |
| Gemcitabine | Nivolumab | Low dose Gemcitabine Combined with Nivolumab for Second-line and Above-line Treatment of Non-small Cell Lung Cancer Metastatic | Not yet recruiting | IV | NCT04331626 |
| Cyclophosphamide | Pembrolizumab | CHEMOIMMUNE Study: Evaluation of Pembrolizumab in Lymphopenic Metastatic Breast Cancer Patients Treated with Metronomic Cyclophosphamide (Safety Run-in Phase) | Completed | II | EudraCT n. 2016-002736-33 |
| Cyclophosphamide | Avelumab | CONFRONT Phase I-II Trial: Multimodality Immunotherapy with Avelumab, Short-Course Radiotherapy, and Cyclophosphamide in Patients with Relapsed/metastatic Head and Neck Cancer | Ongoing | I and II | EudraCT n. 2017-000353-39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muraro, E.; Vinante, L.; Fratta, E.; Bearz, A.; Höfler, D.; Steffan, A.; Baboci, L. Metronomic Chemotherapy: Anti-Tumor Pathways and Combination with Immune Checkpoint Inhibitors. Cancers 2023, 15, 2471. https://doi.org/10.3390/cancers15092471
Muraro E, Vinante L, Fratta E, Bearz A, Höfler D, Steffan A, Baboci L. Metronomic Chemotherapy: Anti-Tumor Pathways and Combination with Immune Checkpoint Inhibitors. Cancers. 2023; 15(9):2471. https://doi.org/10.3390/cancers15092471
Chicago/Turabian StyleMuraro, Elena, Lorenzo Vinante, Elisabetta Fratta, Alessandra Bearz, Daniela Höfler, Agostino Steffan, and Lorena Baboci. 2023. "Metronomic Chemotherapy: Anti-Tumor Pathways and Combination with Immune Checkpoint Inhibitors" Cancers 15, no. 9: 2471. https://doi.org/10.3390/cancers15092471
APA StyleMuraro, E., Vinante, L., Fratta, E., Bearz, A., Höfler, D., Steffan, A., & Baboci, L. (2023). Metronomic Chemotherapy: Anti-Tumor Pathways and Combination with Immune Checkpoint Inhibitors. Cancers, 15(9), 2471. https://doi.org/10.3390/cancers15092471






