Favorable Effect of High-Density Lipoprotein Cholesterol on Gastric Cancer Mortality by Sex and Treatment Modality
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Data Extraction and Content
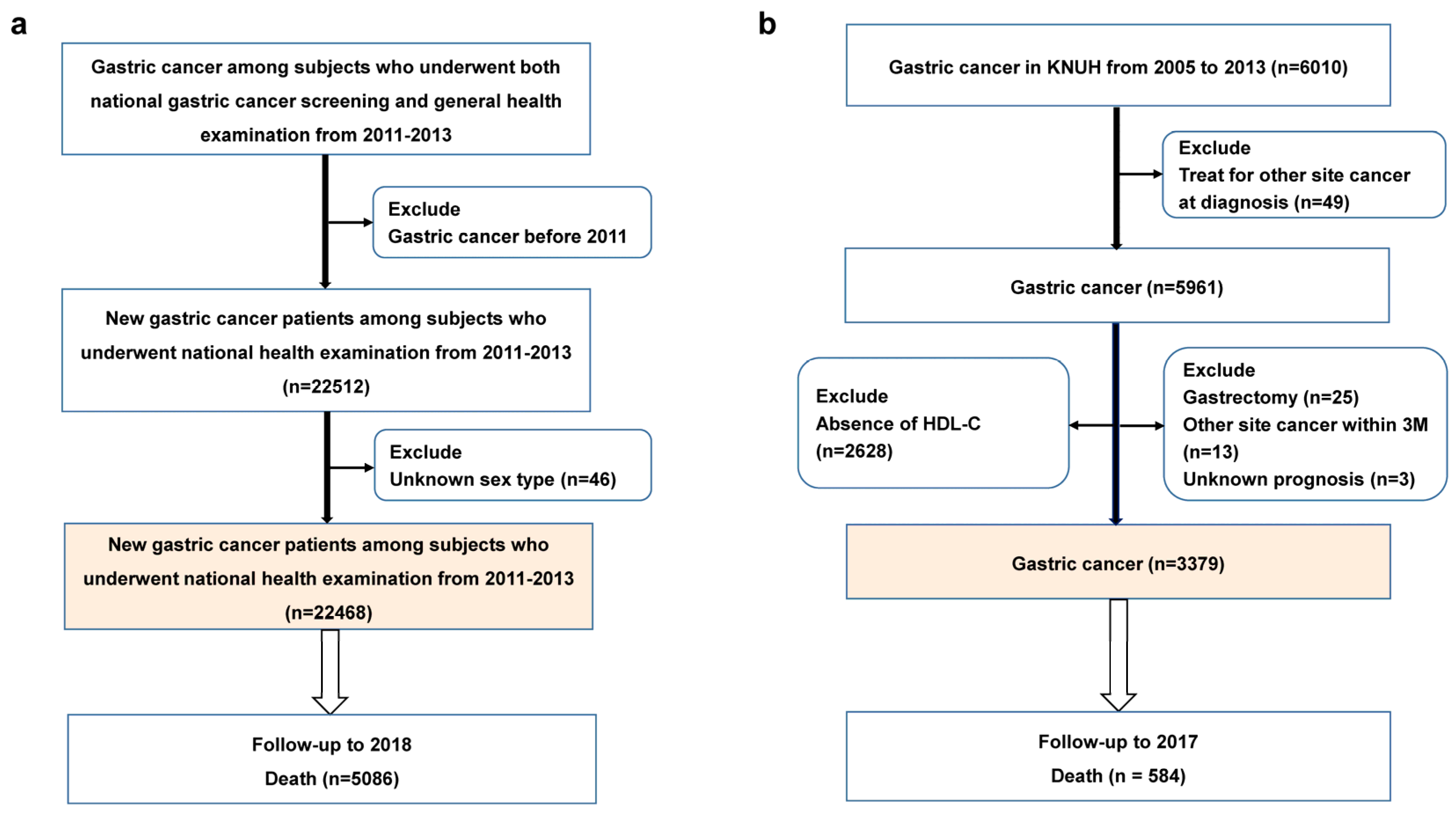
2.2. Baseline Enrollment and Follow-Up in the NHISS Cohort
2.3. Study Population and Follow-Up in the Validation Cohort
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Patients
3.2. Gastric Cancer Mortality According to HDL-C Levels in the NHISS Cohort
3.3. Gastric Cancer Mortality According to HDL-C Levels in the KNUH Cohort
3.4. Sensitivity Analysis by Different HDL-C Levels
3.5. Sub-Group Analysis by Treatment Method
3.6. Sub-Group Analysis in Stage I
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Di Angelantonio, E.; Sarwar, N.; Perry, P.; Kaptoge, S.; Ray, K.K.; Thompson, A.; Wood, A.M.; Lewington, S.; Sattar, N.; Packard, C.J.; et al. Major lipids, apolipoproteins, and risk of vascular disease. JAMA 2009, 302, 1993–2000. [Google Scholar]
- Wilson, P.W.; Abbott, R.D.; Castelli, W.P. High density lipoprotein cholesterol and mortality. The Framingham Heart Study. Arteriosclerosis 1988, 8, 737–741. [Google Scholar] [CrossRef]
- Catapano, A.L.; Pirillo, A.; Bonacina, F.; Norata, G.D. HDL in innate and adaptive immunity. Cardiovasc. Res. 2014, 103, 372–383. [Google Scholar] [CrossRef]
- Lupattelli, G.; Marchesi, S.; Lombardini, R.; Siepi, D.; Bagaglia, F.; Pirro, M.; Ciuffetti, G.; Schillaci, G.; Mannarino, E. Mechanisms of high-density lipoprotein cholesterol effects on the endothelial function in hyperlipemia. Metabolism 2003, 52, 1191–1195. [Google Scholar] [CrossRef]
- Katzke, V.A.; Sookthai, D.; Johnson, T.; Kühn, T.; Kaaks, R. Blood lipids and lipoproteins in relation to incidence and mortality risks for CVD and cancer in the prospective EPIC-Heidelberg cohort. BMC Med. 2017, 15, 218. [Google Scholar] [CrossRef]
- Cedó, L.; Reddy, S.T.; Mato, E.; Blanco-Vaca, F.; Escolà-Gil, J.C. HDL and LDL: Potential New Players in Breast Cancer Development. J. Clin. Med. 2019, 8, 853. [Google Scholar] [CrossRef]
- Nam, S.Y.; Park, B.J.; Nam, J.H.; Kook, M.C. Effect of Helicobacter pylori eradication and high-density lipoprotein on the risk of de novo gastric cancer development. Gastrointest. Endosc. 2019, 90, 448–456. [Google Scholar] [CrossRef]
- Yang, Y.; Han, K.; Park, S.H.; Kim, M.K.; Yoon, K.H.; Lee, S.H. High-Density Lipoprotein Cholesterol and the Risk of Myocardial Infarction, Stroke, and Cause-Specific Mortality: A Nationwide Cohort Study in Korea. J. Lipid Atheroscler. 2021, 10, 74–87. [Google Scholar] [CrossRef]
- Nishiyama, H.; Funamizu, T.; Iwata, H.; Endo, H.; Chikata, Y.; Doi, S.; Wada, H.; Naito, R.; Ogita, M.; Kato, Y.; et al. Low apolipoprotein A1 was associated with increased risk of cancer mortality in patients following percutaneous coronary intervention: A 10-year follow-up study. Int. J. Cancer 2022, 151, 1482–1490. [Google Scholar] [CrossRef]
- Zhong, G.C.; Huang, S.Q.; Peng, Y.; Wan, L.; Wu, Y.Q.L.; Hu, T.Y.; Hu, J.-J.; Hao, F.B. HDL-C is associated with mortality from all causes, cardiovascular disease and cancer in a J-shaped dose-response fashion: A pooled analysis of 37 prospective cohort studies. Eur. J. Prev. Cardiol. 2020, 27, 1187–1203. [Google Scholar] [CrossRef]
- Tamura, T.; Inagawa, S.; Hisakura, K.; Enomoto, T.; Ohkohchi, N. Evaluation of serum high-density lipoprotein cholesterol levels as a prognostic factor in gastric cancer patients. J. Gastroenterol. Hepatol. 2012, 27, 1635–1640. [Google Scholar] [CrossRef]
- Shen, J.G.; Jin, L.D.; Dong, M.J.; Wang, L.B.; Zhao, W.H.; Shen, J. Low level of serum high-density lipoprotein cholesterol in gastric cancer correlates with cancer progression but not survival. Transl. Cancer Res. 2020, 9, 6206–6213. [Google Scholar] [CrossRef]
- Bhaskaran, K.; Douglas, I.; Forbes, H.; dos-Santos-Silva, I.; Leon, D.A.; Smeeth, L. Body-mass index and risk of 22 specific cancers: A population-based cohort study of 5·24 million UK adults. Lancet 2014, 384, 755–765. [Google Scholar] [CrossRef]
- Nam, S.Y.; Jeon, S.W.; Kwon, Y.H.; Kwon, O.K. Sex difference of mortality by age and body mass index in gastric cancer. Dig. Liver Dis. 2021, 53, 1185–1191. [Google Scholar] [CrossRef]
- Nam, S.Y.; Jeong, J.; Lee, W.K.; Jeon, S.W. Sex-specific effect of body mass index and fasting glucose on gastric cancer risk and all causes mortality; a cohort study of 5.17 million. Int. J. Obes. 2022, 46, 1644–1651. [Google Scholar] [CrossRef]
- Kim, S.Y.; Song, Y.S.; Wee, J.H.; Min, C.; Yoo, D.M.; Lee, C.H.; Song, C.M.; Park, B.J.; Choi, H.G. Evaluation of the relationship between previous statin use and thyroid cancer using Korean National Health Insurance Service-Health Screening Cohort data. Sci. Rep. 2021, 11, 7912. [Google Scholar] [CrossRef]
- Chung, H.S.; Lee, J.S.; Song, E.; Kim, J.A.; Roh, E.; Yu, J.H.; Kim, N.H.; Yoo, H.J.; Seo, J.A.; Kim, S.G.; et al. Effect of Metabolic Health and Obesity Phenotype on the Risk of Pancreatic Cancer: A Nationwide Population-Based Cohort Study. Cancer Epidemiol. Biomark. Prev. 2021, 30, 521–528. [Google Scholar] [CrossRef]
- Pan, W.H.; Yeh, W.T. How to define obesity? Evidence-based multiple action points for public awareness, screening, and treatment: An extension of Asian-Pacific recommendations. Asia Pac. J. Clin. Nutr. 2008, 17, 370–374. [Google Scholar]
- Kim, Y.; Jun, J.K.; Choi, K.S.; Lee, H.Y.; Park, E.C. Overview of the National Cancer screening programme and the cancer screening status in Korea. Asian Pac. J. Cancer Prev. 2011, 12, 725–730. [Google Scholar]
- Washington, K. 7th edition of the AJCC cancer staging manual: Stomach. Ann. Surg. Oncol. 2010, 17, 3077–3079. [Google Scholar] [CrossRef]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [CrossRef]
- Li, C.; Fu, Y.; Li, Q.; Yang, X.; Wang, W.; Jin, X.; Bian, L.; Zhao, H.; Li, D.; Gao, J.; et al. Postoperative high-density lipoprotein cholesterol level: An independent prognostic factor for gastric cancer. Front. Oncol. 2022, 12, 884371. [Google Scholar] [CrossRef]
- Hong, J.S.; Yi, S.W.; Yi, J.J.; Hong, S.; Ohrr, H. Body Mass Index and Cancer Mortality Among Korean Older Middle-Aged Men: A Prospective Cohort Study. Medicine 2016, 95, e3684. [Google Scholar] [CrossRef]
- Reeves, G.K.; Pirie, K.; Beral, V.; Green, J.; Spencer, E.; Bull, D. Cancer incidence and mortality in relation to body mass index in the Million Women Study: Cohort study. BMJ 2007, 335, 1134. [Google Scholar] [CrossRef]
- Schlansky, B.; Sonnenberg, A. Epidemiology of noncardia gastric adenocarcinoma in the United States. Am. J. Gastroenterol. 2011, 106, 1978–1985. [Google Scholar] [CrossRef]
- Yu, X.Q.; O’Connell, D.L.; Gibberd, R.W.; Armstrong, B.K. Assessing the impact of socio-economic status on cancer survival in New South Wales, Australia 1996–2001. Cancer Causes Control 2008, 19, 1383–1390. [Google Scholar] [CrossRef]
- Tian, T.; Zhang, L.Q.; Ma, X.H.; Zhou, J.N.; Shen, J. Diabetes mellitus and incidence and mortality of gastric cancer: A meta-analysis. Exp. Clin. Endocrinol. Diabetes 2012, 120, 217–223. [Google Scholar] [CrossRef]
- Li, T.; Wei, S.; Shi, Y.; Pang, S.; Qin, Q.; Yin, J.; Deng, Y.; Chen, Q.; Wei, S.; Nie, S.; et al. The dose-response effect of physical activity on cancer mortality: Findings from 71 prospective cohort studies. Br. J. Sports Med. 2016, 50, 339–345. [Google Scholar] [CrossRef]
- Oh, M.G.; Kim, J.H.; Han, M.A.; Park, J.; Ryu, S.Y.; Choi, S.W. Family history and survival of patients with gastric cancer: A meta-analysis. Asian Pac. J. Cancer Prev. 2014, 15, 3465–3470. [Google Scholar] [CrossRef]
- Yang, P.R.; Tsai, Y.Y.; Chen, K.J.; Yang, Y.H.; Shih, W.T. Statin Use Improves Overall Survival of Patients with Gastric Cancer after Surgery and Adjuvant Chemotherapy in Taiwan: A Nationwide Matched Cohort Study. Cancers 2020, 12, 2055. [Google Scholar] [CrossRef]
- Spence, A.D.; Busby, J.; Hughes, C.M.; Johnston, B.T.; Coleman, H.G.; Cardwell, C.R. Statin use and survival in patients with gastric cancer in two independent population-based cohorts. Pharmacoepidemiol. Drug. Saf. 2019, 28, 460–470. [Google Scholar] [CrossRef]
- Lou, D.; Fu, R.; Gu, L.; Su, H.; Guan, L. Association between statins’ exposure with incidence and prognosis of gastric cancer: An updated meta-analysis. Expert Rev. Clin. Pharmacol. 2022, 15, 1127–1138. [Google Scholar] [CrossRef]
- Yuan, M.; Han, S.; Jia, Y.; Feng, J.; Liu, D.; Su, Z.; Liu, X. Statins Are Associated with Improved Survival of Patients with Gastric Cancer: A Systematic Review and Meta-Analysis. Int. J. Clin. Pract. 2022, 17, 4938539. [Google Scholar] [CrossRef]
- Kwon, M.J.; Kang, H.S.; Kim, J.H.; Kim, S.H.; Kim, N.Y.; Nam, E.S.; Min, K.W.; Choi, H.G. Association between Statin Use and Gastric Cancer: A Nested Case-Control Study Using a National Health Screening Cohort in Korea. Pharmaceuticals 2021, 14, 1283. [Google Scholar] [CrossRef]
| HDL-C, mg/dL | |||||
|---|---|---|---|---|---|
| <40 (n = 3562) | 40–49 (n = 6970) | 50–59 (n = 6133) | 60–69 (n = 3552) | ≥70 (n = 2251) | |
| Person years | 22,382.0 | 45,223.1 | 40,267.9 | 23,513.6 | 14,958.1 |
| Men, no (%) | 2840 (80.0) | 5050 (72.7) | 3920 (64.2) | 2130 (60.2) | 1280 (57.2) |
| Age, mean (SD) | 65.9 (9.8) | 64.8 (9.7) | 64.5 (9.8) | 64.1 (10.0) | 63.5 (10.1) |
| Economic status, mean (SD) † | 12.3 (5.8) | 12.4 (5.8) | 12.2 (5.9) | 12.1 (5.8) | 12.0 (5.8) |
| Incomes, median (IQR) † | 14 (8, 17) | 14 (8, 17) | 13 (7, 17) | 13 (7, 17) | 13 (7, 17) |
| HDL-C, mg/dL, median (IQR) | 36 (32,38) | 45 (42, 47) | 54 (52, 56) | 64 (61, 66) | 76 (73, 83) |
| BMI, kg/m2, median (IQR) | 25 (23, 26) | 24 (22, 26) | 24 (22, 26) | 23 (21, 25) | 23 (21, 25) |
| BMI, kg/m2, no (%) | |||||
| <18.5 | 50 (1.6) | 160 (2.3) | 200 (3.4) | 140 (4.0) | 130 (6.1) |
| 18.5–22.4 | 970 (27.4) | 2190 (31.5) | 2290 (37.5) | 1500 (42.5) | 1120 (50.0) |
| 22.5–24.9 | 990 (27.9) | 1910 (27.5) | 1620 (26.5) | 920 (26.1) | 490 (22.0) |
| 25–29.9 | 1400 (39.5) | 2460 (35.4) | 1830 (30.0) | 890 (25.3) | 450 (20.2) |
| ≥30 | 130 (3.7) | 220 (3.2) | 160 (2.6) | 70 (2.1) | 30 (1.6) |
| Hypertension, no (%) | 1600 (45.1) | 2950 (42.4) | 2430 (39.8) | 1320 (37.3) | 790 (35.4) |
| Heart disease, no (%) | 210 (6.0) | 360 (5.2) | 270 (4.4) | 140 (4.0) | 70 (3.3) |
| Diabetes mellitus, no (%) | 880 (24.8) | 1290 (18.6) | 850 (13.9) | 420 (11.9) | 220 (10.2) |
| Cerebrovascular disease, no (%) | 100 (2.9) | 160 (2.3) | 120 (2.0) | 50 (1.7) | 20 (1.2) |
| Lipid-lowering drug, no (%) | 110 (3.2) | 250 (3.6) | 260 (4.4) | 150 (4.3) | 90 (4.3) |
| Smoking status, no (%) | |||||
| Never | 1590 (44.8) | 3400 (48.9) | 3340 (54.5) | 2070 (58.4) | 1360 (60.4) |
| Past | 990 (28.0) | 1920 (27.7) | 1530 (25.0) | 840 (23.7) | 430 (19.5) |
| Current | 970 (27.3) | 1620 (23.4) | 1250 (20.4) | 630 (17.9) | 450 (20.1) |
| Drinking frequency, no (%) | |||||
| None | 2280 (64.3) | 4120 (29.3) | 3550 (57.9) | 1980 (55.8) | 1110 (49.7) |
| 1/week | 750 (21.3) | 1610 (23.2) | 1330 (21.9) | 750 (21.2) | 460 (20.4) |
| 2–3/week | 290 (8.4) | 690 (10.0) | 690 (11.4) | 440 (12.4) | 300 (13.7) |
| 4–5/week | 100 (3.0) | 270 (4.0) | 260 (4.3) | 200 (5.7) | 170 (7.8) |
| ≥6/week | 110 (3.1) | 240 (3.5) | 280 (4.6) | 170 (4.9) | 180 (8.4) |
| Family History of GC, no (%) | 400 (11.5) | 870 (12.6) | 740 (12.3) | 490 (13.9) | 310 (14.0) |
| Moderate activity, no (%) ‡ | |||||
| None | 2240 (63.2) | 4310 (61.9) | 3730 (60.9) | 2170 (61.3) | 1340 (59.6) |
| 1–2 days/week | 560 (15.8) | 1170 (16.8) | 1010 (16.5) | 580 (16.4) | 380 (17.2) |
| 3–5 days/week | 510 (14.4) | 990 (14.3) | 970 (15.9) | 550 (15.5) | 350 (15.6) |
| 6–7 days/week | 230 (6.6) | 480 (6.9) | 400 (6.7) | 240 (6.9) | 170 (7.6) |
| Total * | Men ** | Women † | ||||
|---|---|---|---|---|---|---|
| aHR (95% CI) | p-Value | aHR (95% CI) | p-Value | aHR (95% CI) | p-Value | |
| HDL-C, mg/dL | ||||||
| <40 | 1 | 1 | 1 | |||
| 40–49 | 0.90 (0.83–0.98) | 0.011 | 0.91 (0.83–0.99) | 0.035 | 0.86 (0.72–1.03) | 0.109 |
| 50–59 | 0.86 (0.79–0.93) | 0.001 | 0.90 (0.81–0.99) | 0.030 | 0.74 (0.62–0.89) | 0.002 |
| 60–69 | 0.82 (0.74–0.90) | <0.001 | 0.80 (0.71–0.90) | 0.001 | 0.81 (0.66–0.99) | 0.042 |
| ≥70 | 0.78 (0.69–0.87) | <0.001 | 0.80 (0.70–0.92) | 0.001 | 0.69 (0.55–0.87) | 0.002 |
| Women | 0.73 (0.68–0.79) | <0.001 | ||||
| Age, yr | 1.07 (1.07–1.07) | <0.001 | 1.08 (1.07–1.08) | <0.001 | 1.07 (1.06–1.08) | <0.001 |
| Incomes | 0.99 (0.98–0.99) | <0.001 | 0.98 (0.98–0.99) | <0.001 | 0.99 (0.98–1.00) | 0.023 |
| BMI, kg/m2 | ||||||
| <18.5 | 2.44 (2.14–2.78) | <0.001 | 2.55 (2.20–2.96) | <0.001 | 2.12 (1.61–2.78) | <0.001 |
| 18.5–22.4 | 1.48 (1.37–1.59) | <0.001 | 1.47 (1.34–1.60) | <0.001 | 1.45 (1.26–1.67) | <0.001 |
| 22.5–24.9 | 1.17 (1.08–1.26) | 0.001 | 1.18 (1.07–1.30) | 0.001 | 1.12 (0.95–1.31) | 0.177 |
| 25–29.9 | 1 | 1 | 1 | |||
| ≥30 | 1.07 (0.87–1.30) | 0.540 | 1.10 (0.84–1.43) | 0.499 | 1.04 (0.76–1.42) | 0.808 |
| Hypertension | 0.92 (0.87–0.98) | 0.009 | 0.91 (0.85–0.98) | 0.008 | 0.98 (0.86–1.10) | 0.685 |
| Heart disease | 1.08 (0.96–1.21) | 0.197 | 1.01 (0.88–1.16) | 0.844 | 1.24 (1.00–1.54) | 0.048 |
| Diabetes | 1.21 (1.13–1.30) | <0.001 | 1.22 (1.13–1.33) | <0.001 | 1.20 (1.04–1.39) | 0.014 |
| Stroke | 1.15 (0.98–1.34) | 0.091 | 1.21 (1.01–1.43) | 0.037 | 0.94 (0.65–1.35) | 0.724 |
| Moderate activity | ||||||
| None | 1 | 1 | 1 | |||
| 1–2 days/week | 0.88 (0.81–0.96) | 0.004 | 0.91 (0.83–1.00) | 0.053 | 0.81 (0.68–0.97) | 0.019 |
| 3–5 days/week | 0.75 (0.69–0.83) | <0.001 | 0.76 (0.68–0.84) | <0.001 | 0.76 (0.62–0.93) | 0.007 |
| 6–7 days/week | 0.80 (0.71–0.89) | 0.001 | 0.83 (0.73–0.94) | 0.003 | 0.66 (0.49–0.90) | 0.009 |
| Smoking status | ||||||
| Never | 1 | 1 | 1 | 1 | ||
| Past | 0.97 (0.90–1.05) | 0.451 | 0.98 (0.91–1.07) | 0.691 | 0.82 (0.49–1.38) | 0.459 |
| Current | 1.12 (1.03–1.21) | 0.008 | 1.14 (1.05–1.25) | 0.002 | 1.06 (0.73–1.54) | 0.752 |
| Drinking frequency | ||||||
| None | 1 | 1 | 1 | |||
| 1/week | 0.87 (0.80–0.94) | 0.001 | 0.86 (0.79–0.93) | 0.001 | 1.00 (0.80–1.25) | 0.994 |
| 2–3/week | 0.80 (0.72–0.89) | <0.001 | 0.82 (0.73–0.91) | 0.001 | 0.55 (0.29–1.04) | 0.065 |
| 4–5/week | 1.02 (0.89–1.16) | 0.798 | 1.02 (0.89–1.17) | 0.827 | 1.26 (0.52–3.04) | 0.615 |
| ≥6/week | 1.02 (0.90–1.15) | 0.782 | 1.03 (0.90–1.17) | 0.704 | 0.18 (0.03–1.24) | 0.082 |
| Family History of GC | 0.82 (0.74–0.90) | <0.001 | 0.83 (0.74–0.92) | 0.001 | 0.81 (0.67–0.97) | 0.021 |
| Lipid-lowering drug | 0.79 (0.67–0.93) | 0.004 | 0.88 (0.72–1.07) | 0.196 | 0.68 (0.51–0.92) | 0.013 |
| Unadjusted Analysis | Adjusted Analysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Total (n = 3379) | Men (n = 2252) | Women (n = 1126) | ||||||
| Event/Total No | HR (95% CI) | p | aHR (95% CI) | p * | aHR (95% CI) | p † | aHR (95% CI) | p † | |
| HDL-C, mg/dL | |||||||||
| <40 | 235/1006 | 1 | 1 | 1 | 1 | ||||
| 40–49 | 170/1001 | 0.67 (0.55–0.81) | <0.001 | 0.86 (0.69–1.07) | 0.185 | 0.91 (0.71–1.17) | 0.461 | 0.84 (0.52–1.36) | 0.477 |
| 50–59 | 109/719 | 0.58 (0.46–0.72) | <0.001 | 0.69 (0.54–0.89) | 0.004 | 0.72 (0.54–0.96) | 0.024 | 0.55 (0.33–0.94) | 0.027 |
| 60–69 | 44/359 | 0.44 (0.32–0.61) | <0.001 | 0.54 (0.38–0.77) | 0.0006 | 0.54 (0.35–0.83) | 0.005 | 0.52 (0.28–0.95) | 0.035 |
| ≥70 | 26/294 | 0.30 (0.20–0.45) | <0.001 | 0.42 (0.27–0.66) | 0.0002 | 0.42 (0.24–0.75) | 0.003 | 0.40 (0.18–0.87) | 0.021 |
| BMI, kg/m2 | |||||||||
| <18.5 | 59/138 | 4.11 (3.00–5.58) | <0.001 | 2.39 (1.73–3.29) | <0.001 | 2.45 (1.67–3.56) | <0.001 | 1.95 (1.02–3.78) | 0.046 |
| 18.5–22.9 | 233/1276 | 1.42 (1.15–1.76) | 0.001 | 1.35 (1.09–1.69) | 0.006 | 1.36 (1.06–1.77) | 0.016 | 1.22 (0.77–1.95) | 0.397 |
| 23–24.9 | 140/898 | 1.16 (0.91–1.47) | 0.224 | 1.16 (0.91–1.47) | 0.233 | 1.24 (0.94–1.63) | 0.133 | 1 | |
| 25–29.9 | 132/968 | 1 | 1 | 1 | 1.05 (0.63–1.74) | 0.864 | |||
| ≥30 | 13/69 | 1.48 (0.84–2.61) | 0.179 | 1.74 (0.98–3.08) | 0.059 | 2.53 (1.31–4.88) | 0.006 | 0.96 (0.29–3.21) | 0.944 |
| Unadjusted | Adjusted | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Event/Total | Total | Men | Women | ||||||
| NHISS Cohort | aHR (95% CI) | p-Value | aHR (95% CI) | p-Value * | aHR (95% CI) | p-Value ** | aHR (95% CI) | p-Value † | |
| HDL-C by ATP III | |||||||||
| Low | 1337/5457 | 1 | 1 | 1 | 1 | ||||
| Normal | 3745/16965 | 0.88 (0.83–0.94) | <0.001 | 0.86 (0.81–0.92) | <0.001 | 0.88 (0.81–0.95) | 0.002 | 0.84 (0.74–0.95) | 0.004 |
| HDL-C by NECP | |||||||||
| Low | 1338/5462 | 1 | 1 | 1 | 1 | ||||
| Intermediate | 2563/11174 | 0.92 (0.86–0.99) | 0.018 | 0.88 (0.83–0.95) | 0.001 | 0.90 (0.83–0.98) | 0.017 | 0.80 (0.69–0.93) | 0.004 |
| High (≥60) | 1184/5803 | 0.81 (0.75–0.88) | <0.001 | 0.82 (0.75–0.89) | <0.001 | 0.80 (0.72–0.89) | <0.001 | 0.87 (0.75–1.01) | 0.068 |
| HDL-C by 4 groups | |||||||||
| Very low (<30) | 132/377 | 1 | 1 | 1 | 1 | ||||
| Low | 1206/5082 | 0.62 (0.51–0.74) | <0.001 | 0.68 (0.57–0.82) | <0.001 | 0.68 (0.55–0.84) | 0.001 | 0.65 (0.42–1.00) | 0.051 |
| Intermediate | 2563/11174 | 0.59 (0.49–0.71) | <0.001 | 0.62 (0.52–0.75) | <0.001 | 0.65 (0.53–0.79) | <0.001 | 0.53 (0.34–0.82) | 0.005 |
| High (≥60) | 1184/5803 | 0.52 (0.43–0.62) | <0.001 | 0.58 (0.48–0.69) | <0.001 | 0.57 (0.46–0.70) | <0.001 | 0.58 (0.37–0.89) | 0.014 |
| KNUH cohort | Event/total | aHR (95% CI) | p-value | aHR (95% CI) | p-value § | aHR (95% CI) | p-value € | aHR (95% CI) | p-value € |
| HDL-C by ATP III | |||||||||
| Low | 282/1326 | 1 | 1 | 1 | 1 | ||||
| Normal | 302/2053 | 0.62 (0.53–0.73) | <0.001 | 0.72 (0.6–0.86) | <0.001 | 0.75 (0.6–0.92) | 0.007 | 0.56 (0.39–0.83) | 0.003 |
| HDL-C by NECP | |||||||||
| Low | 282/1326 | 1 | 1 | 1 | 1 | ||||
| Intermediate | 232/1400 | 0.72 (0.61–0.86) | <0.001 | 0.81 (0.67–0.99) | 0.035 | 0.82 (0.66–1.03) | 0.088 | 0.61 (0.38–0.97) | 0.035 |
| High (≥60) | 70/653 | 0.42 (0.33–0.55) | <0.001 | 0.52 (0.39–0.69) | <0.001 | 0.49 (0.34–0.71) | <0.001 | 0.52 (0.33–0.84) | 0.007 |
| HDL-C by 4 groups | |||||||||
| Very low (<30) | 82/244 | 1 | 1 | 1 | 1 | ||||
| Low | 200/1082 | 0.52 (0.4–0.67) | <0.001 | 1.04 (0.78–1.4) | 0.784 | 1.38 (0.97–1.96) | 0.072 | 0.66 (0.37–1.18) | 0.163 |
| Intermediate | 232/1400 | 0.44 (0.34–0.56) | <0.001 | 0.83 (0.63–1.11) | 0.208 | 1.02 (0.74–1.42) | 0.901 | 0.44 (0.24–0.83) | 0.011 |
| High (≥60) | 70/653 | 0.26 (0.19–0.35) | <0.001 | 0.53 (0.37–0.76) | <0.001 | 0.61 (0.39–0.94) | 0.025 | 0.37 (0.19–0.72) | 0.003 |
| KNUH | ||||||
|---|---|---|---|---|---|---|
| Gastrectomy (n = 2358) | ESD (n = 922) | |||||
| HR (95% CI) * | p-Value | p for Trend | HR (95% CI) * | p-Value | p for Trend | |
| HDL-C by ATP III | ||||||
| Low | 1 | 1 | ||||
| Normal | 0.63 (0.51–0.78) | <0.001 | 0.59 (0.39–0.88) | 0.011 | ||
| HDL-C by 4 groups | ||||||
| Very low (<30) | 1 | 1 | ||||
| Low | 0.90 (0.64–1.27) | 0.547 | 0.68 (0.36–1.30) | 0.241 | ||
| Intermediate | 0.63 (0.46–0.87) | 0.004 | 0.48 (0.24–0.94) | 0.031 | ||
| High (≥60) | 0.44 (0.30–0.64) | <0.001 | <0.001 | 0.06 (0.01–0.50) | 0.009 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nam, S.Y.; Jeon, S.W.; Jo, J.; Kwon, O.K. Favorable Effect of High-Density Lipoprotein Cholesterol on Gastric Cancer Mortality by Sex and Treatment Modality. Cancers 2023, 15, 2463. https://doi.org/10.3390/cancers15092463
Nam SY, Jeon SW, Jo J, Kwon OK. Favorable Effect of High-Density Lipoprotein Cholesterol on Gastric Cancer Mortality by Sex and Treatment Modality. Cancers. 2023; 15(9):2463. https://doi.org/10.3390/cancers15092463
Chicago/Turabian StyleNam, Su Youn, Seong Woo Jeon, Junwoo Jo, and Oh Kyoung Kwon. 2023. "Favorable Effect of High-Density Lipoprotein Cholesterol on Gastric Cancer Mortality by Sex and Treatment Modality" Cancers 15, no. 9: 2463. https://doi.org/10.3390/cancers15092463
APA StyleNam, S. Y., Jeon, S. W., Jo, J., & Kwon, O. K. (2023). Favorable Effect of High-Density Lipoprotein Cholesterol on Gastric Cancer Mortality by Sex and Treatment Modality. Cancers, 15(9), 2463. https://doi.org/10.3390/cancers15092463






