Surgical Resection of Pulmonary Metastases from Melanoma in Oligometastatic Patients: Results from a Multicentric Study in the Era of Immunoncology and Targeted Therapy
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
Statistical Analysis
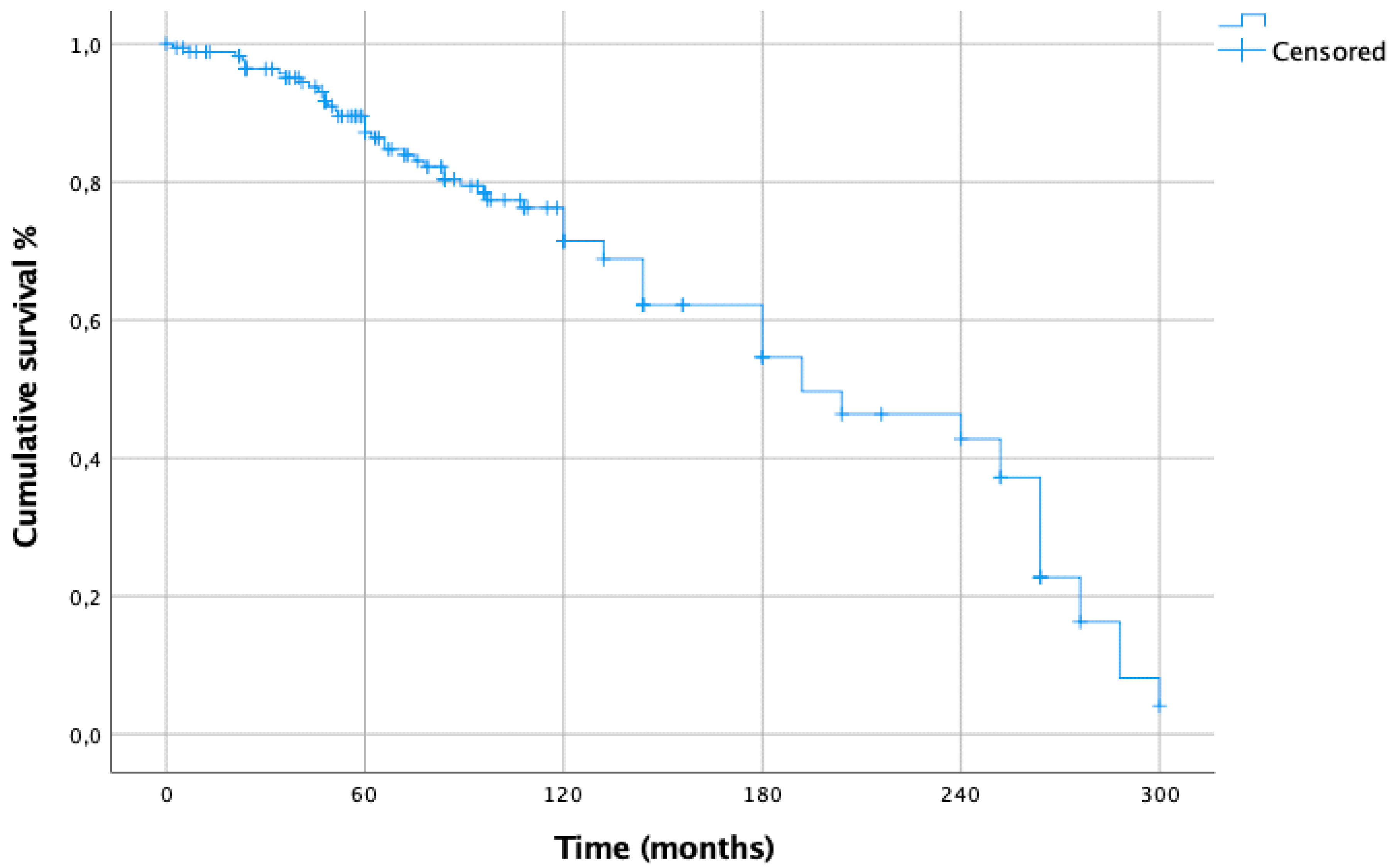
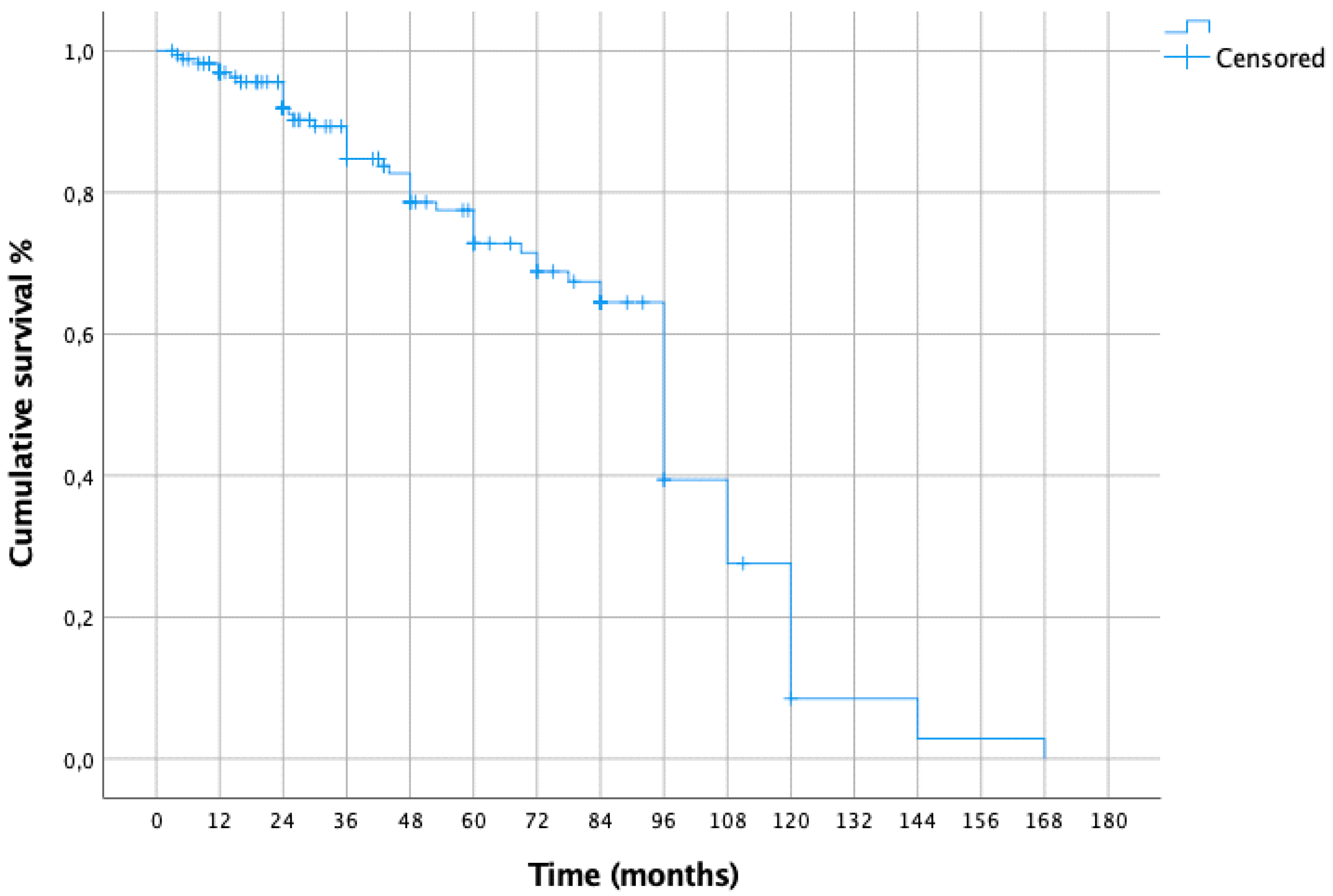
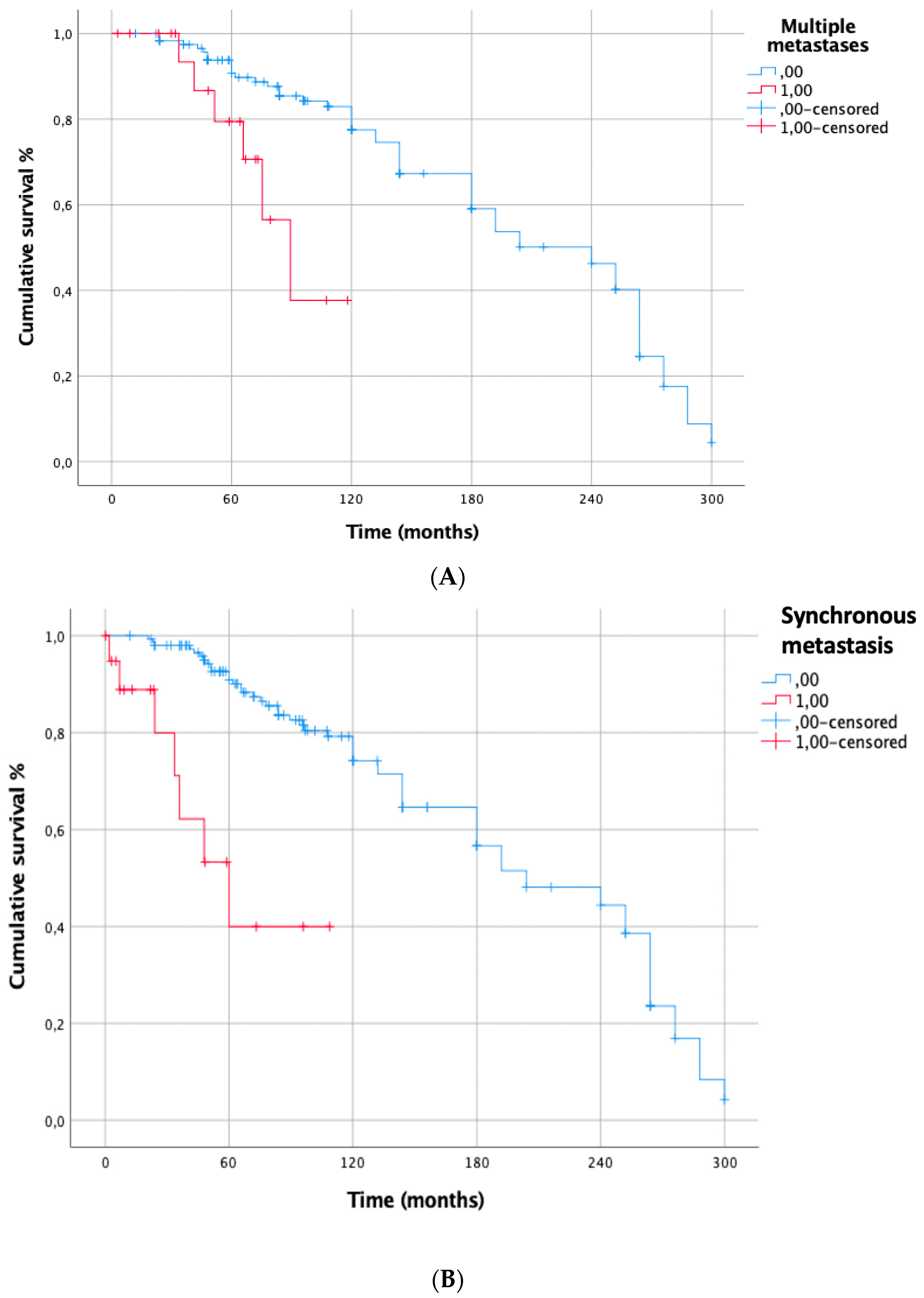
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Balch, C.M.; Gershenwald, J.E.; Soong, S.J.; Thompson, J.F.; Atkins, M.B.; Byrd, D.R.; Buzaid, A.C.; Cochran, A.J.; Coit, D.G.; Ding, S.; et al. Final version of 2009 AJCC melanoma staging and classification. J. Clin. Oncol. 2009, 27, 6199–6206. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Atkins, M.B.; Hsu, J.; Lee, S.; Cohen, G.I.; Flaherty, L.E.; Sosman, J.A.; Sondak, V.K.; Kirkwood, J.M. Phase III trial comparing concurrent biochemotherapy with cisplatin, vinblastine, dacarbazine, interleukin-2, and interferon alfa-2b with cisplatin, vinblastine, and dacarbazine alone in patients with metastatic malignant melanoma (E3695): A trial coordinated by the Eastern Cooperative Oncology Group. J. Clin. Oncol. 2008, 26, 5748–5754. [Google Scholar] [PubMed]
- Viehofa, J.; Livingstone, E.; Loschaa, E.; Stockhammera, P.; Bankfalvid, A.; Plo¨nesa, T.; Mardanzaia, K.; Zimmerb, L.; Suckerb, A.; Schadendorfb, D.; et al. Prognostic factors for pulmonary metastasectomy in malignant melanoma: Size matters. Eur. J. Cardiothorac. Surg. 2019, 56, 1104–1109. [Google Scholar] [CrossRef]
- Hanna, T.P.; Chauvin, C.; Miao, Q.; Rizkalla, M.; Ried, K.; Peng, Y.; Nguyen, P.; Jalink, D.; An Nanki, S. Clinical Outcomes After Pulmonary Metastasectomy for Melanoma: A Population-Based Study. Ann. Thorac. Surg. 2018, 106, 1675–1681. [Google Scholar] [CrossRef]
- Bhatia, S.; Tykodi, S.S.; Thompson, J.A. Treatment of metastatic melanoma: An overview. Oncology 2009, 2, 488–496. [Google Scholar]
- Passarelli, A.; Mannavola, F.; Stucci, L.S.; Tucci, M.; Silvestris, F. Immune system and melanoma biology: A balance between immune surveillance and immune escape. Oncotarget 2017, 8, 106132–160142. [Google Scholar] [CrossRef]
- Deutsch, G.B.; Flaherty, D.C.; Kirchoff, D.D.; Bailey, M.; Vitug, S.; Foshag, L.J.; Fraies, M.B.; Bilchick, A.J. Association of Surgical Treatment, Systemic Therapy, and Survival in Patients with Abdominal Visceral Melanoma Metastases, 1965–2014: Relevance of Surgical Cure in the Era of Modern Systemic Therapy. JAMA Surg. 2017, 152, 672–678, Erratum in JAMA Surg. 2018, 153, 1064. [Google Scholar] [CrossRef] [PubMed]
- Meacci, E.; Nachira, D.; Zanfrini, E.; Evangelista, J.; Triumbari, E.K.A.; Congedo, M.T.; Petracca Ciavarella, L.; Chiappetta, M.; Vita, M.L.; Schinzari, G.; et al. Prognostic Factors Affecting Survival after Pulmonary Resection of Metastatic Renal Cell Carcinoma: A Multicenter Experience. Cancers 2021, 13, 3258. [Google Scholar] [CrossRef]
- Oliaro, A.; Filosso, P.L.; Bruna, M.C.; Mossetti, C.; Ruffini, E. Pulmonary metastasectomy for melanoma. J. Thorac. Oncol. 2010, 5, S187–S191. [Google Scholar] [CrossRef]
- Wasif, N.; Bagaria, S.P.; Ray, P.; Morton, D.L. Does metastasectomy improve survival in patients with Stage IV melanoma? A cancer registry analysis of outcomes. J. Surg. Oncol. 2011, 104, 111e5. [Google Scholar] [CrossRef]
- Sosman, J.A.; Moon, J.; Tuthill, R.J.; Warneke, J.A.; Vetto, T.J.; Redman, B.G.; Liu, P.Y.; Unger, J.M.; Flaherty, L.E.; Sondak, V.K. A phase 2 trial of complete resection for stage IV melanoma: Results of Southwest Oncology Group Clinical Trial S9430. Cancer 2011, 117, 4740-06. [Google Scholar] [CrossRef]
- Harrison Howard, J.; Thompson, J.F.; Mozzillo, N.; Nieweg, O.E.; Howkstra, H.J.; Roses, D.F.; Sondak, V.K.; Reintgen, D.S.; Kashani-Sabet, M.; Karakousis, C.P.; et al. Metastasectomy for distant metastatic melanoma: Analysis of data from the first Multicenter Selective Lymphadenectomy Trial (MSLT-I). Ann. Surg. Oncol. 2012, 19, 2547–2555. [Google Scholar] [CrossRef]
- Schuhan, C.; Muley, T.; Dienemann, H.; Pfannschmidt, J. Survival after pulmonary metastasectomy in patients with malignant melanoma. Thorac. Cardiovasc. Surg. 2011, 59, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Van Akkooj, A.C.J.; Atkins, M.B.; Agarwala, S.S.; Lorigan, P. Surgical Management and Adjuvant Therapy for High-Risk and Metastatic Melanoma. Am. Soc. Clin. Oncol. Educ. Book 2016, 35, e505–e514. [Google Scholar] [CrossRef] [PubMed]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef]
- Chapman, P.B.; Hauschild, A.; Robert, C.; Haanen, J.B.; Ascierto, P.; Larkin, J.; Dummer, R.; Garbe, C.; Testori, A.; Maio, M.; et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N. Engl. J. Med. 2011, 364, 2507–2516. [Google Scholar] [CrossRef]
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Rutkowski, P.; Jrob, J.J.; Cowey, L.; Lao, C.D.; Wagstaff, J.; Schadendorf, D.; Ferrucci, P.F.; et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2017, 377, 1345–1356. [Google Scholar] [CrossRef] [PubMed]
- Hodj, F.S.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Rutkowski, P.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Wagstaff, D.; Dummer, R.; et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 1480–1492. [Google Scholar]
- Michielin, O.; van Akkooi, A.C.J.; Ascierto, P.A.; Dummer, R.; on behalf of the ESMO Guidelines Committee. Cutaneous melanoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 1884–1901. [Google Scholar] [CrossRef] [PubMed]
- Faries, M.B.; Mozzillo, N.; Kashani-Sabet, M.; Thompson, J.F.; Kelley, M.C.; DeConti, R.C.; Lee, J.E.; Huth, J.F.; Wagner, J.; Dalgleish, A.; et al. Long-Term Survival after Complete Surgical Resection and Adjuvant Immunotherapy for Distant Melanoma Metastases. Ann. Surg. Oncol. 2017, 24, 3991–4000. [Google Scholar] [CrossRef] [PubMed]
- Leo, F.; Cagini, L.; Rocmans, P.; Cappello, M.; Geel, A.N.; Maggi, G.; Goldstraw, P.; Pastorino, U. Lung metastases from melanoma: When is surgical treatment warranted? British J. Cancer 2000, 83, 569–572. [Google Scholar] [CrossRef]
- Meyer, T.; Merkel, S.; Goehl, J.; Hohenberger, W. Surgical therapy for distant metastases of malignant melanoma. Cancer 2000, 89, 1983–1991. [Google Scholar] [CrossRef] [PubMed]
- Feun, L.G.; Gutterman, J.; Burgess, M.A.; Hersh, E.M.; Mavligit, G.; McBride, C.M.; Benjamin, R.S.; Richman, S.P.; Murphy, W.K.; Bodey, G.P.; et al. The natural history of resectable metastatic melanoma (Stage IVA melanoma). Cancer 1982, 50, 1656–1663. [Google Scholar] [CrossRef]
- Overett, T.K.; Shiu, M.H. Surgical treatment of distant metastatic melanoma. Indications and results. Cancer 1985, 56, 1222–1230. [Google Scholar] [CrossRef]
- Wong, J.H.; Euhus, D.M.; Morton, D.L. Surgical resection for metastatic melanoma to the lung. Arch. Surg. 1988, 123, 1091–1095. [Google Scholar] [CrossRef] [PubMed]
- Karp, N.S.; Boyd, A.; DePan, H.J.; Harris, M.N.; Ros, D.F. Thoracotomy for metastatic malignant melanoma of the lung. Surgery 1990, 107, 256–261. [Google Scholar]
- Gorenstein, L.A.; Putnam, J.B.; Nataraian, G.; Balch, C.A.; Roth, J.A. Improved survival after resection of pulmonary metastases from malignant melanoma. Ann. Thorac. Surg. 1991, 52, 204–210. [Google Scholar] [CrossRef]
- Harpole, D.H.; Johnson, C.M.; Wolfe, W.G.; George, S.L.; Seigler, H.F. Analysis of 945 cases of pulmonary metastatic melanoma. J. Thorac. Cardiovasc. Surg. 1992, 103, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Wankhede, D.; Grover, S. Outcomes After Curative Metastasectomy for Patients with Malignant Melanoma: A Systematic Review and Metaanalysis. Ann. Surg. Oncol. Jan. 2022, 29, 3709–3723. [Google Scholar] [CrossRef]
- Pastorino, U.; Buyse, M.; Friedel, G.; Ginsberg, R.J.; Girard, P.; Goldstraw, P.; Johnston, M.; McCormack, P.; Pass, H.; Putnam Jr, J.B.; et al. Long-term results of lung metastasectomy: Prognostic analyses based on 5206 cases. J. Thorac. Cardiovasc. Surg. 1997, 113, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Dalrymple-Hay, M.J.; Rome, P.D.; Kennedy, C.; Fulham, M.; McCaughan, B.C. Pulmonary metastatic melanoma—the survival benefit associated with positron emission tomography scanning. Eur. J. Cardiothorac. Surg. 2002, 21, 611–614; discussion 614–615. [Google Scholar] [CrossRef]
- Andrews, S.; Robinson, L.; Cantor, A.; De Conti, R.C. Survival after surgical resection of isolated pulmonary metastases from malignant melanoma. Cancer Control 2006, 13, 218–223. [Google Scholar] [CrossRef]
- Neuman, H.B.; Patel, A.; Hanlon, C.; Wolchok, J.D.; Houghton, A.N.; Coit, D.G. Stage-IV melanoma and pulmonary metastases: Factors predictive of survival. Ann Surg. Oncol. 2007, 14, 2847–2853. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.P.; Hanish, S.I.; Haney, J.C.; Miller, C.C.; Burfeind, W.R., Jr.; Tyler, D.S.; Seiger, H.F.; Wolfe, W.; D’Amico, T.A.; Harpole, D.H., Jr. Improved survival with pulmonary metastasectomy: An analysis of 1720 patients with pulmonary metastatic melanoma. J. Thorac. Cardiovasc. Surg. 2007, 133, 104–110. [Google Scholar] [CrossRef]
- Chua, T.C.; Scolver, R.A.; Kennedy, C.W.; Yan, T.D.; McCaughan, B.C.; Thompson, J.F. Surgical management of melanoma lung metastasis: An analysis of survival outcomes in 292 consecutive patients. Ann. Surg. Oncol. 2012, 19, 1774–1781. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.J.; Smith, H.G.; Joshi, K.; Gore, M.; Strauss, D.C.; Hayes, A.J.; Larkin, J. The impact of effective systemic therapies on surgery for stage IV melanoma. Eur. J. Cancer 2018, 103, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Bello, D.M.; Panageas, K.S.; Hollmann, T.; Shoushtari, A.N.; Momtaz, P.; Chapman, P.B.; Postow, M.A.; Callahan, M.K.; Wolchock, J.D.; Brady, M.S.; et al. Survival outcomes after metastasectomy in melanoma patients categorized by response to checkpoint blockade. Ann. Surg. Oncol. 2020, 27, 1180–1188. [Google Scholar] [CrossRef]
- Day, C.L., Jr.; Lew, R.A.; Mihm, M.C., Jr.; Sober, A.J.; Harris, M.N.; Kopf, A.W.; Fitzpatrick, T.B.; Harrist, T.J.; Golomb, F.M.; Postel, A.; et al. A multivariate analysis of prognostic factors for melanoma patients with lesions greater than or equal to 3.65 mm in thickness. The importance of revealing alternate Cox models. Ann. Surg. 1982, 195, 44–49. [Google Scholar] [CrossRef]
- Day, C.L., Jr.; Mihm, M.C., Jr.; Sober, A.J.; Harris, M.N.; Kopf, A.W.; Fitzpatrik, T.B.; Lew, R.A.; Harrist, T.J.; Golomb, F.M.; Postel, A.; et al. Prognostic factors for melanoma patients with lesions 0.76–1.69 mm in thickness. An appraisal of ‘thin’ level IV lesions. Ann. Surg. 1982, 195, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Day, C.L., Jr.; Mihm, M.C.; Lew, R.A.; Harris, M.N.; Kopf, A.W.; Fitzpatrick, T.B.; Harrist, T.J.; Golomb, F.M.; Postel, A.; Hennessey, P.; et al. Prognostic factors for clinical stage I melanoma of intermediate thickness (1.51–3.39 mm). A conceptual model for tumor growth and metastasis. Ann. Surg. 1982, 195, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Crowson, A.N.; Magro, C.; Mihm, M.C., Jr. The biology of melanoma progression: From melanocyte to metastatic seed. In From Melanocytes to Malignant Melanoma; Hearing, V.J., Leong, S.P.L., Eds.; Human Press: Totowa, NJ, USA, 2006; pp. 365–399. [Google Scholar]
- Bedrosian, I.; Faries, M.B.; Guerry, D.; Elenitsas, R.; Schuchter, L.; Mick, R.; Spitz, F.R.; Bucky, L.P.; Alavi, A.; Elder, D.E.; et al. Incidence of sentinel node metastasis in patients with thin primary melanoma (£1 mm) with vertical growth phase. Ann. Surg. Oncol. 2000, 7, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Green, A.C.; Williams, G.M.; Logan, V.; Strutton, G.M. Reduced melanoma after regular sunscreen use: Randomized trial follow-up. J Clin. Oncol. 2011, 29, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Boniol, M.; Autier, P.; Boyle, P.; Gandini, S. Cutaneous melanoma attribuible to sunbeds use: Systematic review and metanalysis. BMJ 2012, 345, e4757. [Google Scholar] [CrossRef] [PubMed]
- Aberg, T. Selection mechanisms as major determinants of survival after pulmonary metastasectomy. Ann. Thorac. Surg. 1997, 63, 611e2. [Google Scholar]
- Aberg, T.; Malmberg, K.A.; Nilsson, B.; Nou, E. The effect of metastasectomy: Fact or fiction? Ann. Thorac. Surg. 1980, 30, 378e84. [Google Scholar] [CrossRef]
- Treasure, T.; Milosevic, M.; Fiorentino, F.; Macbeth, F. Pulmonary metastasectomy: What is the practice and where is the evidence for effectiveness? Thorax 2014, 69, 946e9. [Google Scholar] [CrossRef]


| Variables | #183 Patients |
|---|---|
| Sex (male) | 76 (41.5%) |
| Previous oncological history | 21 (11.4%) |
| Melanoma primary site: | |
| Skin | 172 (94.0%) |
| Uvea | 9 (4.9%) |
| Mucosae | 2 (1.1%) |
| Breslow thickness (mm) | 3.11 ± 3.26 |
| Histology | |
| Acral | 19 (10.4%) |
| Superficial spreading | 95 (51.9%) |
| Nodular | 65 (35.5%) |
| Lentigo | 4 (2.2%) |
| B-RAF mutation | 64 (34.9%) |
| Stage at diagnosis | 10 (5.5%) |
| IA | 36 (19.7%) |
| IB | 48 (26.2%) |
| IIA | 22 (12.0%) |
| IIB | 14 (7.7%) |
| IIC | 20 (10.9%) |
| IIIA | 13 (7.1%) |
| IIIB | 4 (2.2%) |
| IIIC | 1 (0.5%) |
| IIID | 15 (8.2%) |
| IV | |
| Type of adjuvant therapy: | |
| None | 87 (47.6%) |
| Immunotherapy | 75 (41.0%) |
| Targeted Therapy | 18 (9.8%) |
| Chemotherapy | 3 (1.6%) |
| Patients with previous metastases other than lung | 24 (13.1%) |
| Diameter of pulmonary metastases (mm) | 12.4 ± 5.6 |
| Multiple lung metastases | 21 (11.5%) |
| Synchronous metastases | 26 (14.2%) |
| Type of lung resection: | |
| Wedge | 175 (95.6%) |
| Segmentectomy/Lobectomy | 8 (4.4%) |
| Minor complications after metastasectomy | 21 (11.5%) |
| Major complications after metastasectomy | null |
| In-hospital stay (days) | 4.46 ± 2.8 |
| Thirty-day mortality | null |
| Adjuvant therapy after metastasectomy: | |
| None | 19 (10.4%) |
| Immunotherapy | 86 (47.0%) |
| Targeted therapy | 78 (42.6%) |
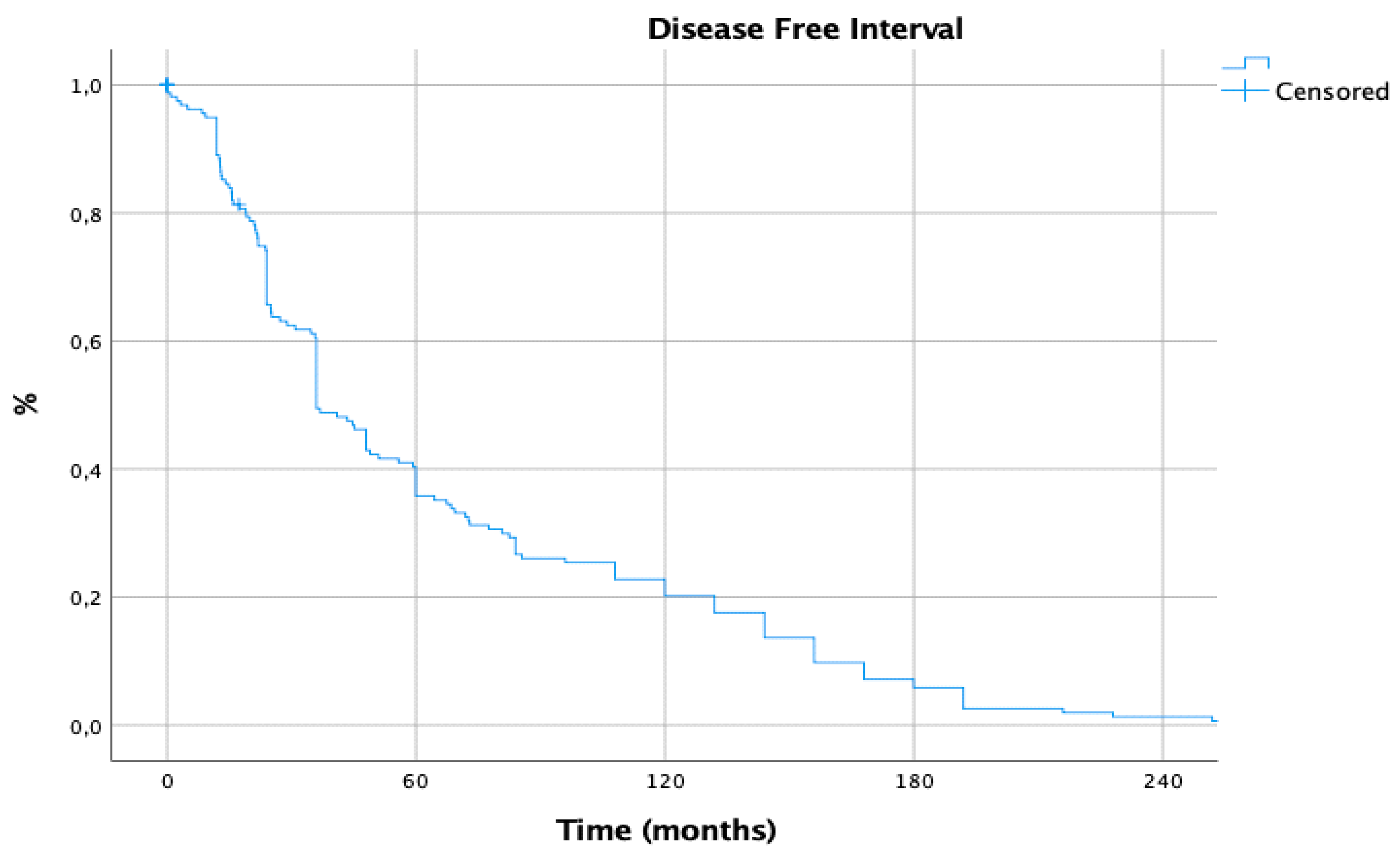
| Recurrence of disease after metastasectomy | 73 (39.9%) |
| Variables | Univariate Analysis | Multivariable Analysis | |
|---|---|---|---|
| p-Value | HR [95% CI] | p-Value | |
| Previous oncological history | 0.370 | ||
| Melanoma histology | 0.052 | 1.295 [0.699–2.398] | 0.411 |
| Vertical growth | 0.122 | ||
| Radial growth | 0.524 | ||
| Breslow thickness | 0.636 | ||
| BRAF mutation | 0.103 | ||
| Neurotropism | 0.858 | ||
| Vascular invasion | 0.999 | ||
| Multiple lung metastases | 0.002 | 1.508 [0.540–4.206] | 0.433 |
| Synchronous lung metastasis | <0.001 | 1.130 [0.326–3.923] | 0.847 |
| Previous metastatic sites other than lung | 0.0002 | 1.607 [0.443–5.834] | 0.471 |
| Diameter of metastasis > 2 cm | 0.547 | ||
| Type of lung resection | 0.986 | ||
| Therapy after metastasectomy | 0.185 | ||
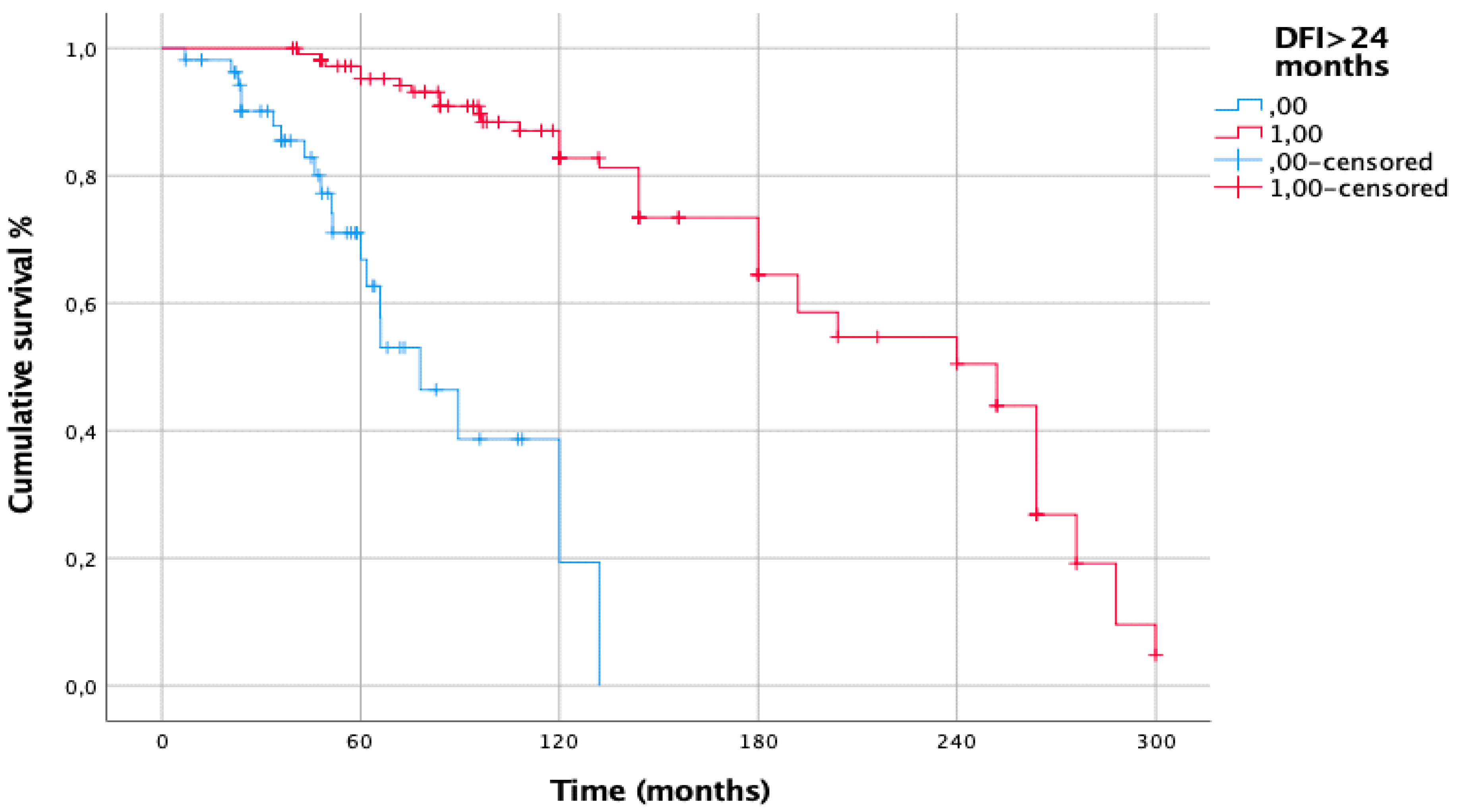
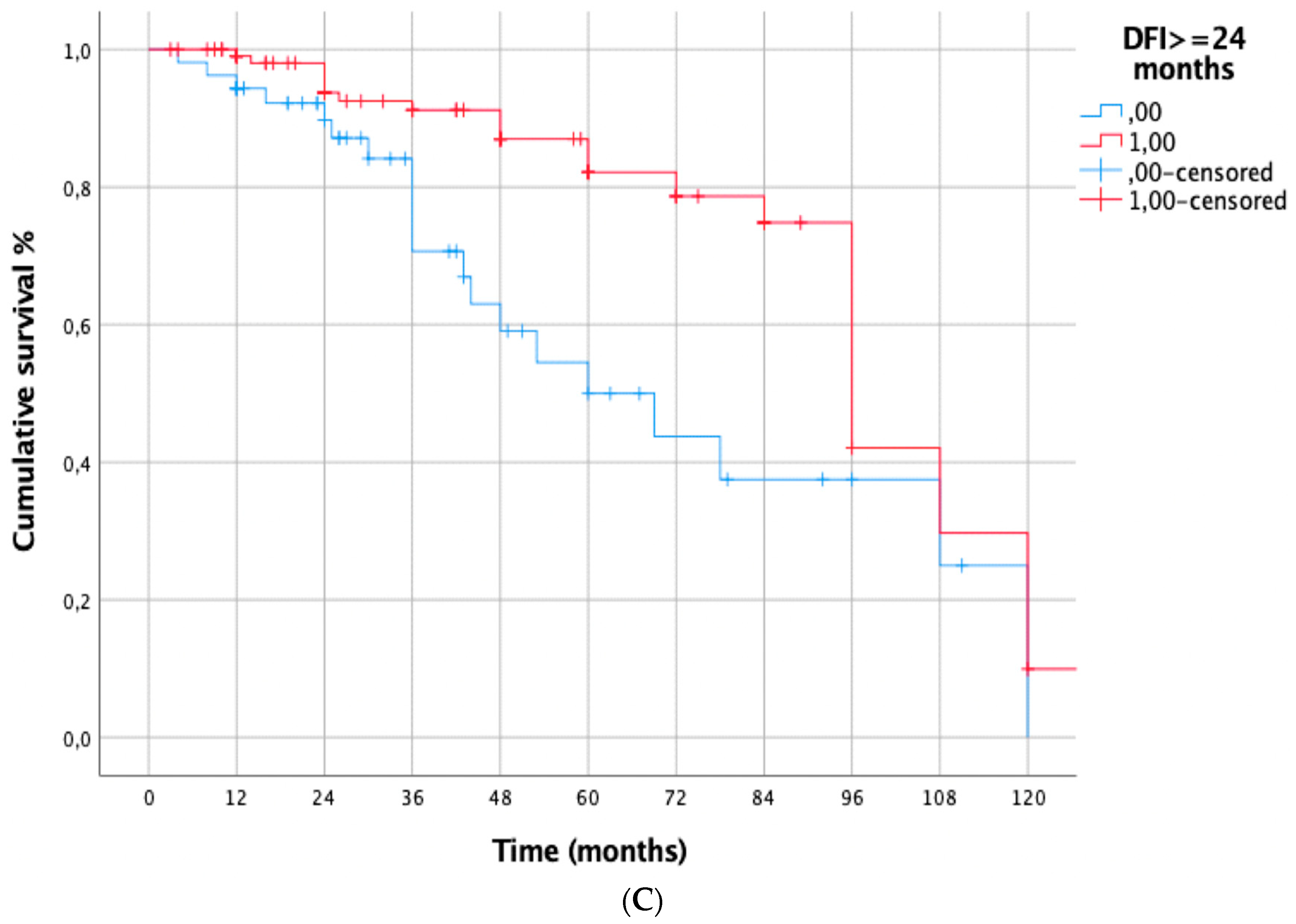
| DFI < 24 months | 0.002 | 7.813 [2.787–21.902] | <0.001 |
| Variables | Univariate Analysis | Multivariable Analysis | |
|---|---|---|---|
| p-Value | HR [95% CI] | p-Value | |
| Previous oncological history | 0.883 | ||
| Melanoma histology | 0.069 | ||
| Vertical growth | 0.018 | 9.688 [1.410–66.551] | 0.021 |
| Radial growth | 0.784 | ||
| Breslow thickness | 0.320 | ||
| BRAF mutation | 0.067 | ||
| Neurotropism | 0.854 | ||
| Vascular invasion | 0.680 | ||
| Multiple lung metastases | 0.054 | 2.687 [0.614-11.753] | 0.189 |
| Synchronous lung metastasis | 0.146 | ||
| Previous metastatic sites other than lung | <0.001 | 3.154 [1.332–7.470] | 0.009 |
| Diameter of metastasis > 2 cm | 0.592 | ||
| Type of lung resection | 0.902 | ||
| Therapy after metastasectomy | 0.435 | ||
| DFI < 24 months | 0.007 | 1.670 [0.267–10.456] | 0.584 |
| Variables | Univariate Analysis | Multivariable Analysis | |
|---|---|---|---|
| p-Value | HR [95% CI] | p-Value | |
| Male sex | 0.914 | ||
| Melanoma histology | 0.143 | ||
| Vertical growth | 0.182 | ||
| Breslow thickness | 0.636 | ||
| BRAF mutation | 0.029 | 1.285 [0.894–1.846] | 0.176 |
| c-KIT mutation | 0.429 | ||
| Variables | Univariate Analysis | Multivariable Analysis | |
|---|---|---|---|
| p-Value | HR [95% CI] | p-Value | |
| Male sex | 0.298 | ||
| Melanoma histology | 0.099 | ||
| Vertical growth | 0.616 | ||
| BRAF mutation | 0.131 | ||
| c-KIT mutation | 0.685 | ||
| Breslow thickness | 0.407 | ||
| Synchronous lung metastasis | 0.484 | ||
| Diameter of metastasis > 2 cm | 0.848 | ||
| Type of lung resection | 0.241 | ||
| Therapy after metastasectomy | <0.001 | 0.106 [0.028–0.404] | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meacci, E.; Nachira, D.; Congedo, M.T.; Ibrahim, M.; Pariscenti, G.; Petrella, F.; Casiraghi, M.; De Stefani, A.; del Regno, L.; Peris, K.; et al. Surgical Resection of Pulmonary Metastases from Melanoma in Oligometastatic Patients: Results from a Multicentric Study in the Era of Immunoncology and Targeted Therapy. Cancers 2023, 15, 2462. https://doi.org/10.3390/cancers15092462
Meacci E, Nachira D, Congedo MT, Ibrahim M, Pariscenti G, Petrella F, Casiraghi M, De Stefani A, del Regno L, Peris K, et al. Surgical Resection of Pulmonary Metastases from Melanoma in Oligometastatic Patients: Results from a Multicentric Study in the Era of Immunoncology and Targeted Therapy. Cancers. 2023; 15(9):2462. https://doi.org/10.3390/cancers15092462
Chicago/Turabian StyleMeacci, Elisa, Dania Nachira, Maria Teresa Congedo, Mohsen Ibrahim, Gianluca Pariscenti, Francesco Petrella, Monica Casiraghi, Alessandro De Stefani, Laura del Regno, Ketty Peris, and et al. 2023. "Surgical Resection of Pulmonary Metastases from Melanoma in Oligometastatic Patients: Results from a Multicentric Study in the Era of Immunoncology and Targeted Therapy" Cancers 15, no. 9: 2462. https://doi.org/10.3390/cancers15092462
APA StyleMeacci, E., Nachira, D., Congedo, M. T., Ibrahim, M., Pariscenti, G., Petrella, F., Casiraghi, M., De Stefani, A., del Regno, L., Peris, K., Triumbari, E. K. A., Schinzari, G., Rossi, E., Petracca-Ciavarella, L., Vita, M. L., Chiappetta, M., Siciliani, A., Peritore, V., Manitto, M., ... Margaritora, S. (2023). Surgical Resection of Pulmonary Metastases from Melanoma in Oligometastatic Patients: Results from a Multicentric Study in the Era of Immunoncology and Targeted Therapy. Cancers, 15(9), 2462. https://doi.org/10.3390/cancers15092462