Simple Summary
Radiotherapy, commonly used to treat thoracic cancers, can induce adverse effects in the surrounding normal tissue, which become dose limiting factors and narrow the therapeutic window. Polyphenols, a type of natural plant compounds, have been proposed to radiosensitize the tumor and, at the same time, protect the normal tissue against radiotherapy-induced adverse effects. This review summarizes the current knowledge of the radioprotective effects of polyphenols, especially on normal thoracic tissues, and their underlying molecular mechanisms.
Abstract
Radiotherapy is one of the standard treatment approaches used against thoracic cancers, occasionally combined with chemotherapy, immunotherapy and molecular targeted therapy. However, these cancers are often not highly sensitive to standard of care treatments, making the use of high dose radiotherapy necessary, which is linked with high rates of radiation-induced adverse effects in healthy tissues of the thorax. These tissues remain therefore dose-limiting factors in radiation oncology despite recent technological advances in treatment planning and delivery of irradiation. Polyphenols are metabolites found in plants that have been suggested to improve the therapeutic window by sensitizing the tumor to radiotherapy, while simultaneously protecting normal cells from therapy-induced damage by preventing DNA damage, as well as having anti-oxidant, anti-inflammatory or immunomodulatory properties. This review focuses on the radioprotective effect of polyphenols and the molecular mechanisms underlying these effects in the normal tissue, especially in the lung, heart and esophagus.
1. Introduction
More than half of cancer patients receive radiotherapy, either as monotherapy or in combination with other anti-cancer therapies. Many factors are involved in determining the final clinical outcome of radiotherapy, including the radiation type, dose, schedule and radiation delivery technique, as well as the final dose received by both tumor and normal tissue [1]. High-intensity radiotherapy is frequently needed due to cancer cell resistance to treatment, which increases the probability for radiation-induced toxicities in the normal tissues. In case of thoracic cancers, the most affected organs by radiation are the lungs, heart and esophagus [2,3,4]. These adverse effects are known as RILT, RIHD and radiation-induced esophagitis, respectively, and can be developed both at early stages, starting within approximately three weeks after the start of radiotherapy, and/or at late stages, with an onset of months to years after treatment [3,4,5]. In RILT, the most frequent toxicities are lung pneumonitis and fibrosis due to the high radiosensitivity of the lung parenchyma [6,7]. The incidence of pneumonitis after irradiation treatment in lung cancer patients stands at up to 50%, and pulmonary fibrosis is diagnosed in 70% to 80% of the patients [2,8]. Pneumonitis is developed at early stages and may be reversible, whereas pulmonary fibrosis is a late effect and considered irreversible due to the inexistence of an approved treatment. This leads to poorer prognosis and impaired quality of life [2]. RIHD includes a wide range of morbidities, including pericarditis, coronary artery disease, valvular heart disease, conduction abnormalities as well as cardiomyopathies [5,9]. RIHD is more frequent in long-term survivors, as it has a late onset and requires a long incubation period. Recent studies have shown that lung cancer patients receiving a heart radiation dose above 20 Gy showed a higher risk of RIHD and lower survival. More than 10% of lung cancer patients treated with thorax radiation suffered myocardial infarction, heart failure or cardiac death, with an onset of 18 months post-treatment [10,11]. In case of radiation-induced esophagitis, the most common early effects are dysphagia and odynophagia, which are described as difficulties to swallow or painful swallowing, respectively, and are developed in most patients within two months after treatment [4,12]. Other complications such as esophagus ulceration and perforation, as well as fibrosis at late stages, are less common, but more likely to occur when radiotherapy is combined with chemotherapy [4,13]. In fact, 1.3% of patients receiving radiotherapy as monotherapy develop severe esophagitis (>grade 3), while the incidence for patients receiving radiochemotherapy is up to 34% [12].
Radiotherapy adverse effects are therefore a major impediment to cancer treatment, as they become dose-limiting factors and narrow the therapeutic window [3], defined as the balance between TCP and NTCP [14]. The therapeutic ratio can be increased by either enhancing TCP, reducing NTCP or preferably a combination of both. In terms of TCP, efforts have been focused on increasing treatment response through, for example, the use of novel compounds, named radiosensitizers, which in combination with radiotherapy enhance the overall treatment effect, improving patient prognosis and survival [15,16]. On the other hand, chemical radioprotectors and improved dose conformity techniques are being investigated to decrease NTCP [3,17]. Furthermore, many studies are focused on widening the therapeutic window by increasing TCP as well as reducing NTCP. For example, fractionated irradiation, in which smaller radiation doses are delivered to the patient in multiple fractions, reduces NTCP as it causes less DNA damage in both tumor and normal tissues. As the normal tissue has a functional DNA repair system, it will be able to repair the damage in between the fractions and therefore decrease the normal tissue toxicities [18]. However, tumors have more aberrant DNA repair systems, which will result in insufficient repair by the next fraction, causing accumulation of sub-lethal DNA damage, which will likely become lethal at the end [18]. Another example is the use of modern high-precision radiation techniques such as IMRT to improve dose conformity, which permits the treatment of smaller and more accurate target volumes, reducing the dose delivered to the normal surrounding tissue [19,20]. Furthermore, state-of-the-art irradiation machines are capable of delivering a non-uniform dose beam, permitting the design of dose painting plans [20]. Dose painting, which consists of the application of different intensity radiation doses in the target, permits the delivery of a higher dose in the tumor, while sparing maximumly the normal tissue [21,22,23].
Pharmacological interventions using radioprotectors are being studied to prevent or reduce radiation-induced toxicities in the organs at risk within the irradiation field. Radiation leads to DNA damage as well as ROS formation, which in turn cause oxidation of DNA, lipids and proteins [24]. Radiation-induced DNA damage will induce the activation of many signaling pathways, which will cause inflammation and will modulate immune response [7]. A perfect radioprotector should therefore induce DNA repair mechanisms, have antioxidant properties as well as inflammatory and immunomodulatory effects, while having neither radioprotective effects on the tumor nor toxicity in other parts of the body [25]. Up to date, aminothiols and its derivatives, including amifostine, are the most investigated radioprotectors. Although these compounds have been shown to reduce and protect the normal tissue against radiation injuries in cancer patients, their clinical application is limited due to high toxicity [26,27]. So far, only amifostine has been FDA-approved, despite the controversy regarding its benefits and harms [26,28]. Evidence supports that amifostine could act as a ROS scavenger and an inducer of DNA damage repair mechanisms in normal, healthy cells [27,29]. However, its mechanism of action is complex and unclear, and its overall effect is being questioned due to the lack of complete specificity to the normal tissue and contradicting evidence regarding its radioprotective effects [25,26,27]. Its clinical use is also hampered due to its limited bioavailability and therefore difficulties in treatment administration and duration, toxicities in the healthy organs and elevated costs [26,30]. Further research is therefore required in order to identify novel radioprotectors which surpass these limitations.
Several plant-derived products such as polyphenols have gained interest within oncology due to their effects on both sides of the therapeutic window [31,32]. Polyphenols can sensitize tumors to radiation by interacting with several intracellular signaling pathways involved in tumor initiation and growth, such as the MAPK, the NF-κB and the Wnt/β-catenin pathway [33,34], activating tumor suppressor genes while downregulating oncogenes and pro-survival genes [31,35,36]. On the other hand, polyphenols’ protective effects have been studied in normal tissue including lung [37,38,39,40,41], breast [42,43], esophagus [44,45], skin [46,47] and intestine [48,49]. They behave as free radical scavengers, have anti-oxidant effects in DNA, lipids and proteins, and induce DNA repair mechanisms [24,31]. Previous research has shown that combined treatment of radiotherapy and polyphenols could increase the therapeutic window in thoracic cancer patients by radiosensitizing tumors as well as protecting the normal tissue, improving patient survival, outcome and quality of life [36,43,50]. This review focuses on summarizing the protective properties of polyphenols against radiation-induced thoracic organ toxicities (Figure 1).
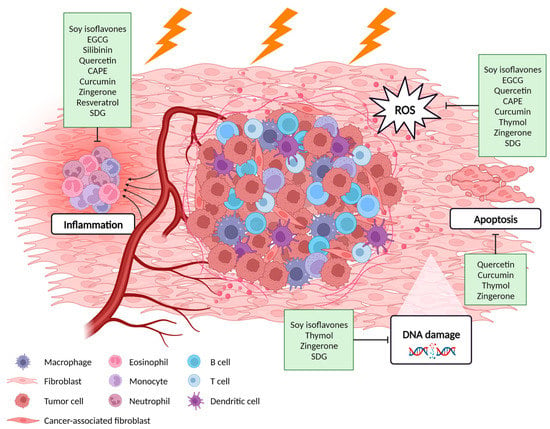
Figure 1.
Potential molecular mechanisms of polyphenols against radiation-induced adverse effects in the thoracic normal tissue. Created with BioRender.com.
2. Radioprotective Effects of Polyphenols in Thoracic Normal Tissues
Despite being a very large and heterogeneous family, polyphenols have been classified in four different categories—flavonoids (genistein, EGCG, silibinin, quercetin), phenolic acids (caffeic acid phenethyl ester, curcumin, thymol, zingerone), stilbenes (resveratrol) and lignans (secoisolariciresinol diglucoside) (Figure 2) [51]. In this review we summarize the radioprotective effects of each compound in thoracic organs in in vitro models, preclinical models and clinical trials.
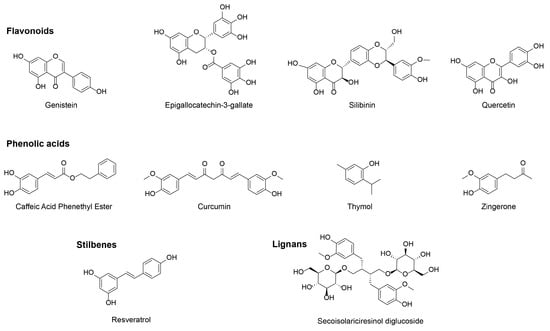
Figure 2.
Chemical structure of the discussed polyphenols.
2.1. Flavonoids
2.1.1. Soy Isoflavones—Genistein
Soy isoflavones, such as genistein, are flavonoids (Table 1) mainly found in beans and legumes (including soybeans), and are considered estrogenic polyphenols as they mimic the action of estrogens on the estrogen receptors. In preclinical models of lung cancer, soy isoflavones have been shown to enhance the radiation cytotoxic effect by decreasing tumor growth and cell proliferation. In addition, soy isoflavones reduced radiation-related lung adverse effects including inflammation, hemorrhages and fibrosis [52,53]. Likewise, the administration of a genistein-enriched diet in both mice and rats has been shown to protect the animals against radiation-induced lung toxicities by reducing collagen deposition and consequently lung fibrosis [54,55]. Evidence supports that soy isoflavones, and specifically genistein, have an impact on the inflammatory responses that drive radiation-induced lung toxicities. The exact mechanism of action however is not clear yet. It was observed that genistein mitigates the radiation-induced immune response in mice by reducing leukocyte infiltrate in the lungs after daily soy isoflavones administration [56,57]. Soy isoflavones have been shown to affect blood vessel structure and expression of adhesion molecules by preventing nuclear activation of the transcription factor NF-κB [57,58]. Radiation-induced heart toxicities were mitigated upon soy isoflavones pre-treatment, improving heart structure and integrity, reducing damage in heart arteries as well as collagen deposition in arteries and myocardium, indicative for a reduction in radiation-induced heart fibrosis [59]. Soy isoflavones have also been proposed to reduce radiation-induced esophagitis in mouse models of lung cancer by reducing radiation-induced damage in different esophageal layers as well as immune cell infiltration [45]. Table 1 summarizes the preclinical studies on the radioprotective effects of soy isoflavones and their underlying molecular mechanisms.

Table 1.
Radioprotective effects of soy isoflavones and its potential molecular mechanisms.
2.1.2. Epigallocatechin-3-Gallate (EGCG)
EGCG is one of the main bioactive components of green tea and has been reported to have cytotoxic and radiosensitizing effects by decreasing cell proliferation and inducing apoptosis in different cancers including lung and esophagus [24,60]. In normal tissues, EGCG has been suggested to protect against radiation-induced adverse effects (Table 2). In vivo, thorax irradiation rat models showed that EGCG improved animal survival as well as ameliorated radiotherapy-induced lung fibrosis due to its effects on oxidative stress and the Nrf2 signaling pathway [61].
Despite few in vitro and in vivo preclinical studies, several clinical trials have been performed in lung and esophagus cancer patients testing the radioprotective effects of EGCG. A phase I study in chemoradiotherapy-treated NSCLC patients revealed a lower degree of esophagitis as well as a reduced pain score upon EGCG supplementation [62]. This has been confirmed in two independent phase II clinical trials in lung cancer patients. EGCG not only decreased radiation-induced esophagitis but also other characteristic radiation injury symptoms such as pain, nausea and dysphagia [63,64]. In a prospective, three-arm phase II clinical trial comparing the effects of EGCG and placebo treatment in NSCLC patients receiving chemoradiotherapy, the degree of esophagitis was lower when EGCG was administered prophylactically, compared to a therapeutic treatment setup [50]. Similarly, a phase II trial in esophagus cancer patients treated with radiotherapy or chemoradiotherapy showed a reduced esophagitis score and pain upon EGCG treatment. Continuous EGCG treatment led to ameliorated esophagitis over time without affecting the anti-tumor therapy efficacy [44]. Phase III clinical studies are awaited. Table 2 summarizes the studies on the radioprotective effects of EGCG and its underlying molecular mechanisms.

Table 2.
Radioprotective effects of epigallocatechin-3-gallate and its potential molecular mechanisms.
Table 2.
Radioprotective effects of epigallocatechin-3-gallate and its potential molecular mechanisms.
| Model | Tissue | Treatment | Effect | Reference |
|---|---|---|---|---|
| Rat | Lung |
|
| [61] |
| Clinical trial (phase I and II) | Esophagus |
|
| NCT02577393 [50,64]; NCT01481818 [62,63] |
| Clinical trial (phase I and II) | Esophagus |
|
| NCT01481818 [44] |
2.1.3. Silibinin
Silibinin, one of the main active components of milk thistle (Silybum marianum), has been extensively studied for its anti-tumor effects in several types of carcinomas. Its ability to inhibit tumor growth has been demonstrated in both in vitro and in vivo models of lung cancer [65,66]. However, literature supporting its radioprotective effects is sparse (Table 3). An in vivo study using murine lung cancer models undergoing thorax irradiation demonstrated a reduction in radiation-induced lung toxicity and improved animal survival after oral administration of silibinin, as well as a reduced number of lung tumor nodules. Mice exhibited decreased inflammatory response in lungs as well as in BALF, and a mitigated lung fibrosis score [67]. Table 3 summarizes the studies on the radioprotective effects of silibinin and its underlying molecular mechanisms.

Table 3.
Radioprotective effects of silibinin and its potential molecular mechanisms.
2.1.4. Quercetin
Quercetin is a flavonoid present in fruits and vegetables, especially onions, berries and apples, as well as in green tea and red wine (Table 4). It has been suggested to have cytotoxic and radiosensitizing effects in many cancer types, including lung, by decreasing cell proliferation and inducing apoptosis in vitro [68,69]. In vivo models confirmed the radioprotective properties of quercetin against RILT as well as against other radiation-induced toxicities. The administration of quercetin (injected or inhaled) in murine and rat models of RILT showed a decrease in lung fibrosis severity and inflammatory infiltrate in BALF, plasma and lung tissue after total body irradiation [70,71,72]. One of the proposed mechanisms of action of quercetin is a decrease in the activity of NF-κB and MAPK pathways, supporting its anti-inflammatory effects [71,73]. The administration of quercetin has also been suggested to reduce radiation-induced oxidative stress and apoptosis in murine models [70,71].

Table 4.
Radioprotective effects of quercetin and its potential molecular mechanisms.
No clinical trials have been performed so far testing the potential of quercetin to reduce radiation-induced thoracic toxicities. However, its safety and tolerance have been proven in patients with COPD [74]. Table 4 summarizes the preclinical studies on the radioprotective effects of quercetin and its underlying molecular mechanisms.
2.2. Phenolic Acids
2.2.1. Caffeic Acid Phenethyl Ester (CAPE)
CAPE (Table 5), obtained from propolis and considered one of its main bioactive components, has been proposed as a radiosensitizer for different cancer types. In case of lung cancer, CAPE has been proposed to induce cell death and decreased cell division in vitro [75,76]. CAPE is also considered a radioprotective agent due to its anti-inflammatory, anti-oxidant and immunomodulatory properties [77]. One of its mechanisms of action is the inhibition of the radiation-induced NF-κB pathway, which results in a reduced inflammatory response, i.e., reduction of pro-inflammatory cytokines combined with an upregulation of anti-inflammatory markers, in normal tissues and mitigated irradiation-induced pneumonitis [78]. Another mechanism of action of CAPE is the reduction of oxidative stress via its antioxidant properties, which has been shown in rat models of RILT. Animals showed decreased radiation-induced ROS as well as increased antioxidant enzymes [79]. CAPE has also been suggested to have radioprotective effects against radiation-induced heart toxicities. In vivo studies in rats showed that the administration of CAPE led to decreased oxidative stress as well as decreased pro-oxidant and increased anti-oxidant activity in heart tissue. Radiation-induced hyperlipidemia, which leads to oxidative stress, was also reduced. CAPE has also been suggested to mitigate radiation-induced heart toxicities by preventing the increase in serum cardiac enzymes [80]. Table 5 summarizes the studies on the radioprotective effects of CAPE and its underlying molecular mechanisms.

Table 5.
Radioprotective effects of CAPE and its potential molecular mechanisms.
2.2.2. Curcumin
Curcumin, one of the major components of Indian turmeric (Curcuma longa), is a dietary polyphenol (Table 6) characterized for its antioxidant protective effects in normal tissue [31,81]. Furthermore, curcumin has also been proposed to have cytotoxic and radiosensitizing effects against different types of tumors, such as lung and cervical cancer by sensitizing cells to radiation as well as increasing apoptosis both in vitro and in vivo [31,81,82]. Curcumin has been suggested to have radioprotective effects in the normal tissue via scavenging ROS and preventing lipid peroxidation. Preliminary in vitro results showed that curcumin reduced the fraction of apoptotic lung cells and did not sensitize them to radiotherapy, which could be explained via its antioxidant properties [83,84]. In mouse lung fibroblasts, curcumin has been suggested to increase antioxidant enzymes activity as well as to prevent irradiation-induced ROS formation [85].

Table 6.
Radioprotective effects of curcumin and its potential molecular mechanisms.
In mouse models of lung irradiation, both curcumin supplemented diet as well as liposome-delivered curcumin ameliorated RILT by reducing pneumonitis and lung fibrosis [81,85]. Curcumin has also been proposed to mitigate inflammatory responses via suppressing NF-κB activation as well as reducing pro-inflammatory cytokine levels [81]. Curcumin ameliorated RILT in rats independently of the administration route, including intragastric administration and curcumin-containing nanoparticle inhalation. Rats receiving curcumin treatment showed reduced radiation-induced pneumonitis as well as lung fibrotic tissue. Furthermore, curcumin exerted anti-inflammatory effects with a reduction in immune infiltration in the lung [83,86,87]. Inhaled curcumin also decreased oxidative stress via increasing antioxidant enzymes expression and reducing lipid peroxidation [83]. Curcumin has also been suggested to mitigate RIHD in rat models of thoracic irradiation by decreasing the inflammatory infiltrate as well as pro-cytokine levels in heart tissue [88]. Table 6 summarizes the studies on the radioprotective effects of curcumin and its underlying molecular mechanisms.
2.2.3. Thymol
Thymol (Table 7), obtained from thyme (Thymus vulgaris), has been suggested to sensitize certain tumor types to radiation, while protecting against treatment adverse effects in the normal tissue [24,89]. In vitro studies have confirmed its radioprotective effects in lung tissue as a result of its anti-oxidant properties. The administration of thymol prior to irradiation in hamster lung fibroblasts resulted in a reduction of radiation-induced apoptosis and necrosis [89,90], mediated through the prevention of radiation-induced DNA damage and mitochondrial membrane collapse [90]. In addition, thymol mitigated radiation-induced oxidative stress via a decrease in ROS levels and lipid peroxidation, as well as a prevention of the radiation-mediated reduction of antioxidant enzymes [89]. Table 7 summarizes the in vitro studies on the radioprotective effects of thymol and its underlying molecular mechanisms. So far, no preclinical studies have been performed to investigate the radioprotective effects of thymol in lung, heart and esophagus.

Table 7.
Radioprotective effects of thymol and its potential molecular mechanisms.
2.2.4. Zingerone
Zingerone, one of the active compounds of ginger (Zingiber officinale), has also been suggested to have radioprotective effects (Table 8). However, literature supporting zingerone radioprotective effects in thoracic normal tissues is sparse. An in vitro study in hamster lung fibroblast cells proved zingerone to have anti-apoptotic and anti-oxidant properties. Pretreatment of zingerone improved cell survival after irradiation by inhibiting caspase-3 activation, reducing radiation-induced ROS levels and lipid peroxidation, as well as by increasing antioxidant enzymes levels [91].

Table 8.
Radioprotective effects of zingerone and its potential molecular mechanisms.
The radioprotective effects of zingerone against RIHD have been evaluated in vivo in rat models. Intragastric administration of zingerone prior to radiation mitigated the radiation-induced changes in architecture of the myocardial muscle fibers. The levels of cardiac toxicity and apoptotic markers decreased compared to radiation alone. Zingerone also reduced the immune infiltration and inflammation in heart tissue. Lastly, zingerone increased antioxidant enzyme activity and reduced lipid peroxidation [92]. Table 8 summarizes the studies on the radioprotective effects of zingerone and its underlying molecular mechanisms.
2.3. Stillbenes
Resveratrol
Resveratrol, extracted from grapes and wine, berries and peanuts (Table 9), has been shown to have cytotoxic effects against tumor cells [31,93], while protection against radiation adverse effects in normal tissues due to its anti-oxidant properties [24,31]. In RILT mouse models, resveratrol has been proven to reduce the degree of radiation-induced pneumonitis and lung fibrosis, by decreasing the inflammatory response and immune cell infiltration in the lungs [94,95]. Although the radioprotective effects of resveratrol against RIHD are poorly understood, one study demonstrated that resveratrol is able to restore the heart metabolic profile of mice receiving radiation treatment. The levels of choline-containing compounds as well as lipids with unsaturated fatty chains, involved in the structure of the cellular membrane, were restored after resveratrol treatment [96]. Further confirmatory research however is warranted. Table 9 summarizes the preclinical studies on the radioprotective effects of resveratrol and its underlying molecular mechanisms.

Table 9.
Radioprotective effects of resveratrol and its potential molecular mechanisms.
2.4. Lignans
Secoisolariciresinol Diglucoside (SDG)
SDG is one of the most common lignans and it is found in sesame, sunflower and pumpkin seeds, and flaxseeds (Table 10). SDG has been proposed to have cytotoxic effects against different cancer types, such as breast, colon and prostate [97,98,99]. In case of lung cancer, it has only been demonstrated that SDG does not have radioprotective effects in the tumor [100,101,102]. However, many studies support that SDG mitigates the radiation-induced adverse effects in the lungs due to its anti-inflammatory and anti-oxidant properties [100]. In vitro, SDG has been proposed to reduce radiation-induced DNA damage as well as oxidative stress by inducing antioxidant enzymes in normal lung cell lines [103]. In in vitro models of lung vasculature, SDG was observed to mitigate radiation-induced inflammation markers [104] as well as oxidative stress levels [103]. Ex vivo models of human precision cut lung slices also showed ameliorated radiation-induced adverse effects after proton irradiation and SDG treatment. SDG reduced radiation induced senescence, inflammation and oxidative stress [102].

Table 10.
Radioprotective effects of SDG and its potential molecular mechanisms.
In RILT mouse models, SDG supplemented diet prior to irradiation improved animal survival and welfare, together with reduced lung inflammation and fibrosis as well as oxidative stress [100,101,105]. Interestingly, the administration of SDG supplemented diet after several weeks post irradiation also mitigated the radiation-induced lung adverse effects in preclinical mouse models [106]. Table 10 summarizes the studies on the radioprotective effects of SDG and its underlying molecular mechanisms.
3. Discussion and Future Directions
In this review, we describe the effects of polyphenols against radiation-induced toxicity in lung, heart and esophagus. Polyphenols have been shown to have radioprotective effects also on other tissues including brain, breast, intestine, kidney, liver, prostate and skin (see Table S1 [42,43,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198,199,200]). Furthermore, polyphenols have also been proposed to have radiosensitizing effects at the level of the tumor. This dual effect combined with its low toxicity is an interesting avenue in order to increase the therapeutic window, as these compounds could be able to increase tumor control and protect the normal tissue against the therapy-related adverse effects, increasing patient survival and quality of life.
However, the administration of most polyphenols is hampered by their poor bioavailability due to their low solubility in water, which limits the delivery. For example, the average bioavailability of polyphenols when administered orally is 2 to 20% [201]. Furthermore, the content of polyphenols in certain types of food can also vary and therefore affect bioavailability when administered via diet, such as environmental factors, food processing and interaction with other molecules as well as human intestinal and general systemic factors (age, gender, previous pathologies, etc.) [202]. Another limitation frequently encountered when using polyphenols is their short half-life and therefore low tissue and plasma concentrations. Despite the fact that distribution of polyphenols varies depending on the administration route, most compounds are rapidly metabolized and excreted by the liver and kidneys into bile and urine, respectively [203]. Different strategies are being studied to overcome these limitations. Nanoencapsulation of polyphenols is becoming more common and studied in the research field to improve absorption and stability of these compounds. For example, many efforts have been made to encapsulate curcumin in lipoprotein particles, which resulted in improving its solubility while maintaining its radiosensitizing and radioprotective effects [81,84]. Another strategy is the development of polyphenol derivatives that have the same biological function with a better bioavailability and stability [204,205,206]. Derivatives of zingerone with higher water solubility were shown to have similar radioprotective effects as the natural compound, such as improved survival rate and prevented radiation-induced intestine toxicities in vivo [184].
Most studies testing the radioprotective effects of polyphenols in preclinical studies employed radiation techniques such as hemithorax, whole-thorax or whole-body irradiation. These experimental set-ups however do not represent current clinical practice, as here the irradiated area is larger and therefore the radiotherapy-adverse toxicities in the normal tissue might be more extensive [207]. This could lead to an underestimation of the radioprotective effects of polyphenols. Overall, further research should be performed to confirm the radioprotective effects of the discussed polyphenols and/or their novel derivatives with improved pharmacokinetic profiles both in preclinical studies, using state-of-the-art irradiation techniques reflecting the clinical situation which becomes possible with the development of small animal irradiators [208], and especially in clinical trials.
4. Conclusions
In conclusion, the use of polyphenols could be a promising strategy to increase the therapeutic window. Their dual radiosensitizing and radioprotective effect, combined with their low toxicity, suggests that they can increase tumor control, as well as protect the normal tissue against radiotherapy-induced toxicities, ultimately improving patient survival and quality of life. However, their poor bioavailability and short half-life may limit their effectiveness and administration. These limitations could be overcome via, for example, encapsulation of the compound or by developing derivatives of polyphenols with better solubility and equal biological effect. In addition, most preclinical studies performed do not mimic the current clinical practice, which could lead to an underestimation of their protective properties. Therefore, further research is needed to confirm their radio-protective effects in vivo as well as in clinical trials.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers15092412/s1, Table S1: Radioprotective effects of polyphenols in other healthy tissues.
Author Contributions
Writing—È.P.-S.; review and editing—A.Y. and L.J.D. All authors have read and agreed to the published version of the manuscript.
Funding
E.P.S. has received funding from the European’s Union Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement No #860245 (to L.J.D.).
Conflicts of Interest
LJD has, outside of the submitted work, shares in the companies Convert Pharmaceuticals and LivingMed Biotech, he is co-inventor of a non-issue, non-licensed patent on LSRT (N2024889). The other authors declare no conflict of interest.
Abbreviations
| 8-OHdG | 8-Oxo-2’-deoxyguanosin | MAPK | Mitogen-Activated Protein Kinase |
| ADA | Adenosine Deaminase | MDA | Malondialdehyde |
| AST | Aspartate transaminase | MPO | Myeloperoxidase |
| BALF | Bronchoalveolar Lavage Fluid | NCT | National Clinical Trial |
| Bax | Bcl-2-associated X protein | NF-κB | Nuclear Factor kappa B |
| Bcl-2 | B-cell lymphoma 2 | NLRP3 | NLR family pyrin domain containing 3 |
| BNP | Brain natriuretic peptide | NO | Nitric Oxide |
| BW | Body Weight | NQO1 | NAD(P)H Quinone Dehydrogenase 1 |
| CAPE | Caffeic Acid Phenethyl Ester | Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| CAT | Catalase | NTCP | Normal Tissue Complication Probability |
| CDK | Cyclin-dependent kinase | PO | Per os |
| CDKN2A | cyclin-dependent kinase inhibitor 2A | pRb | Retinoblastoma protein |
| CK-MB | Creatine phosphokinase-MB | QD | Quaque die, once a day |
| COPD | Chronic obstructive pulmonary disease | QDx–yW | Once a day for x days/week and for y weeks |
| COX-2 | Prostaglandin-endoperoxide synthase 2 | RIHD | Radiation-induced heart disease |
| CPK | Creatine phosphokinase | RILT | Radiation-induced lung toxicity |
| cTNT | Cardiac muscle troponin T | ROS | Reactive Oxygen Species |
| DUOX1/2 | Dual oxidase 1/2 | RT | Radiotherapy |
| Erk1/2 | Extracellular signal-regulated kinases | SAPK | Stress-activated protein kinases |
| ETC | Electron Transport Chain | SDG | Secoisolariciresinol diglucoside |
| GSH | Glutathione | Smad3 | Mothers against decapentaplegic homolog 3 |
| GST | Glutathione S-transferase | SOD | Superoxide Dismutase |
| Gstm1 | Glutathione S-transferase mu 1 | TBARS | Thiobarbituric acid reactive substances |
| HMOX1 | Heme Oxygenase 1 gene | TCP | Tumor Control Probability |
| H2AX HO-1 | H2A histone family member X Heme Oxygenase 1 | TGF-β | Transforming growth factor beta |
| ICAM-1 | Intercellular Adhesion Molecule 1 | TNFR1 | Tumor necrosis factor receptor 1 |
| IFN-γ | Interferon γ | TNF-α | Tumor necrosis factor α |
| IL | Interleukin | VCAM | Vascular cell adhesion protein |
| IMRT | Intensity Modulated RadioTherapy | VEGF | Vascular endothelial growth factor |
| IP | Intraperitoneal | XO | Xanthine Oxidase |
| IRCT | Iranian registry of clinical trials | ↑ | Increase |
| JNK | Jun amino-terminal kinases | ↓ | Decrease |
| LDH | Lactate Dehydrogenase | ||
| LDL | Low Density Lipoprotein |
References
- Allen, C.; Her, S.; Jaffray, D.A. Radiotherapy for Cancer: Present and Future. Adv. Drug Deliv. Rev. 2017, 109, 1–2. [Google Scholar] [CrossRef]
- Jin, H.; Yoo, Y.; Kim, Y.; Kim, Y.; Cho, J.; Lee, Y.-S. Radiation-Induced Lung Fibrosis: Preclinical Animal Models and Therapeutic Strategies. Cancers 2020, 12, 1561. [Google Scholar] [CrossRef] [PubMed]
- Ruysscher, D.; Niedermann, G.; Burnet, N.; Siva, S.; Lee, A.; Hegi-Johnson, F. Radiotherapy toxicity. Nat. Rev. Dis. Prim. 2019, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Bradley, J.; Movsas, B. Radiation Pneumonitis and Esophagitis in Thoracic Irradiation, in Radiation Toxicity: A Practical Guide; Small, W., Woloschak, G.E., Eds.; Springer: Boston, MA, USA, 2006; pp. 43–64. [Google Scholar]
- Wang, H.; Wei, J.; Zheng, Q.; Meng, L.; Xin, Y.; Yin, X.; Jiang, X. Radiation-induced heart disease: A review of classification, mechanism and prevention. Int. J. Biol. Sci. 2019, 15, 2128–2138. [Google Scholar] [CrossRef] [PubMed]
- Bledsoe, T.J.; Nath, S.; Decker, R. Radiation Pneumonitis. Clin. Chest. Med. 2017, 38, 201–208. [Google Scholar] [CrossRef]
- Giuranno, L.; Ient, J.; De Ruysscher, D.; Vooijs, M.A. Radiation-Induced Lung Injury (RILI). Front Oncol. 2019, 9, 877. [Google Scholar] [CrossRef]
- Ueki, N.; Matsuo, Y.; Togashi, Y.; Kubo, T.; Shibuya, K.; Iizuka, Y.; Mizowaki, T.; Togashi, K.; Mishima, M.; Hiraoka, M. Impact of pretreatment interstitial lung disease on radiation pneumonitis and survival after stereotactic body radiation therapy for lung cancer. J. Thorac. Oncol. 2015, 10, 116–125. [Google Scholar] [CrossRef]
- Zou, B.; Schuster, J.P.; Niu, K.; Huang, Q.; Rühle, A.; Huber, P.E. Radiotherapy-induced heart disease: A review of the literature. Precis. Clin. Med. 2019, 2, 270–282. [Google Scholar] [CrossRef]
- Yegya-Raman, N.; Berlin, E.; Feigenberg, S.J.; Ky, B.; Sun, L. Cardiovascular Toxicity and Risk Mitigation with Lung Cancer Treatment. Curr. Oncol. Rep. 2023, 25, 433–444. [Google Scholar] [CrossRef]
- Atkins, K.M.; Rawal, B.; Chaunzwa, T.L.; Lamba, N.; Bitterman, D.S.; Williams, C.L.; Kozono, D.E.; Baldini, E.H.; Chen, A.B.; Nguyen, P.L.; et al. Cardiac Radiation Dose, Cardiac Disease, and Mortality in Patients with Lung Cancer. J. Am. Coll. Cardiol. 2019, 73, 2976–2987. [Google Scholar] [CrossRef]
- Werner-Wasik, M.; Paulus, R.; Curran, W.J.; Byhardt, R. Acute Esophagitis and Late Lung Toxicity in Concurrent Chemoradiotherapy Trials in Patients with Locally Advanced Non–Small-Cell Lung Cancer: Analysis of the Radiation Therapy Oncology Group (RTOG) Database. Clin. Lung Cancer 2011, 12, 245–251. [Google Scholar] [CrossRef]
- Murro, D.; Jakate, S. Radiation esophagitis. Arch. Pathol. Lab. Med. 2015, 139, 827–830. [Google Scholar] [CrossRef] [PubMed]
- Benson, R.M.S. Therapeutic Index and Its Clinical Significance; In Practical Radiation Oncology; Springer: Singapore, 2020. [Google Scholar]
- Buckley, A.M.; Lynam-Lennon, N.; O’neill, H.; O’sullivan, J. Targeting hallmarks of cancer to enhance radiosensitivity in gastrointestinal cancers. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 298–313. [Google Scholar] [CrossRef] [PubMed]
- Wardman, P. Chemical radiosensitizers for use in radiotherapy. Clin. Oncol. 2007, 19, 397–417. [Google Scholar] [CrossRef]
- De Ruysscher, D.; Faivre-Finn, C.; Moeller, D.; Nestle, U.; Hurkmans, C.W.; Le Péchoux, C.; Belderbos, J.; Guckenberger, M.; Senan, S. European Organization for Research and Treatment of Cancer (EORTC) recommendations for planning and delivery of high-dose, high precision radiotherapy for lung cancer. Radiother. Oncol. 2017, 124, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ghaderi, N.; Jung, J.; Brüningk, S.; Subramanian, A.; Nassour, L.; Peacock, J. A Century of Fractionated Radiotherapy: How Mathematical Oncology Can Break the Rules. Int. J. Mol. Sci. 2022, 23, 1316. [Google Scholar] [CrossRef]
- van der Heide, U.A.; Houweling, A.C.; Groenendaal, G.; Beets-Tan, R.G.; Lambin, P. Functional MRI for radiotherapy dose painting. Magn. Reson. Imaging 2012, 30, 1216–1223. [Google Scholar] [CrossRef]
- Chun, S.G.; Hu, C.; Choy, H.; Komaki, R.U.; Timmerman, R.D.; Schild, S.E.; Bogart, J.A.; Dobelbower, M.C.; Bosch, W.; Galvin, J.M.; et al. Impact of Intensity-Modulated Radiation Therapy Technique for Locally Advanced Non-Small-Cell Lung Cancer: A Secondary Analysis of the NRG Oncology RTOG 0617 Randomized Clinical Trial. J. Clin. Oncol. 2017, 35, 56–62. [Google Scholar] [CrossRef]
- Trani, D.; Yaromina, A.; Dubois, L.J.; Granzier, M.; Peeters, S.G.; Biemans, R.; Nalbantov, G.; Lieuwes, N.G.; Reniers, B.R.; Troost, E.E.; et al. Preclinical Assessment of Efficacy of Radiation Dose Painting Based on Intratumoral FDG-PET Uptake. Clin. Cancer Res. 2015, 21, 5511–5518. [Google Scholar] [CrossRef]
- Trani, D.; Reniers, B.; Persoon, L.; Podesta, M.; Nalbantov, G.; Leijenaar, R.T.; Granzier, M.; Yaromina, A.; Dubois, L.; Verhaegen, F.; et al. What level of accuracy is achievable for preclinical dose painting studies on a clinical irradiation platform? Radiat. Res. 2015, 183, 501–510. [Google Scholar] [CrossRef]
- Yaromina, A.; Granzier, M.; Biemans, R.; Lieuwes, N.; van Elmpt, W.; Shakirin, G.; Dubois, L.; Lambin, P. A novel concept for tumour targeting with radiation: Inverse dose-painting or targeting the Low Drug Uptake Volume. Radiother. Oncol. 2017, 124, 513–520. [Google Scholar] [CrossRef]
- Fischer, N.; Seo, E.-J.; Efferth, T. Efferth, Prevention from radiation damage by natural products. Phytomedicine 2017, 47, 192–200. [Google Scholar] [CrossRef]
- Obrador, E.; Salvador, R.; Villaescusa, J.I.; Soriano, J.M.; Estrela, J.M.; Montoro, A. Radioprotection and Radiomitigation: From the Bench to Clinical Practice. Biomedicines 2020, 8, 461. [Google Scholar] [CrossRef] [PubMed]
- Kuruba, V.; Gollapalli, P. Natural radioprotectors and their impact on cancer drug discovery. Radiat. Oncol. J. 2018, 36, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Devine, A.; Marignol, L. Potential of Amifostine for Chemoradiotherapy and Radiotherapy-associated Toxicity Reduction in Advanced NSCLC: A Meta-Analysis. Anticancer. Res. 2016, 36, 5–12. [Google Scholar]
- Hanania, A.N.; Mainwaring, W.; Ghebre, Y.; Hanania, N.; Ludwig, M. Radiation-Induced Lung Injury: Assessment and Management. Chest 2019, 156, 150–162. [Google Scholar] [CrossRef]
- King, M.; Joseph, S.; Albert, A.; Thomas, T.V.; Nittala, M.R.; Woods, W.C.; Vijayakumar, S.; Packianathan, S. Use of Amifostine for Cytoprotection during Radiation Therapy: A Review. Oncology 2019, 98, 61–80. [Google Scholar] [CrossRef]
- Bourhis, J.; Rosine, D. Radioprotective effect of amifostine in patients with head and neck squamous cell carcinoma. Semin. Oncol. 2002, 29 (Suppl. S19), 61–62. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Seong, K.M.; Youn, B. Phenylpropanoids in radioregulation: Double edged sword. Exp. Mol. Med. 2011, 43, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Faramarzi, S.; Piccolella, S.; Manti, L.; Pacifico, S. Could Polyphenols Really Be a Good Radioprotective Strategy? Mol. Basel Switz. 2021, 26, 4969. [Google Scholar] [CrossRef]
- Xiang, D.; Wang, D.; He, Y.; Xie, J.; Zhong, Z.; Li, Z.; Xie, J. Caffeic acid phenethyl ester induces growth arrest and apoptosis of colon cancer cells via the beta-catenin/T-cell factor signaling. Anticancer Drugs 2006, 17, 753–762. [Google Scholar] [CrossRef]
- Surh, Y.-J. Cancer chemoprevention with dietary phytochemicals. Nat. Rev. Cancer 2003, 3, 768–780. [Google Scholar] [CrossRef] [PubMed]
- Ramos, S. Cancer chemoprevention and chemotherapy: Dietary polyphenols and signalling pathways. Mol. Nutr. Food Res. 2008, 52, 507–526. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Pan, H.; Li, J. Molecular Insights into Potential Contributions of Natural Polyphenols to Lung Cancer Treatment. Cancers 2019, 11, 1565. [Google Scholar] [CrossRef] [PubMed]
- Kilic, T.; Ciftci, O.; Cetin, A.; Kahraman, H. Preventive effect of chrysin on bleomycin-induced lung fibrosis in rats. Inflammation 2014, 37, 2116–2124. [Google Scholar] [CrossRef]
- Chen, L.; Zhao, W. Apigenin protects against bleomycin-induced lung fibrosis in rats. Exp. Ther. Med. 2015, 11, 230–234. [Google Scholar] [CrossRef]
- Yao, L.; Hou, G.; Wang, L.; Zuo, X.-S.; Liu, Z. Protective effects of thymol on LPS-induced acute lung injury in mice. Microb. Pathog. 2018, 116, 8–12. [Google Scholar] [CrossRef]
- Zhang, J.; Chao, L.; Liu, X.; Shi, Y.; Zhang, C.; Kong, L.; Li, R. The potential application of strategic released apigenin from polymeric carrier in pulmonary fibrosis. Exp. Lung Res. 2017, 43, 359–369. [Google Scholar] [CrossRef]
- Christofidou-Solomidou, M.; Pietrofesa, R.A.; Arguiri, E.; Koumenis, C.; Segal, R. Radiation Mitigating Properties of Intranasally Administered KL(4) Surfactant in a Murine Model of Radiation-Induced Lung Damage. Radiat. Res. 2017, 188, 571–584. [Google Scholar] [CrossRef]
- Becker-Schiebe, M.; Mengs, U.; Schaefer, M.; Bulitta, M.; Hoffmann, W. Topical use of a silymarin-based preparation to prevent radiodermatitis: Results of a prospective study in breast cancer patients. Strahlenther. Onkol. 2011, 187, 485–491. [Google Scholar] [CrossRef]
- Ryan, J.L.; Heckler, C.E.; Ling, M.; Katz, A.; Williams, J.P.; Pentland, A.P.; Morrow, G.R. Curcumin for radiation dermatitis: A randomized, double-blind, placebo-controlled clinical trial of thirty breast cancer patients. Radiat. Res. 2013, 180, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xing, L.; Zhang, Y.; Xie, P.; Zhu, W.; Meng, X.; Wang, Y.; Kong, L.; Zhao, H.; Yu, J. Phase II Trial of Epigallocatechin-3-Gallate in Acute Radiation-Induced Esophagitis for Esophagus Cancer. J. Med. Food 2020, 23, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Fountain, M.D.; Abernathy, L.M.; Lonardo, F.; Rothstein, S.E.; Dominello, M.M.; Yunker, C.K.; Chen, W.; Gadgeel, S.; Joiner, M.C.; Hillman, G.G. Radiation-Induced Esophagitis is Mitigated by Soy Isoflavones. Front. Oncol. 2015, 5, 238. [Google Scholar] [CrossRef]
- Camouse, M.M.; Hanneman, K.K.; Conrad, E.P.; Baron, E.D. Protective effects of tea polyphenols and caffeine. Expert Rev. Anticancer. Ther. 2005, 5, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Hwang, K.; Lee, J.; Han, S.Y.; Kim, E.-M.; Park, J.; Cho, J.Y. Skin Protective Effect of Epigallocatechin Gallate. Int. J. Mol. Sci. 2018, 19, 173. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, K.; Uehara, Y.; Nakata, E.; Inoue, M.; Shimazu, K.; Yoshida, T.; Kanda, H.; Nanjo, H.; Hosoi, Y.; Yamakoshi, H.; et al. A diarylpentanoid curcumin analog exhibits improved radioprotective potential in the intestinal mucosa. Int. J. Radiat. Biol. 2016, 92, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-T.; Cui, W.-Q.; Pan, D.; Jiang, M.; Chang, B.; Sang, L.-X. Tea polyphenols and their chemopreventive and therapeutic effects on colorectal cancer. World J. Gastroenterol. 2020, 26, 562–597. [Google Scholar] [CrossRef]
- Zhao, H.; Jia, L.; Chen, G.; Li, X.; Meng, X.; Zhao, X.; Xing, L.; Zhu, W. A prospective, three-arm, randomized trial of EGCG for preventing radiation-induced esophagitis in lung cancer patients receiving radiotherapy. Radiother. Oncol. 2019, 137, 186–191. [Google Scholar] [CrossRef]
- Fresco, P.; Borges, F.; Diniz, C.; Marques, M. New insights on the anticancer properties of dietary polyphenols. Med. Res. Rev. 2006, 26, 747–766. [Google Scholar] [CrossRef]
- Hillman, G.G.; Singh-Gupta, V.; Runyan, L.; Yunker, C.K.; Rakowski, J.T.; Sarkar, F.H.; Miller, S.; Gadgeel, S.M.; Sethi, S.; Joiner, M.C.; et al. Soy isoflavones radiosensitize lung cancer while mitigating normal tissue injury. Radiother. Oncol. 2011, 101, 329–336. [Google Scholar]
- Hillman, G.G.; Singh-Gupta, V.; Hoogstra, D.J.; Abernathy, L.; Rakowski, J.; Yunker, C.K.; Rothstein, S.E.; Sarkar, F.H.; Gadgeel, S.; Konski, A.A.; et al. Differential effect of soy isoflavones in enhancing high intensity radiotherapy and protecting lung tissue in a pre-clinical model of lung carcinoma. Radiother. Oncol. 2013, 109, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Para, A.E.; Bezjak, A.; Yeung, I.W.; Van Dyk, J.; Hill, R.P. Effects of genistein following fractionated lung irradiation in mice. Radiother. Oncol. 2009, 92, 500–510. [Google Scholar] [CrossRef]
- Calveley, V.L.; Jelveh, S.; Langan, A.; Mahmood, J.; Yeung, I.W.T.; Van Dyk, J.; Hill, R.P. Genistein can mitigate the effect of radiation on rat lung tissue. Radiat. Res. 2010, 173, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Abernathy, L.M.; Fountain, M.D.; Rothstein, S.E.; David, J.M.; Yunker, C.K.; Rakowski, J.; Lonardo, F.; Joiner, M.C.; Hillman, G.G. Soy Isoflavones Promote Radioprotection of Normal Lung Tissue by Inhibition of Radiation-Induced Activation of Macrophages and Neutrophils. J. Thorac. Oncol. 2015, 10, 1703–1712. [Google Scholar] [CrossRef]
- Fountain, M.D.; McLellan, L.A.; Smith, N.L.; Loughery, B.F.; Rakowski, J.T.; Tse, H.Y.; Hillman, G.G. Isoflavone-mediated radioprotection involves regulation of early endothelial cell death and inflammatory signaling in Radiation-Induced lung injury. Int. J. Radiat. Biol. 2019, 96, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Abernathy, L.M.; Fountain, M.D.; Joiner, M.C.; Hillman, G.G. Hillman, Innate Immune Pathways Associated with Lung Radioprotection by Soy Isoflavones. Front. Oncol. 2017, 7, 7. [Google Scholar] [CrossRef]
- Dominello, M.M.; Fountain, M.D.; Rothstein, S.E.; Cannon, A.C.; Abernathy, L.M.; Hoogstra, D.; Chen, W.; Joiner, M.C.; Hillman, G.G. Radiation injury to cardiac arteries and myocardium is reduced by soy isoflavones. J. Radiat. Oncol. 2017, 6, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Gan, R.-Y.; Li, H.-B.; Sui, Z.-Q.; Corke, H. Absorption, metabolism, anti-cancer effect and molecular targets of epigallocatechin gallate (EGCG): An updated review. Crit. Rev. Food Sci. Nutr. 2018, 58, 924–941. [Google Scholar] [CrossRef]
- You, H.; Wei, L.; Sun, W.-L.; Wang, L.; Yang, Z.-L.; Liu, Y.; Zheng, K.; Wang, Y.; Zhang, W.-J. The green tea extract epigallocatechin-3-gallate inhibits irradiation-induced pulmonary fibrosis in adult rats. Int. J. Mol. Med. 2014, 34, 92–102. [Google Scholar] [CrossRef]
- Zhao, H.; Zhu, W.; Xie, P.; Li, H.; Zhang, X.; Sun, X.; Yu, J.; Xing, L. A phase I study of concurrent chemotherapy and thoracic radiotherapy with oral epigallocatechin-3-gallate protection in patients with locally advanced stage III non-small-cell lung cancer. Radiother. Oncol. 2014, 110, 132–136. [Google Scholar] [CrossRef]
- Zhao, H.; Xie, P.; Li, X.; Zhu, W.; Sun, X.; Sun, X.; Chen, X.; Xing, L.; Yu, J. A prospective phase II trial of EGCG in treatment of acute radiation-induced esophagitis for stage III lung cancer. Radiother. Oncol. 2015, 114, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Zhao, Y.; Zhang, S.; Li, X.; Xing, L.; Zhao, H.; Yu, J. Evaluation of Epigallocatechin-3-Gallate as a Radioprotective Agent During Radiotherapy of Lung Cancer Patients: A 5-Year Survival Analysis of a Phase 2 Study. Front. Oncol. 2021, 11, 686950. [Google Scholar] [CrossRef]
- Bosch-Barrera, J.; Queralt, B.; Menendez, J.A. Targeting STAT3 with silibinin to improve cancer therapeutics. Cancer Treat. Rev. 2017, 58, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Cufí, S.; Bonavia, R.; Vazquez-Martin, A.; Corominas-Faja, B.; Oliveras-Ferraros, C.; Cuyàs, E.; Martín-Castillo, B.; Barrajón-Catalán, E.; Visa, J.; Carretero, A.S.; et al. Silibinin meglumine, a water-soluble form of milk thistle silymarin, is an orally active anti-cancer agent that impedes the epithelial-to-mesenchymal transition (EMT) in EGFR-mutant non-small-cell lung carcinoma cells. Food Chem. Toxicol. 2013, 60, 360–368. [Google Scholar] [CrossRef]
- Son, Y.; Lee, H.J.; Rho, J.K.; Chung, S.Y.; Lee, C.G.; Yang, K.; Kim, S.H.; Lee, M.; Shin, I.S.; Kim, J.S. The ameliorative effect of silibinin against radiation-induced lung injury: Protection of normal tissue without decreasing therapeutic efficacy in lung cancer. BMC Pulm. Med. 2015, 15, 68. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, Y.; Lu, H.; Wang, H.; Feng, H.; Xu, J.; Zhang, B. Quercetin radiosensitizes non-small cell lung cancer cells through the regulation of miR-16-5p/WEE1 axis. IUBMB Life 2020, 72, 1012–1022. [Google Scholar] [CrossRef]
- Reyes-Farias, M.; Carrasco-Pozo, C. The Anti-Cancer Effect of Quercetin: Molecular Implications in Cancer Metabolism. Int. J. Mol. Sci. 2019, 20, 3177. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xue, J.-X.; Li, X.; Ao, R.; Lu, Y. Quercetin liposomes protect against radiation-induced pulmonary injury in a murine model. Oncol. Lett. 2013, 6, 453–459. [Google Scholar] [CrossRef]
- Verma, S.; Dutta, A.; Dahiya, A.; Kalra, N. Quercetin-3-Rutinoside alleviates radiation-induced lung inflammation and fibrosis via regulation of NF-kappaB/TGF-beta1 signaling. Phytomedicine 2022, 99, 154004. [Google Scholar] [CrossRef]
- Qin, M.; Chen, W.; Cui, J.; Li, W.; Liu, D.; Zhang, W. Protective efficacy of inhaled quercetin for radiation pneumonitis. Exp. Ther. Med. 2017, 14, 5773–5778. [Google Scholar] [CrossRef][Green Version]
- Wang, J.; Zhang, Y.-Y.; Cheng, J.; Zhang, J.-L.; Li, B.-S. Preventive and therapeutic effects of quercetin on experimental radiation induced lung injury in mice. Asian Pac. J. Cancer Prev. 2015, 16, 2909–2914. [Google Scholar] [CrossRef] [PubMed]
- Han, M.K.; A Barreto, T.; Martinez, F.J.; Comstock, A.T.; Sajjan, U.S. Randomised clinical trial to determine the safety of quercetin supplementation in patients with chronic obstructive pulmonary disease. BMJ Open Respir. Res. 2020, 7, e000392. [Google Scholar] [CrossRef]
- Chen, M.-F.; Wu, C.-T.; Chen, Y.-J.; Keng, P.C.; Chen, W.-C. Cell killing and radiosensitization by caffeic acid phenethyl ester (CAPE) in lung cancer cells. J. Radiat. Res. 2004, 45, 253–260. [Google Scholar] [CrossRef]
- Omene, C.O.; Wu, J.; Frenkel, K. Caffeic Acid Phenethyl Ester (CAPE) derived from propolis, a honeybee product, inhibits growth of breast cancer stem cells. Investig. New Drugs 2011, 30, 1279–1288. [Google Scholar] [CrossRef] [PubMed]
- Çalikoglu, M. The effects of caffeic acid phenethyl ester on tissue damage in lung after hindlimb ischemia-reperfusion. Pharmacol. Res. 2003, 48, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-F.; Keng, P.C.; Lin, P.-Y.; Yang, C.-T.; Liao, S.-K.; Chen, W.-C. Caffeic acid phenethyl ester decreases acute pneumonitis after irradiation In Vitro and In Vivo. BMC Cancer 2005, 5, 158. [Google Scholar] [CrossRef]
- Yildiz, O.G.; Soyuer, S.; Saraymen, R.; Eroglu, C. Protective effects of caffeic acid phenethyl ester on radiation induced lung injury in rats. Clin. Investig. Med. 2008, 31, E242–E247. [Google Scholar] [CrossRef]
- Mansour, H.H.; Tawfik, S.S. Early treatment of radiation-induced heart damage in rats by caffeic acid phenethyl ester. Eur. J. Pharmacol. 2012, 692, 46–51. [Google Scholar] [CrossRef]
- Gao, X.X.; Shi, H.-S.; Li, D.; Zhang, Q.-W.; Wang, Y.-S.; Zheng, Y.; Cai, L.-L.; Zhong, R.-M.; Rui, A.; Li, Z.-Y.; et al. A systemic administration of liposomal curcumin inhibits radiation pneumonitis and sensitizes lung carcinoma to radiation. Int. J. Nanomed. 2012, 7, 2601–2611. [Google Scholar] [CrossRef]
- Javvadi, P.; Segan, A.T.; Tuttle, S.W.; Koumenis, C. The chemopreventive agent curcumin is a potent radiosensitizer of human cervical tumor cells via increased reactive oxygen species production and overactivation of the mitogen-activated protein kinase pathway. Mol. Pharmacol. 2008, 73, 1491–1501. [Google Scholar] [CrossRef]
- Chen, T.; Zhuang, B.; Huang, Y.; Liu, Y.; Yuan, B.; Wang, W.; Yuan, T.; Du, L.; Jin, Y. Inhaled curcumin mesoporous polydopamine nanoparticles against radiation pneumonitis. Acta Pharm. Sin. B 2021, 12, 2522–2532. [Google Scholar] [CrossRef]
- Evans, A.C.; Martin, K.A.; Saxena, M.; Bicher, S.; Wheeler, E.; Cordova, E.J.; Porada, C.D.; Almeida-Porada, G.; Kato, T.A.; Wilson, P.F.; et al. Curcumin Nanodiscs Improve Solubility and Serve as Radiological Protectants against Ionizing Radiation Exposures in a Cell-Cycle Dependent Manner. Nanomaterials 2022, 12, 3619. [Google Scholar] [CrossRef]
- Lee, J.C.; Kinniry, P.A.; Arguiri, E.; Serota, M.; Kanterakis, S.; Chatterjee, S.; Solomides, C.C.; Javvadi, P.; Koumenis, C.; Cengel, K.A.; et al. Dietary curcumin increases antioxidant defenses in lung, ameliorates radiation-induced pulmonary fibrosis, and improves survival in mice. Radiat. Res. 2010, 173, 590–601. [Google Scholar] [CrossRef] [PubMed]
- Amini, P.; Saffar, H.; Nourani, M.R.; Motevaseli, E.; Najafi, M.; Taheri, R.A.; Qazvini, A. Curcumin Mitigates Radiation-induced Lung Pneumonitis and Fibrosis in Rats. Int. J. Mol. Cell. Med. 2019, 7, 212–219. [Google Scholar]
- Cho, Y.J.; Yi, C.O.; Jeon, B.T.; Jeong, Y.Y.; Kang, G.M.; Lee, J.E.; Roh, G.S.; Lee, J.D. Curcumin attenuates radiation-induced inflammation and fibrosis in rat lungs. Korean J. Physiol. Pharmacol. 2013, 17, 267–274. [Google Scholar] [CrossRef]
- Kolivand, S.; Amini, P.; Saffar, H.; Rezapoor, S.; Motevaseli, E.; Najafi, M.; Nouruzi, F.; Shabeeb, D.; Musa, A.E. Evaluating the Radioprotective Effect of Curcumin on Rat’s Heart Tissues. Curr. Radiopharm. 2019, 12, 23–28. [Google Scholar] [CrossRef]
- Archana, P.; Rao, B.N.; Ballal, M. Thymol, a naturally occurring monocyclic dietary phenolic compound protects Chinese hamster lung fibroblasts from radiation-induced cytotoxicity. Mutat. Res. Toxicol. Environ. Mutagen. 2009, 680, 70–77. [Google Scholar] [CrossRef]
- Archana, P.R.; Rao, B.N.; Rao, B.S. Modulation of gamma ray-induced genotoxic effect by thymol, a monoterpene phenol derivative of cymene. Integr. Cancer Ther. 2011, 10, 374–383. [Google Scholar] [CrossRef]
- Nageshwar Rao, B.; Satish Rao, B. Antagonistic effects of Zingerone, a phenolic alkanone against radiation-induced cytotoxicity, genotoxicity, apoptosis and oxidative stress in Chinese hamster lung fibroblast cells growing In Vitro. Mutagenesis 2010, 25, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Soliman, A.F.; Anees, L.M.; Ibrahim, D.M. Cardioprotective effect of zingerone against oxidative stress, inflammation, and apoptosis induced by cisplatin or gamma radiation in rats. Naunyn Schmiedebergs Arch. Pharm. 2018, 391, 819–832. [Google Scholar] [CrossRef] [PubMed]
- Banegas, Y.C.; Ocolotobiche, E.E.; Padula, G.; Córdoba, E.E.; Fernández, E.; Güerci, A.M. Evaluation of resveratrol radiomodifying potential for radiotherapy treatment. Mutat. Res. Toxicol. Environ. Mutagen. 2018, 836, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Azmoonfar, R.; Amini, P.; Yahyapour, R.; Rezaeyan, A.; Tavassoli, A.; Motevaseli, E.; Khodamoradi, E.; Shabeeb, D.; Musa, A.E.; Najafi, M. Mitigation of Radiation-induced Pneumonitis and Lung Fibrosis using Alpha-lipoic Acid and Resveratrol. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2020, 19, 149–157. [Google Scholar] [CrossRef]
- Yahyapour, R.; Amini, P.; Saffar, H.; Motevaseli, E.; Farhood, B.; Pooladvand, V.; Shabeeb, D.; Musa, A.E.; Najafi, M. Protective Effect of Metformin, Resveratrol and Alpha-lipoic Acid on Radiation- Induced Pneumonitis and Fibrosis: A Histopathological Study. Curr. Drug Res. Rev. 2019, 11, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Gramatyka, M.; Widłak, P.; Gabryś, D.; Kulik, R.; Sokół, M. Resveratrol administration prevents radiation-related changes in metabolic profiles of hearts 20 weeks after irradiation of mice with a single 2 Gy dose. Acta Biochim. Pol. 2020, 67, 629–632. [Google Scholar] [CrossRef] [PubMed]
- Bowers, L.W.; Lineberger, C.G.; Ford, N.A.; Rossi, E.L.; Punjala, A.; Camp, K.K.; Kimler, B.K.; Fabian, C.J.; Hursting, S.D. The flaxseed lignan secoisolariciresinol diglucoside decreases local inflammation, suppresses NFκB signaling, and inhibits mammary tumor growth. Breast Cancer Res. Treat. 2018, 173, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Jenab, M.; Thompson, L.U. The influence of flaxseed and lignans on colon carcinogenesis and beta-glucuronidase activity. Carcinogenesis 1996, 17, 1343–1348. [Google Scholar] [CrossRef] [PubMed]
- Demark-Wahnefried, W.; Polascik, T.J.; George, S.L.; Switzer, B.R.; Madden, J.F.; Ruffin, M.T.; Snyder, D.C.; Owzar, K.; Hars, V.; Albala, D.M.; et al. Flaxseed supplementation (not dietary fat restriction) reduces prostate cancer proliferation rates in men presurgery. Cancer Epidemiol. Biomark. Prev. 2008, 17, 3577–3587. [Google Scholar] [CrossRef]
- Christofidou-Solomidou, M.; Tyagi, S.; Pietrofesa, R.; Dukes, F.; Arguiri, E.; Turowski, J.; Grieshaber, P.A.; Solomides, C.C.; Cengel, K.A. Radioprotective role in lung of the flaxseed lignan complex enriched in the phenolic secoisolariciresinol diglucoside (SDG). Radiat. Res. 2012, 178, 568–580. [Google Scholar] [CrossRef]
- Lee, J.C.; Krochak, R.; Blouin, A.; Kanterakis, S.; Chatterjee, S.; Arguiri, E.; Vachani, A.; Solomides, C.C.; A Cengel, K.; Christofidou-Solomidou, M. Dietary flaxseed prevents radiation-induced oxidative lung damage, inflammation and fibrosis in a mouse model of thoracic radiation injury. Cancer Biol. Ther. 2009, 8, 47–53. [Google Scholar] [CrossRef]
- Velalopoulou, A.; Chatterjee, S.; Pietrofesa, R.A.; Koziol-White, C.; Panettieri, R.A.; Lin, L.; Tuttle, S.; Berman, A.; Koumenis, C.; Christofidou-Solomidou, M. Synthetic Secoisolariciresinol Diglucoside (LGM2605) Protects Human Lung in an Ex Vivo Model of Proton Radiation Damage. Int. J. Mol. Sci. 2017, 18, 2525. [Google Scholar] [CrossRef]
- Velalopoulou, A.; Tyagi, S.; Pietrofesa, R.A.; Arguiri, E.; Christofidou-Solomidou, M. The Flaxseed-Derived Lignan Phenolic Secoisolariciresinol Diglucoside (SDG) Protects Non-Malignant Lung Cells from Radiation Damage. Int. J. Mol. Sci. 2015, 17, 7. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Pietrofesa, R.A.; Park, K.; Tao, J.-Q.; Carabe-Fernandez, A.; Berman, A.T.; Koumenis, C.; Sielecki, T.; Christofidou-Solomidou, M. LGM2605 Reduces Space Radiation-Induced NLRP3 Inflammasome Activation and Damage in In Vitro Lung Vascular Networks. Int. J. Mol. Sci. 2019, 20, 176. [Google Scholar] [CrossRef] [PubMed]
- Pietrofesa, R.; Turowski, J.; Tyagi, S.; Dukes, F.; Arguiri, E.; Busch, T.M.; Gallagher-Colombo, S.M.; Solomides, C.C.; A Cengel, K.; Christofidou-Solomidou, M. Radiation mitigating properties of the lignan component in flaxseed. BMC Cancer 2013, 13, 179. [Google Scholar] [CrossRef] [PubMed]
- Christofidou-Solomidou, M.; Tyagi, S.; Tan, K.-S.; Hagan, S.; Pietrofesa, R.; Dukes, F.; Arguiri, E.; Heitjan, D.F.; Solomides, C.C.; A Cengel, K. Dietary flaxseed administered post thoracic radiation treatment improves survival and mitigates radiation-induced pneumonopathy in mice. BMC Cancer 2011, 11, 269. [Google Scholar] [CrossRef]
- Song, L.; Ma, L.; Cong, F.; Shen, X.; Jing, P.; Ying, X.; Zhou, H.; Jiang, J.; Fu, Y.; Yan, H. Radioprotective effects of genistein on HL-7702 cells via the inhibition of apoptosis and DNA damage. Cancer Lett. 2015, 366, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Uslu, G.H.; Canyilmaz, E.; Serdar, L. Protective effects of genistein and melatonin on mouse liver injury induced by whole-body ionising radiation. Mol. Clin. Oncol. 2018, 10, 261–266. [Google Scholar] [CrossRef]
- Zhang, J.; Pang, Z.; Zhang, Y.; Liu, J.; Wang, Z.; Xu, C.; He, L.; Li, W.; Zhang, K.; Zhang, W.; et al. Genistein From Fructus sophorae Protects Mice from Radiation-Induced Intestinal Injury. Front. Pharmacol. 2021, 12. [Google Scholar] [CrossRef]
- Kim, J.-S.; Heo, K.; Yi, J.-M.; Gong, E.J.; Yang, K.; Moon, C.; Kim, S.-H. Genistein Mitigates Radiation-induced Testicular Injury. Phytotherapy Res. 2011, 26, 1119–1125. [Google Scholar] [CrossRef]
- Raffoul, J.J.; Wang, Y.; Kucuk, O.; Forman, J.D.; Sarkar, F.H.; Hillman, G.G. Genistein inhibits radiation-induced activation of NF-kappaB in prostate cancer cells promoting apoptosis and G2/M cell cycle arrest. BMC Cancer 2006, 6, 107. [Google Scholar] [CrossRef]
- Zhou, Y.; Mi, M.-T. Genistein Stimulates Hematopoiesis and Increases Survival in Irradiated Mice. J. Radiat. Res. 2005, 46, 425–433. [Google Scholar] [CrossRef]
- Ha, C.T.; Li, X.-H.; Fu, D.; Xiao, M.; Landauer, M.R. Genistein Nanoparticles Protect Mouse Hematopoietic System and Prevent Proinflammatory Factors after Gamma Irradiation. Radiat. Res. 2013, 180, 316–325. [Google Scholar] [CrossRef]
- Ahmad, I.U.; Forman, J.D.; Sarkar, F.H.; Hillman, G.G.; Heath, E.; Vaishampayan, U.; Cher, M.L.; Andic, F.; Rossi, P.J.; Kucuk, O. Soy Isoflavones in Conjunction with Radiation Therapy in Patients with Prostate Cancer. Nutr. Cancer 2010, 62, 996–1000. [Google Scholar] [CrossRef]
- Xie, L.-W.; Cai, S.; Zhao, T.-S.; Li, M.; Tian, Y. Green tea derivative (−)-epigallocatechin-3-gallate (EGCG) confers protection against ionizing radiation-induced intestinal epithelial cell death both in vitro and in vivo. Free. Radic. Biol. Med. 2020, 161, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Richi, B.; Kale, R.K.; Tiku, A.B. Radio-modulatory effects of Green Tea Catechin EGCG on pBR322 plasmid DNA and murine splenocytes against gamma-radiation induced damage. Mutat. Res. Toxicol. Environ. Mutagen. 2012, 747, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Xu, J.; Ge, Y.; Cao, H.; Ge, X.; Luo, J.; Xue, J.; Yang, H.; Zhang, S.; Cao, J. Epigallocatechin-3-gallate (EGCG) protects skin cells from ionizing radiation via heme oxygenase-1 (HO-1) overexpression. J. Radiat. Res. 2014, 55, 1056–1065. [Google Scholar] [CrossRef] [PubMed]
- Sulistiyani, E.; Brimson, J.M.; Chansaenroj, A.; Sariya, L.; Urkasemsin, G.; Oonsiri, S.; Tencomnao, T.; Vacharaksa, A.; Chaisuparat, R.; Ferreira, J.N. Epigallocatechin-3-Gallate Protects Pro-Acinar Epithelia Against Salivary Gland Radiation Injury. Int. J. Mol. Sci. 2021, 22, 3162. [Google Scholar] [CrossRef]
- El-Missiry, M.A.; Othman, A.I.; El-Sawy, M.R.; Lebede, M.F. Neuroprotective effect of epigallocatechin-3-gallate (EGCG) on radiation-induced damage and apoptosis in the rat hippocampus. Int. J. Radiat. Biol. 2018, 94, 798–808. [Google Scholar] [CrossRef]
- Yi, J.; Chen, C.; Liu, X.; Kang, Q.; Hao, L.; Huang, J.; Lu, J. Radioprotection of EGCG based on immunoregulatory effect and antioxidant activity against 60Coγ radiation-induced injury in mice. Food Chem. Toxicol. 2019, 135, 111051. [Google Scholar] [CrossRef]
- Yang, S.-W.; Lee, B.R.; Koh, J.-W. Protective Effects of Epigallocatechin Gallate after UV Irradiation in Cultured Human Retinal Pigment Epithelial Cells. Korean J. Ophthalmol. 2007, 21, 232–237. [Google Scholar] [CrossRef]
- Ding, J.; Wang, H.; Wu, Z.-B.; Zhao, J.; Zhang, S.; Li, W. Protection of Murine Spermatogenesis Against Ionizing Radiation-Induced Testicular Injury by a Green Tea Polyphenol1. Biol. Reprod. 2015, 92, 6. [Google Scholar] [CrossRef]
- Tiwari, M.; Dixit, B.; Parvez, S.; Agrawala, P.K. EGCG, a tea polyphenol, as a potential mitigator of hematopoietic radiation injury in mice. Biomed. Pharmacother. 2017, 88, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Mei, H.; Jia, L.; Zhao, H.; Li, X.; Meng, X.; Zhao, X.; Xing, L.; Yu, J. Epigallocatechin-3-gallate mouthwash protects mucosa from radiation-induced mucositis in head and neck cancer patients: A prospective, non-randomised, phase 1 trial. Investig. New Drugs 2019, 38, 1129–1136. [Google Scholar] [CrossRef]
- Zhao, H.; Zhu, W.; Jia, L.; Sun, X.; Chen, G.; Zhao, X.; Li, X.; Meng, X.; Kong, L.; Xing, L.; et al. Phase I study of topical epigallocatechin-3-gallate (EGCG) in patients with breast cancer receiving adjuvant radiotherapy. Br. J. Radiol. 2016, 89, 20150665. [Google Scholar] [CrossRef]
- Zhu, W.; Jia, L.; Chen, G.; Zhao, H.; Sun, X.; Meng, X.; Zhao, X.; Xing, L.; Yu, J.; Zheng, M. Epigallocatechin-3-gallate ameliorates radiation-induced acute skin damage in breast cancer patients undergoing adjuvant radiotherapy. Oncotarget 2016, 7, 48607–48613. [Google Scholar] [CrossRef]
- Adhikari, M.; Dhaker, A.; Adhikari, J.; Ivanov, V.; Singh, V.; Chawla, R.; Kumar, R.; Sharma, R.K.; Karamalakova, Y.; Gadjeva, V.; et al. In vitro studies on radioprotective efficacy of silymarin against γ-irradiation. Int. J. Radiat. Biol. 2012, 89, 200–211. [Google Scholar] [CrossRef]
- Adhikari, M.; Arora, R. Nano-silymarin provides protection against γ-radiation-induced oxidative stress in cultured human embryonic kidney cells. Mutat. Res. Toxicol. Environ. Mutagen. 2015, 792, 1–11. [Google Scholar] [CrossRef]
- Tiwari, P.; Kumar, A.; Ali, M.; Mishra, K. Radioprotection of plasmid and cellular DNA and Swiss mice by silibinin. Mutat. Res. Toxicol. Environ. Mutagen. 2010, 695, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Fatehi, D.; Mohammadi, M.; Shekarchi, B.; Shabani, A.; Seify, M.; Rostamzadeh, A. Radioprotective effects of Silymarin on the sperm parameters of NMRI mice irradiated with γ-rays. J. Photochem. Photobiol. B Biol. 2018, 178, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, L.A.; Roushdy, H.M.; Abu Senna, G.M.; Amin, N.E.; El-Deshw, O.A. Radioprotective effect of silymarin against radiation induced hepatotoxicity. Pharmacol. Res. 2002, 45, 447–454. [Google Scholar] [CrossRef]
- Kim, J.S.; Han, N.-K.; Kim, S.-H.; Lee, H.-J. Silibinin attenuates radiation-induced intestinal fibrosis and reverses epithelial-to-mesenchymal transition. Oncotarget 2017, 8, 69386–69397. [Google Scholar] [CrossRef]
- Marchiori, M.C.L.; Rigon, C.; Camponogara, C.; Oliveira, S.M.; Cruz, L. Hydrogel containing silibinin-loaded pomegranate oil based nanocapsules exhibits anti-inflammatory effects on skin damage UVB radiation-induced in mice. J. Photochem. Photobiol. B Biol. 2017, 170, 25–32. [Google Scholar] [CrossRef]
- Liu, W.; Wang, F.; Li, C.; Otkur, W.; Hayashi, T.; Mizuno, K.; Hattori, S.; Fujisaki, H.; Onodera, S.; Ikejima, T. Silibinin treatment protects human skin cells from UVB injury through upregulation of estrogen receptors. J. Photochem. Photobiol. B Biol. 2021, 216, 112147. [Google Scholar] [CrossRef] [PubMed]
- Marzban, M.; Anjamshoa, M.; Jafari, P.; Masoumi, H.; Ahadi, R.; Fatehi, D. Effects of gamma rays on rat testis tissue according to the morphological parameters and immunohistochemistry: Radioprotective role of silymarin. Electron. Physician 2017, 9, 4524–4532. [Google Scholar] [CrossRef]
- Elyasi, S.; Hosseini, S.; Moghadam, M.R.N.; Aledavood, S.A.; Karimi, G. Effect of Oral Silymarin Administration on Prevention of Radiotherapy Induced Mucositis: A Randomized, Double-Blinded, Placebo-Controlled Clinical Trial. Phytotherapy Res. 2016, 30, 1879–1885. [Google Scholar] [CrossRef] [PubMed]
- Karbasforooshan, H.; Hosseini, S.; Elyasi, S.; Pakdel, A.F.; Karimi, G. Topical silymarin administration for prevention of acute radiodermatitis in breast cancer patients: A randomized, double-blind, placebo-controlled clinical trial. Phytotherapy Res. 2018, 33, 379–386. [Google Scholar] [CrossRef] [PubMed]
- de Siqueira, W.N.; Dos Santos, F.T.J.; de Souza, T.F.; de Vasconcelos Lima, M.; Silva, H.; de Oliveira, P.S.S.; da Rocha Pitta, M.G.; Bezerra, M.; de Salazar, E.F.T.; de Franca, E.J.; et al. Study of the Potential Radiomitigator Effect of Quercetin on Human Lymphocytes. Inflammation 2019, 42, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Devipriya, N.; Sudheer, A.R.; Srinivasan, M.; Menon, V.P. Quercetin ameliorates gamma radiation-induced DNA damage and biochemical changes in human peripheral blood lymphocytes. Mutat. Res. Toxicol. Environ. Mutagen. 2008, 654, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hong, Y.; Liuyang, Z.; Li, H.; Jiang, Z.; Tao, J.; Liu, H.; Xie, A.; Feng, Y.; Dong, X.; et al. Quercetin Prevents Radiation-Induced Oral Mucositis by Upregulating BMI-1. Oxidative Med. Cell. Longev. 2021, 2021, 1–16. [Google Scholar] [CrossRef]
- Özyurt, H.; Çevik, O.; Özgen, Z.; Özden, A.S.; Çadırcı, S.; Elmas, M.A.; Ercan, F.; Gören, M.Z.; Şener, G. Quercetin protects radiation-induced DNA damage and apoptosis in kidney and bladder tissues of rats. Free Radic. Res. 2014, 48, 1247–1255. [Google Scholar] [CrossRef]
- Nikfarjam, B.A.; Hajiali, F.; Adineh, M.; Nassiri-Asl, M. Anti-inflammatory Effects of Quercetin and Vitexin on Activated Human Peripheral Blood Neutrophils: - The effects of quercetin and vitexin on human neutrophils. J Pharmacopuncture 2017, 20, 127–131. [Google Scholar]
- Kimura, S.; Warabi, E.; Yanagawa, T.; Ma, D.; Itoh, K.; Ishii, Y.; Kawachi, Y.; Ishii, T. Essential role of Nrf2 in keratinocyte protection from UVA by quercetin. Biochem. Biophys. Res. Commun. 2009, 387, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Kale, A.; Piskin, Ö.; Bas, Y.; Aydin, B.G.; Can, M.; Elmas, Ö.; Büyükuysal, Ç. Neuroprotective effects of Quercetin on radiation-induced brain injury in rats. J. Radiat. Res. 2018, 59, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Das, D.K.; Chakraborty, A.; Sinha, M.; Manna, K.; Mukherjee, D.; Chakraborty, A.; Bhattacharjee, S.; Dey, S. Modulatory role of quercetin against gamma radiation-mediated biochemical and morphological alterations of red blood cells. Int. J. Radiat. Biol. 2013, 89, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.L.; Somashekarappa, H.; Rajashekhar, K. Radiomodulatory role of Rutin and Quercetin in Swiss Albino mice exposed to the whole body gamma radiation. Indian J. Nucl. Med. 2012, 27, 237–242. [Google Scholar] [CrossRef]
- Patil, S.L.; Mallaiah, S.H.; Patil, R. Antioxidative and radioprotective potential of rutin and quercetin in Swiss albino mice exposed to gamma radiation. J. Med. Phys. 2013, 38, 87–92. [Google Scholar] [CrossRef]
- Sakat, M.S.; Kılıç, K.; Sahin, A.; Ozmen, H.K.; Yıldırım, S.; Kiziltunc, A.; Askin, S.; Saglam, Y.S. The protective efficacy of Quercetin and Naringenin against radiation-related submandibular gland injury in female rats: A histopathological, immunohistochemical, and biochemical study. Arch. Oral Biol. 2022, 142, 105510. [Google Scholar] [CrossRef]
- Dutta, A.; Dahiya, A.; Verma, S. Quercetin-3-rutinoside protects against gamma radiation inflicted hematopoietic dysfunction by regulating oxidative, inflammatory, and apoptotic mediators in mouse spleen and bone marrow. Free. Radic. Res. 2021, 55, 230–245. [Google Scholar] [CrossRef]
- Guven, B.; Can, M.; Piskin, O.; Aydin, B.G.; Karakaya, K.; Elmas, O.; Acikgoz, B. Flavonoids protect colon against radiation induced colitis. Regul. Toxicol. Pharmacol. 2019, 104, 128–132. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, Q.; Dai, W.; Li, S.; Feng, J.; Li, J.; Liu, T.; Xu, S.; Wang, W.; Lu, X.; et al. Quercetin Pretreatment Attenuates Hepatic Ischemia Reperfusion-Induced Apoptosis and Autophagy by Inhibiting ERK/NF-kappaB Pathway. Gastroenterol. Res. Pract. 2017, 2017, 9724217. [Google Scholar] [CrossRef]
- Horton, J.A.; Li, F.; Chung, E.J.; Hudak, K.; White, A.; Krausz, K.; Gonzalez, F.; Citrin, D. Quercetin Inhibits Radiation-Induced Skin Fibrosis. Radiat. Res. 2013, 180, 205–215. [Google Scholar] [CrossRef]
- Lee, Y.; Shin, D.H.; Kim, J.H.; Hong, S.; Choi, D.; Kim, Y.J.; Kwak, M.K.; Jung, Y. Caffeic acid phenethyl ester-mediated Nrf2 activation and IkappaB kinase inhibition are involved in NFkappaB inhibitory effect: Structural analysis for NFkappaB inhibition. Eur. J. Pharmacol. 2010, 643, 21–28. [Google Scholar] [CrossRef]
- Yang, N.; Shi, J.J.; Wu, F.P.; Li, M.; Zhang, X.; Li, Y.P.; Zhai, S.; Jia, X.L.; Dang, S.S. Caffeic acid phenethyl ester up-regulates antioxidant levels in hepatic stellate cell line T6 via an Nrf2-mediated mitogen activated protein kinases pathway. World J. Gastroenterol. 2017, 23, 1203–1214. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.-G.; Chu, J.-J.; Pang, Q.-F.; Zhang, F.-Z.; Wu, G.; Zhou, L.-Y.; Zhang, X.-J.; Xing, C.-G. Caffeic acid phenethyl ester attenuates ionize radiation-induced intestinal injury through modulation of oxidative stress, apoptosis and p38MAPK in rats. Environ. Toxicol. Pharmacol. 2015, 40, 156–163. [Google Scholar] [CrossRef]
- Chu, J.; Zhang, X.; Jin, L.; Chen, J.; Du, B.; Pang, Q. Protective effects of caffeic acid phenethyl ester against acute radiation-induced hepatic injury in rats. Environ. Toxicol. Pharmacol. 2015, 39, 683–689. [Google Scholar] [CrossRef]
- Cikman, O.; Taysi, S.; Gulsen, M.T.; Demir, E.; Akan, M.; Diril, H.; Kiraz, H.A.; Karaayvaz, M.; Tarakcioglu, M. The Radio-protective effects of Caffeic Acid Phenethyl Ester and Thymoquinone in rats exposed to total head irradiation. Wien. Klin. Wochenschr. 2014, 127, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Takakura, K.; Takatou, S.; Tomiyama, R.; Le, T.M.; Nguyen, D.T.; Nakamura, Y.; Konishi, T.; Matsugo, S.; Hori, O. Inhibition of nuclear factor-kappaB p65 phosphorylation by 3,4-dihydroxybenzalacetone and caffeic acid phenethyl ester. J. Pharmacol. Sci. 2018, 137, 248–255. [Google Scholar] [CrossRef]
- Linard, C.; Marquette, C.; Mathieu, J.; Pennequin, A.; Clarençon, D.; Mathé, D. Acute induction of inflammatory cytokine expression after gamma-irradiation in the rat: Effect of an NF-kappaB inhibitor. Int. J. Radiat. Oncol. Biol. Phys. 2004, 58, 427–434. [Google Scholar] [CrossRef]
- Grémy, O.; Benderitter, M.; Linard, C. Caffeic acid phenethyl ester modifies the Th1/Th2 balance in ileal mucosa after γ-irradiation in the rat by modulating the cytokine pattern. World J. Gastroenterol. 2006, 12, 4996–5004. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, M.; Prasad, N.R.; Menon, V.P. Protective effect of curcumin on gamma-radiation induced DNA damage and lipid peroxidation in cultured human lymphocytes. Mutat. Res. 2006, 611, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, M.; Sudheer, A.R.; Rajasekaran, K.; Menon, V.P. Effect of curcumin analog on γ-radiation-induced cellular changes in primary culture of isolated rat hepatocytes in vitro. Chem. Interactions 2008, 176, 1–8. [Google Scholar] [CrossRef]
- Srinivasan, M.; Sudheer, A.R.; Pillai, K.R.; Kumar, P.R.; Sudhakaran, P.; Menon, V. Modulatory effects of curcumin on γ-radiation-induced cellular damage in primary culture of isolated rat hepatocytes. Environ. Toxicol. Pharmacol. 2007, 24, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Wan, M.; Li, H.; Chen, Q.; Li, R.; Liang, B.; Zhu, H. Curcumin protection against ultraviolet-induced photo-damage in Hacat cells by regulating nuclear factor erythroid 2-related factor 2. Bioengineered 2021, 12, 9993–10006. [Google Scholar] [CrossRef]
- Xie, Y.; Zhao, Q.Y.; Li, H.Y.; Zhou, X.; Liu, Y.; Zhang, H. Curcumin ameliorates cognitive deficits heavy ion irradiation-induced learning and memory deficits through enhancing of Nrf2 antioxidant signaling pathways. Pharmacol. Biochem. Behav. 2014, 126, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Ozgen, S.; Dökmeci, D.; Akpolat, M.; Karadağ, C.H.; Gündüz, O.; Erbaş, H.; Benian, O.; Uzal, C.; Turan, F.N. The Protective Effect of Curcumin on Ionizing Radiation-induced Cataractogenesis in Rats. Balkan Med. J. 2012, 29, 358–363. [Google Scholar] [CrossRef]
- Li, W.; Jiang, L.; Lu, X.; Liu, X.; Ling, M. Curcumin protects radiation-induced liver damage in rats through the NF-κB signaling pathway. BMC Complement. Med. Ther. 2021, 21, 10. [Google Scholar] [CrossRef]
- Shabeeb, D.; Musa, A.E.; Ali, H.S.A.; Najafi, M. Curcumin Protects Against Radiotherapy-Induced Oxidative Injury to the Skin. Drug Des. Dev. Ther. 2020, 14, 3159–3163. [Google Scholar] [CrossRef]
- Rafiee, P.; Binion, D.G.; Wellner, M.; Behmaram, B.; Floer, M.; Mitton, E.; Nie, L.; Zhang, Z.; Otterson, M.F. Modulatory effect of curcumin on survival of irradiated human intestinal microvascular endothelial cells: Role of Akt/mTOR and NF-{kappa}B. Am. J. Physiol. Gastrointest Liver Physiol. 2010, 298, G865–G877. [Google Scholar] [CrossRef]
- Okunieff, P.; Xu, J.; Hu, D.; Liu, W.; Zhang, L.; Morrow, G.; Pentland, A.; Ryan, J.L.; Ding, I. Curcumin protects against radiation-induced acute and chronic cutaneous toxicity in mice and decreases mRNA expression of inflammatory and fibrogenic cytokines. Int. J. Radiat. Oncol. 2006, 65, 890–898. [Google Scholar] [CrossRef]
- Kim, J.; Park, S.; Jeon, B.-S.; Jang, W.-S.; Lee, S.-J.; Son, Y.; Rhim, K.-J.; Lee, S.I.; Lee, S.-S. Therapeutic effect of topical application of curcumin during treatment of radiation burns in a mini-pig model. J. Veter- Sci. 2016, 17, 435–444. [Google Scholar] [CrossRef]
- Bagheri, H.; Najafi, M.; Ghanbarzadeh, A.; Farhood, B.; Noodeh, F.A.; Mosaed, R.; Hassanzadeh, G. Histopathological Evaluation of Nanocurcumin for Mitigation of Radiation-Induced Small Intestine Injury. Curr. Radiopharm. 2022. [Google Scholar] [CrossRef] [PubMed]
- Rezvani, M.; Ross, G.A. Modification of radiation induced acute oral mucositis in the rat. Int. J. Radiat. Biol. 2004, 80, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Wolf, J.R.; Heckler, C.E.; Guido, J.J.; Peoples, A.R.; Gewandter, J.S.; Ling, M.; Vinciguerra, V.P.; Anderson, T.; Evans, L.; Wade, J.; et al. Oral curcumin for radiation dermatitis: A URCC NCORP study of 686 breast cancer patients. Supportive Care Cancer 2018, 26, 1543–1552. [Google Scholar] [CrossRef]
- Wolf, J.R.; Gewandter, J.S.; Bautista, J.; Heckler, C.E.; Strasser, J.; Dyk, P.; Anderson, T.; Gross, H.; Speer, T.; Dolohanty, L.; et al. Utility of topical agents for radiation dermatitis and pain: A randomized clinical trial. Support. Care Cancer 2019, 28, 3303–3311. [Google Scholar] [CrossRef]
- Farhood, B.; Najafi, M.; Talakesh, T.; Tabatabaee, N.; Atoof, F.; Aliasgharzadeh, A.; Sarvizade, M. Effect of Nano-Curcumin on Radiotherapy-Induced Skin Reaction in Breast Cancer Patients: A Randomized, Triple-Blind, Placebo-Controlled Trial. Curr. Radiopharm. 2022. [Google Scholar] [CrossRef]
- P.R, A.; Rao, B.N.; Satish Rao, B.S. In vivo radioprotective potential of thymol, a monoterpene phenol derivative of cymene. Mutat. Res. Toxicol. Environ. Mutagen. 2011, 726, 136–145. [Google Scholar] [CrossRef]
- Mahran, Y.F.; Badr, A.M.; Aldosari, A.; Bin-Zaid, R.; Alotaibi, H.N. Carvacrol and Thymol Modulate the Cross-Talk between TNF-α and IGF-1 Signaling in Radiotherapy-Induced Ovarian Failure. Oxidative Med. Cell. Longev. 2019, 2019, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sakat, M.S.; Kılıç, K.; Sahin, A.; Ozmen, H.K.; Yıldırım, S.; Egilmez, E. The Protective Efficacy of Hesperidin and Thymol on Radiation-Induced Submandibular Gland Damage. Laryngoscope 2022. [Google Scholar] [CrossRef]
- Abedi, S.M.; Yarmand, F.; Motallebnejad, M.; Seyedmajidi, M.; Moslemi, D.; Bijani, A.; Hosseinimehr, S.J. Radioprotective Effect of Thymol Against Salivary Glands Dysfunction Induced by Ionizing Radiation in Rats. Iran. J. Pharm. Res. IJPR 2016, 15, 861–866. [Google Scholar]
- Rao, B.N.; Archana, P.R.; Aithal, B.K.; Rao, B.S. Protective effect of zingerone, a dietary compound against radiation induced genetic damage and apoptosis in human lymphocytes. Eur. J. Pharmacol. 2011, 657, 59–66. [Google Scholar]
- Rao, B.N.; Rao, B.S.; Aithal, B.K.; Kumar, M.S. Radiomodifying and anticlastogenic effect of Zingerone on Swiss albino mice exposed to whole body gamma radiation. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2009, 677, 33–41. [Google Scholar]
- Mohamed, H.E.; Badawy, M.M. Modulatory effect of zingerone against cisplatin or γ-irradiation induced hepatotoxicity by molecular targeting regulation. Appl. Radiat. Isot. 2019, 154, 108891. [Google Scholar] [CrossRef]
- Wu, J.; Duan, Y.; Cui, J.; Dong, Y.; Li, H.; Wang, M.; Fan, S.; Li, D.; Li, Y. Protective effects of zingerone derivate on ionizing radiation-induced intestinal injury. J. Radiat. Res. 2019, 60, 740–746. [Google Scholar]
- Dobrzyńska, M.M.; Gajowik, A. Protection and Mitigation by Resveratrol of DNA Damage Induced in Irradiated Human Lymphocytes In Vitro. Radiat. Res. 2021. [Google Scholar] [CrossRef]
- Gao, P.; Li, N.; Ji, K.; Wang, Y.; Xu, C.; Liu, Y.; Wang, Q.; Wang, J.; He, N.; Sun, Z.; et al. Resveratrol targets TyrRS acetylation to protect against radiation-induced damage. FASEB J. 2019, 33, 8083–8093. [Google Scholar] [CrossRef]
- Wei, N.; Shi, Y.; Truong, L.N.; Fisch, K.M.; Xu, T.; Gardiner, E.; Fu, G.; Hsu, Y.-S.O.; Kishi, S.; Su, A.I.; et al. Oxidative Stress Diverts tRNA Synthetase to Nucleus for Protection against DNA Damage. Mol. Cell 2014, 56, 323–332. [Google Scholar] [CrossRef]
- Carsten, R.E.; Bachand, A.M.; Bailey, S.M.; Ullrich, R.L. Resveratrol Reduces Radiation-Induced Chromosome Aberration Frequencies in Mouse Bone Marrow Cells. Radiat. Res. 2008, 169, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Koohian, F.; Shanei, A.; Shahbazi-Gahrouei, D.; Hejazi, S.H.; Moradi, M.T. The Radioprotective Effect of Resveratrol Against Genotoxicity Induced by gamma-Irradiation in Mice Blood Lymphocytes. Dose Response 2017, 15, 1559325817705699. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chan, F.; Sun, H.; Yan, J.; Fan, D.; Zhao, D.; An, J.; Zhou, D. Resveratrol protects human keratinocytes HaCaT cells from UVA-induced oxidative stress damage by downregulating Keap1 expression. Eur. J. Pharmacol. 2011, 650, 130–137. [Google Scholar] [CrossRef]
- Zhang, H.; Zhai, Z.; Wang, Y.; Zhang, J.; Wu, H.; Wang, Y.; Li, C.; Li, D.; Lu, L.; Wang, X.; et al. Resveratrol ameliorates ionizing irradiation-induced long-term hematopoietic stem cell injury in mice. Free. Radic. Biol. Med. 2012, 54, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Feng, L.; Xing, Y.; Wang, Y.; Du, L.; Xu, C.; Cao, J.; Wang, Q.; Fan, S.; Liu, Q.; et al. Radioprotective and Antioxidant Effect of Resveratrol in Hippocampus by Activating Sirt1. Int. J. Mol. Sci. 2014, 15, 5928–5939. [Google Scholar] [CrossRef]
- Zhang, H.; Yan, H.; Zhou, X.; Wang, H.; Yang, Y.; Zhang, J.; Wang, H. The protective effects of Resveratrol against radiation-induced intestinal injury. BMC Complement. Altern. Med. 2017, 17, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Farhood, B.; Hassanzadeh, G.; Amini, P.; Shabeeb, D.; Musa, A.E.; Khodamoradi, E.; Mohseni, M.; Aliasgharzadeh, A.; Moradi, H.; Najafi, M. Mitigation of Radiation-induced Gastrointestinal System Injury using Resveratrol or Alpha-lipoic Acid: A Pilot Histopathological Study. Anti-Inflammatory Anti-Allergy Agents Med. Chem. 2020, 19, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Zhang, H.; Zhang, X.; Zhang, S.; Zhu, S.; Wang, H. Resveratrol protects intestinal epithelial cells against radiation-induced damage by promoting autophagy and inhibiting apoptosis through SIRT1 activation. J. Radiat. Res. 2021, 62, 574–581. [Google Scholar] [CrossRef]
- Prager, I.; Patties, I.; Himmelbach, K.; Kendzia, E.; Merz, F.; Müller, K.; Kortmann, R.-D.; Glasow, A. Dose-dependent short- and long-term effects of ionizing irradiation on neural stem cells in murine hippocampal tissue cultures: Neuroprotective potential of resveratrol. Brain Behav. 2016, 6, e00548. [Google Scholar] [CrossRef]
- Qin, H.; Zhang, H.; Zhang, X.; Zhang, S.; Zhu, S.; Wang, H. Resveratrol attenuates radiation enteritis through the SIRT1/FOXO3a and PI3K/AKT signaling pathways. Biochem. Biophys. Res. Commun. 2021, 554, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Yang, X.; Cai, J.; Ma, J.; Cheng, H.; Zhao, K.; Yang, L.; Cao, Y.; Qin, Q.; Zhang, C.; et al. Resveratrol attenuates radiation-induced salivary gland dysfunction in mice. Laryngoscope 2013, 123, E23–E29. [Google Scholar] [CrossRef]
- Di Franco, R.; Calvanese, M.; Murino, P.; Manzo, R.; Guida, C.; Di Gennaro, D.; Anania, C.; Ravo, V. Skin toxicity from external beam radiation therapy in breast cancer patients: Protective effects of Resveratrol, Lycopene, Vitamin C and anthocianin (Ixor®). Radiat. Oncol. 2012, 7, 12. [Google Scholar] [CrossRef]
- Bhatia, A.L.; Sharma, A.; Patni, S.; Sharma, A.L. Prophylactic effect of flaxseed oil against radiation-induced hepatotoxicity in mice. Phytotherapy Research 2007, 21, 852–859. [Google Scholar] [CrossRef]
- Hu, M. Commentary: Bioavailability of Flavonoids and Polyphenols: Call to Arms. Mol. Pharm. 2007, 4, 803–806. [Google Scholar] [CrossRef]
- D’archivio, M.; Filesi, C.; Varì, R.; Scazzocchio, B.; Masella, R. Bioavailability of the polyphenols: Status and controversies. Int. J. Mol. Sci. 2010, 11, 1321–1342. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar]
- Nikolova, N.; Ivanova, D.; Yaneva, Z. In Vivo Radioprotective Potential of Newly Synthesized Azomethine and Styrylquinoline Derivatives and a Natural Polyphenol: A Preliminary Study. Life 2022, 12, 346. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Sun, L.; Li, H.; Chen, J.; He, P.; Zhao, L.; Tang, W.; Qiu, H. The potent radioprotective agents: Novel nitronyl nitroxide radical spin-labeled resveratrol derivatives. Fitoterapia 2021, 155, 105053. [Google Scholar] [PubMed]
- Uzura, S.; Sekine-Suzuki, E.; Nakanishi, I.; Sonoda, M.; Tanimori, S. A facile and rapid access to resveratrol derivatives and their radioprotective activity. Bioorganic Med. Chem. Lett. 2016, 26, 3886–3891. [Google Scholar] [CrossRef] [PubMed]
- van der Veen, S.J.; Faber, H.; Ghobadi, G.; Brandenburg, S.; Langendijk, J.A.; Coppes, R.P.; van Luijk, P. Decreasing Irradiated Rat Lung Volume Changes Dose-Limiting Toxicity From Early to Late Effects. Int. J. Radiat. Oncol. 2015, 94, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Verhaegen, F.; Butterworth, K.T.; Chalmers, A.J.; Coppes, R.P.; de Ruysscher, D.; Dobiasch, S.; Fenwick, J.D.; Granton, P.V.; Heijmans, S.H.J.; A Hill, M.; et al. Roadmap for Precision preclinical x-ray radiation studies. Phys. Med. Biol. 2022, 68, 06RM01. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).