Radionuclide Therapy of HER2-Expressing Xenografts Using [177Lu]Lu-ABY-027 Affibody Molecule Alone and in Combination with Trastuzumab
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. General
2.2. Labeling Chemistry
2.3. In Vitro Studies
2.4. In Vivo Studies
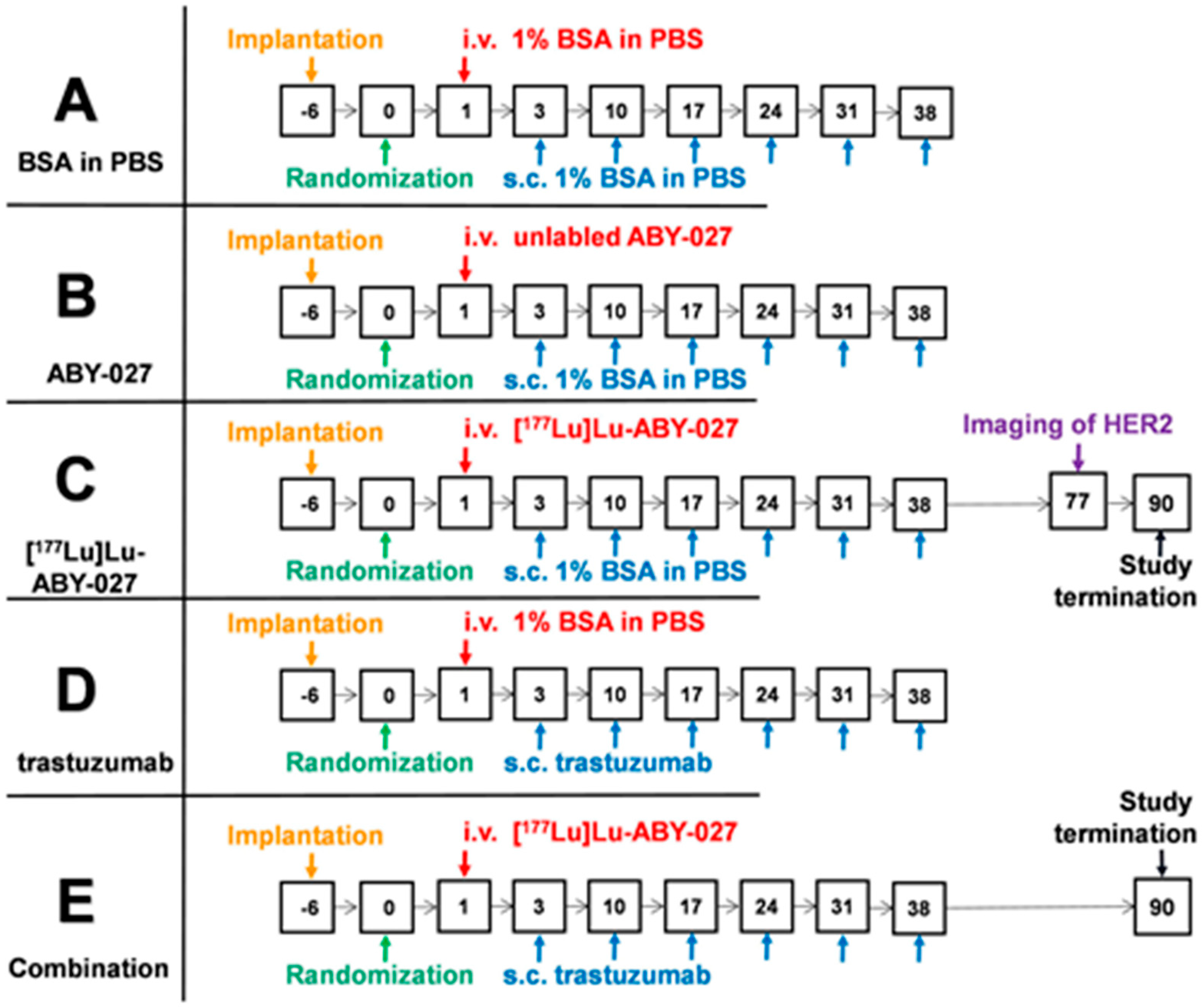
2.5. Experimental Therapy Study
2.6. Statistical Analysis
3. Results
3.1. Radiolabeling and In Vitro Studies
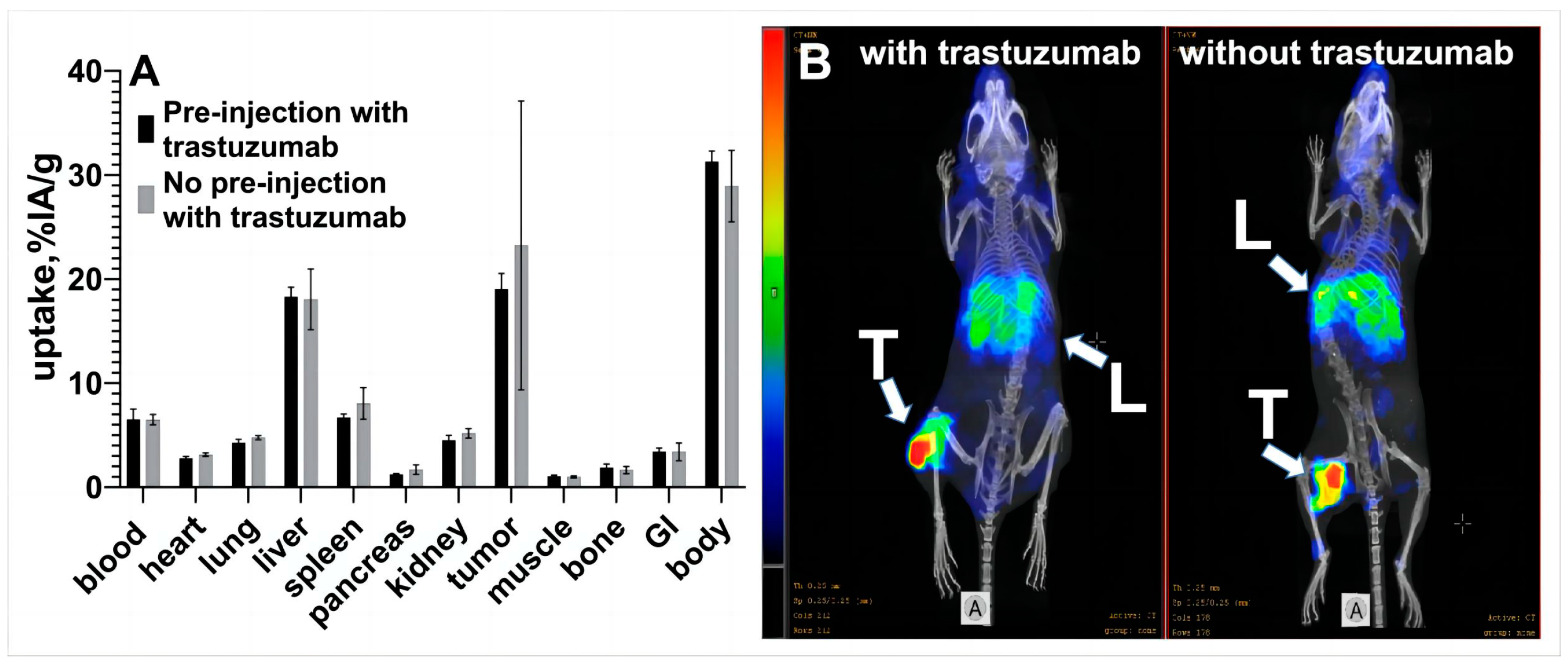
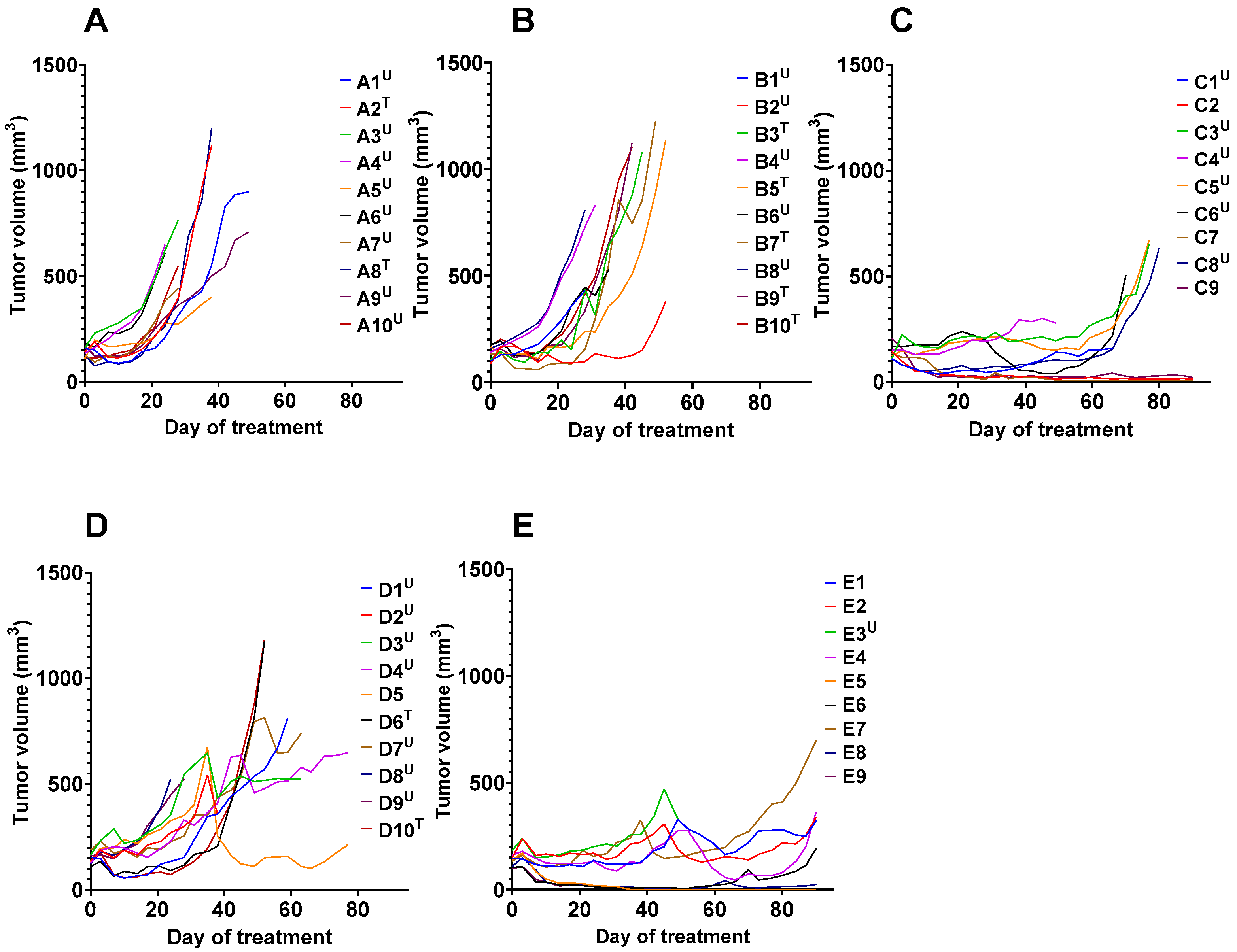
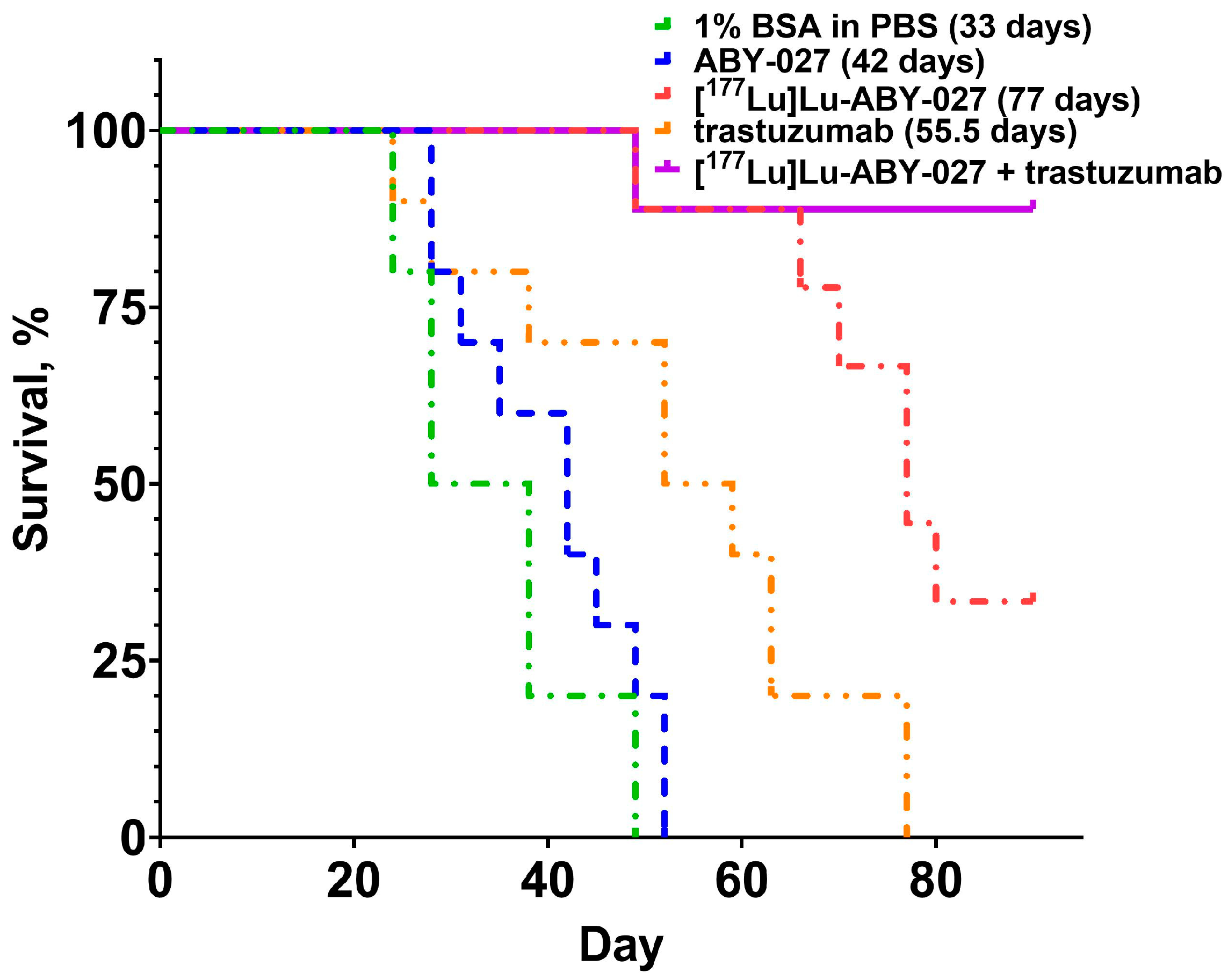
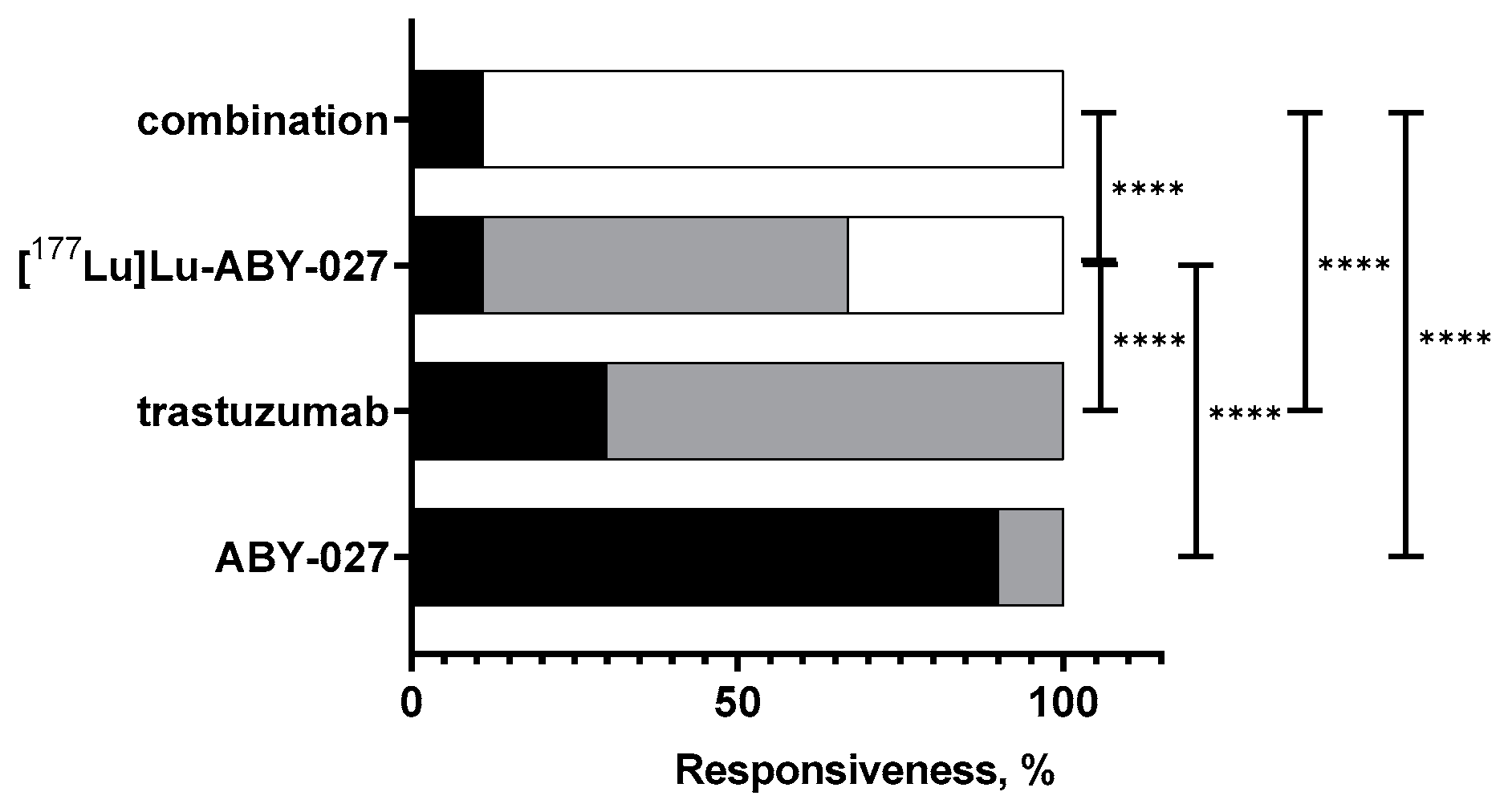
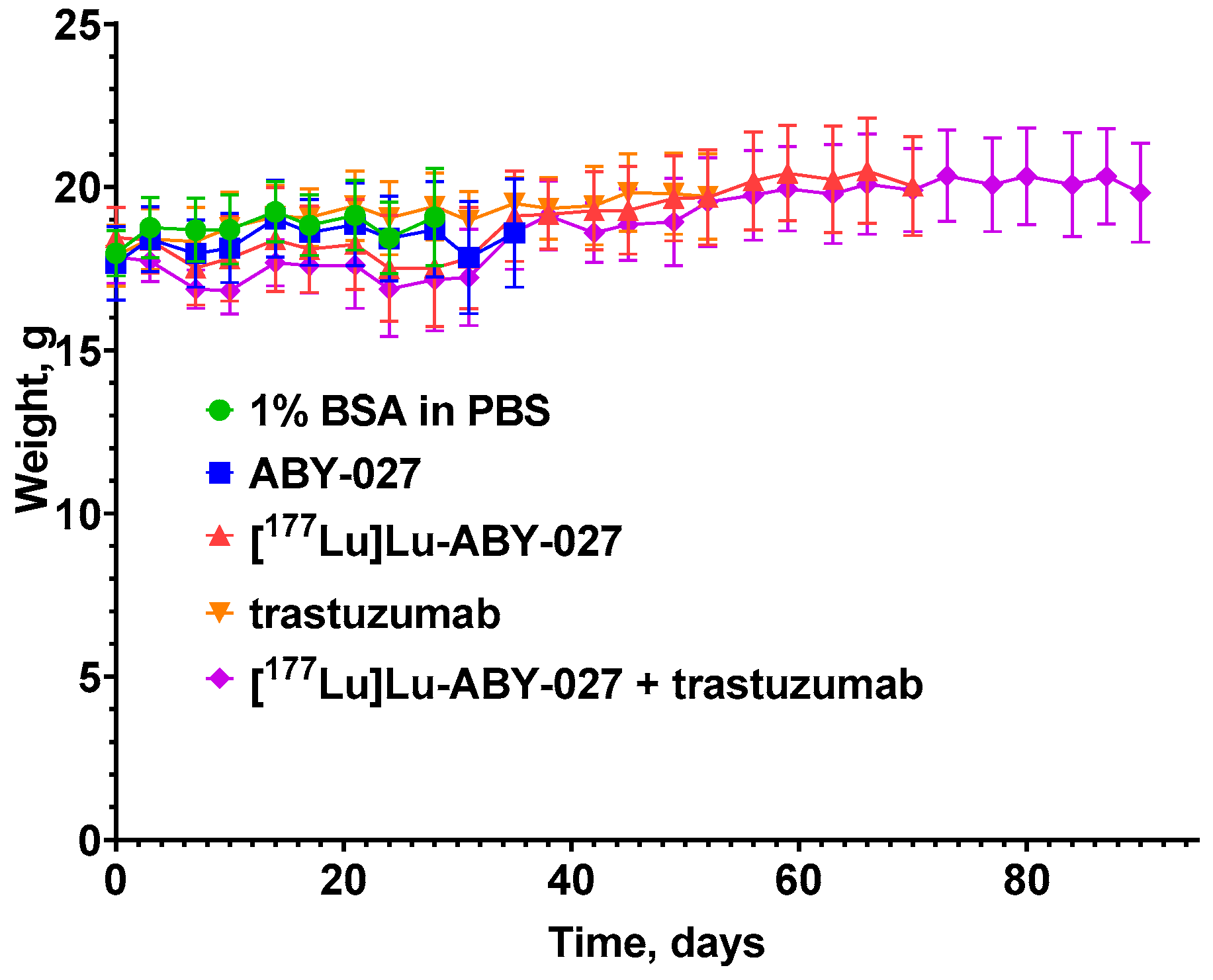
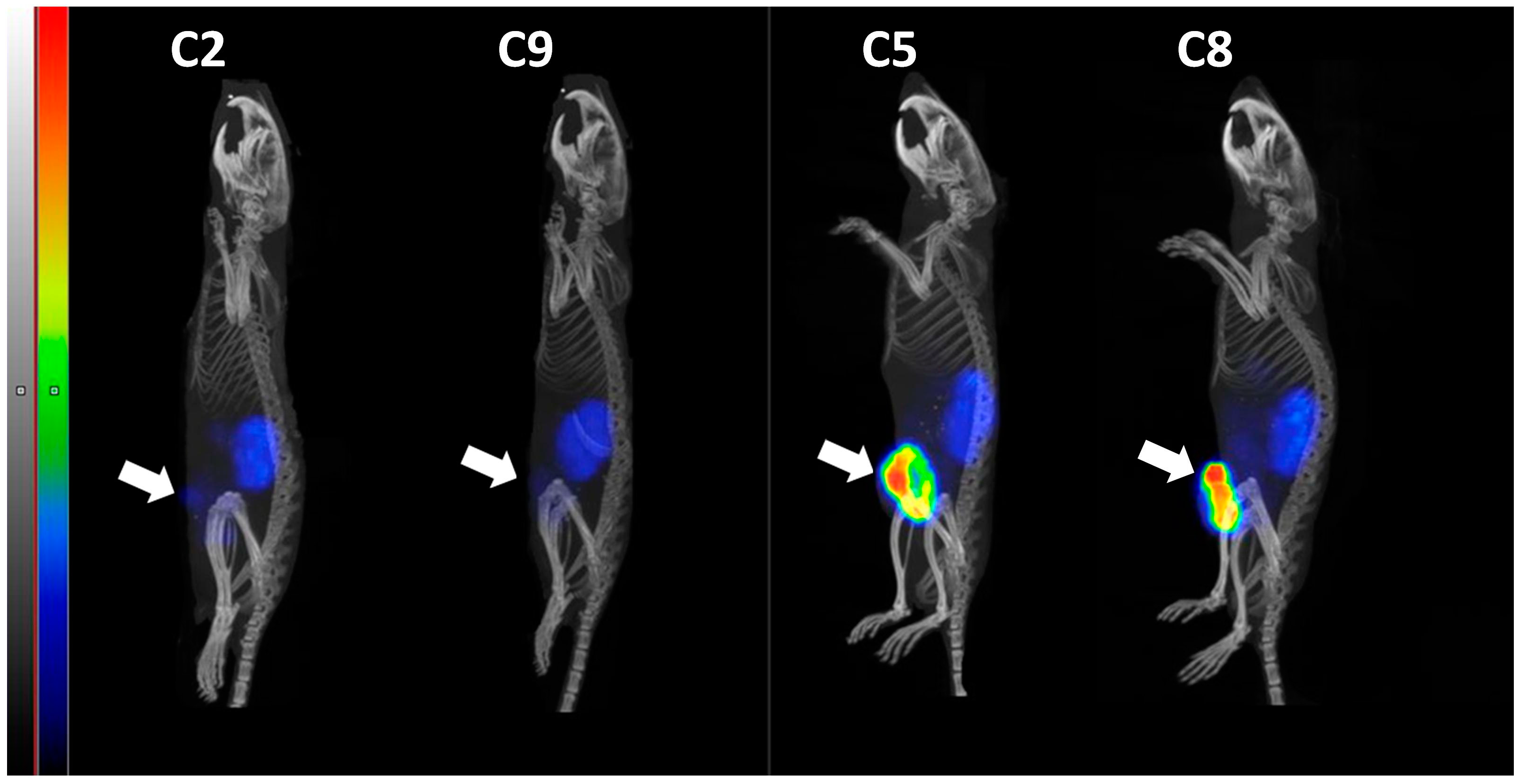
3.2. In Vivo Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Loibl, S.; Gianni, L. HER2-positive breast cancer. Lancet 2017, 389, 2415–2429. [Google Scholar] [CrossRef] [PubMed]
- Von Minckwitz, G.; Procter, M.; de Azambuja, E.; Zardavas, D.; Benyunes, M.; Viale, G.; Suter, T.; Arahmani, A.; Rouchet, N.; Clark, E.; et al. Adjuvant Pertuzumab and Trastuzumab in Early HER2-Positive Breast Cancer. N. Engl. J. Med. 2017, 377, 122–131. [Google Scholar] [CrossRef]
- Tabernero, J.; Hoff, P.M.; Shen, L.; Ohtsu, A.; Shah, M.A.; Cheng, K.; Song, C.; Wu, H.; Eng-Wong, J.; Kim, K.; et al. Pertuzumab plus trastuzumab and chemotherapy for HER2-positive metastatic gastric or gastro-oesophageal junction cancer (JACOB): Final analysis of a double-blind, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2018, 19, 1372–1384. [Google Scholar] [CrossRef]
- Swain, S.M.; Shastry, M.; Hamilton, E. Targeting HER2-positive breast cancer: Advances and future directions. Nat. Rev. Drug Discov. 2023, 22, 101–126. [Google Scholar] [CrossRef]
- Chau, C.H.; Steeg, P.S.; Figg, W.D. Antibody-drug conjugates for cancer. Lancet 2019, 394, 793–804. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, P.; Carmagnani Pestana, R.; Corti, C.; Modi, S.; Bardia, A.; Tolaney, S.M.; Cortes, J.; Soria, J.-C.; Curigliano, G. Antibody-drug conjugates: Smart chemotherapy delivery across tumor histologies. CA Cancer J. Clin. 2022, 72, 165–182. [Google Scholar] [CrossRef] [PubMed]
- Wedam, S.; Fashoyin-Aje, L.; Gao, X.; Bloomquist, E.; Tang, S.; Sridhara, R.; Goldberg, K.B.; King-Kallimanis, B.L.; Theoret, M.R.; Ibrahim, A.; et al. FDA Approval Summary: Ado-Trastuzumab Emtansine for the Adjuvant Treatment of HER2-positive Early Breast Cancer. Clin. Cancer Res. 2020, 26, 4180–4185. [Google Scholar] [CrossRef]
- Hunter, F.W.; Barker, H.R.; Lipert, B.; Rothé, F.; Gebhart, G.; Piccart-Gebhart, M.J.; Sotiriou, C.; Jamieson, S. Mechanisms of resistance to trastuzumab emtansine (T-DM1) in HER2-positive breast cancer. Br. J. Cancer 2020, 122, 603–612. [Google Scholar] [CrossRef]
- Sgouros, G.; Bodei, L.; McDevitt, M.R.; Nedrow, J.R. Radiopharmaceutical therapy in cancer: Clinical advances and challenges. Nat. Rev. Drug Discov. 2020, 19, 589–608. [Google Scholar] [CrossRef]
- Löfblom, J.; Feldwisch, J.; Tolmachev, V.; Carlsson, J.; Ståhl, S.; Frejd, F.Y. Affibody Molecules: Engineered Proteins for Therapeutic, Diagnostic and Biotechnological Applications. FEBS Lett. 2010, 584, 2670–2680. [Google Scholar] [CrossRef]
- Ståhl, S.; Gräslund, T.; Eriksson Karlström, A.; Frejd, F.Y.; Nygren, P.Å.; Löfblom, J. Affibody Molecules in Biotechnological and Medical Applications. Trends Biotechnol. 2017, 35, 691–712. [Google Scholar] [CrossRef]
- Sörensen, J.; Velikyan, I.; Sandberg, D.; Wennborg, A.; Feldwisch, J.; Tolmachev, V.; Orlova, A.; Sandström, M.; Lubberink, M.; Olofsson, H.; et al. Measuring HER2-receptor expression in metastatic breast cancer using [68Ga] ABY-025 Affibody PET/CT. Theranostics 2016, 6, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Alhuseinalkhudhur, A.; Lindman, H.; Liss, P.; Sundin, T.; Frejd, F.Y.; Hartman, J.; Iyer, V.; Lubberink, M.; Velikyan, I.; Sörensen, J. Abstract P3-02-06: A phase II study of 68Ga-ABY-025 PET for non-invasive quantification of HER2 expression in breast cancer. Cancer Res. 2022, 82, P3-02-06. [Google Scholar] [CrossRef]
- Fortin, M.A.; Orlova, A.; Malmström, P.U.; Tolmachev, V. Labelling chemistry and characterization of [90Y/177Lu]-DOTA-ZHER2:342-3 Affibody molecule, a candidate agent for locoregional treatment of urinary bladder carcinoma. Int. J. Mol. Med. 2007, 19, 285–291. [Google Scholar] [CrossRef]
- Tolmachev, V.; Orlova, A.; Pehrson, R.; Galli, J.; Baastrup, B.; Andersson, K.; Sandström, M.; Rosik, D.; Carlsson, J.; Lundqvist, H.; et al. Radionuclide Therapy of HER2-Positive Microxenografts Using a 177Lu-Labeled HER2-Specific Affibody Molecule. Cancer Res. 2007, 67, 2773–2782. [Google Scholar] [CrossRef] [PubMed]
- Dennis, M.S.; Zhang, M.; Meng, Y.G.; Kadkhodayan, M.; Kirchhofer, D.; Combs, D.; Damico, L.A. Albumin Binding as a General Strategy for Improving the Pharmacokinetics of Proteins. J. Biol. Chem. 2002, 277, 35035–35043. [Google Scholar] [CrossRef]
- Orlova, A.; Jonsson, A.; Rosik, D.; Lundqvist, H.; Lindborg, M.; Abrahmsen, L.; Ekblad, C.; Frejd, F.Y.; Tolmachev, V. Site-specific radiometal labeling and improved biodistribution using ABY-027, a novel HER2-targeting affibody molecule-albumin-binding domain fusion protein. J. Nucl. Med. 2013, 54, 961–968. [Google Scholar] [CrossRef]
- Feldwisch, J.; Tolmachev, V.; Lendel, C.; Herne, N.; Sjöberg, A.; Larsson, B.; Rosik, D.; Lindqvist, E.; Fant, G.; Höidén-Guthenberg, I.; et al. Design of an optimized scaffold for affibody molecules. J. Mol. Biol. 2010, 398, 232–247. [Google Scholar] [CrossRef]
- Jonsson, A.; Dogan, J.; Herne, N.; Abrahmsén, L.; Nygren, P.A. Engineering of a femtomolar affinity binding protein to human serum albumin. Protein Eng. Des. Sel. 2008, 21, 515–527. [Google Scholar] [CrossRef]
- Sato, S.; Kajiyama, Y.; Sugano, M.; Iwanuma, Y.; Sonoue, H.; Matsumoto, T.; Sasai, K.; Tsurumaru, M. Monoclonal antibody to HER-2/neu receptor enhances radiosensitivity of esophageal cancer cell lines expressing HER-2/neu oncoprotein. Int. J. Radiat. Oncol. Biol. Phys. 2005, 61, 203–211. [Google Scholar] [CrossRef]
- Kiess, A.P.; McArthur, H.L.; Mahoney, K.; Patil, S.; Morris, P.G.; Ho, A.; Hudis, C.A.; McCormick, B. Adjuvant trastuzumab reduces locoregional recurrence in women who receive breast-conservation therapy for lymph node-negative, human epidermal growth factor receptor 2-positive breast cancer. Cancer 2012, 118, 1982–1988. [Google Scholar] [CrossRef] [PubMed]
- Eigenbrot, C.; Ultsch, M.; Dubnovitsky, A.; Abrahmsén, L.; Härd, T. Structural basis for high-affinity HER2 receptor binding by an engineered protein. Proc. Natl. Acad. Sci. USA 2010, 107, 15039–15044. [Google Scholar] [CrossRef] [PubMed]
- Björke, H.; Andersson, K. Automated, High-Resolution Cellular Retention and Uptake Studies in Vitro. Appl. Radiat. Isot. Data Instrum. Methods Use Agric. Ind. Med. 2006, 64, 901–905. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Vorobyeva, A.; Xu, T.; Orlova, A.; Loftenius, A.; Bengtsson, T.; Jonasson, P.; Tolmachev, V.; Frejd, F.Y. Comparative Preclinical Evaluation of HER2-Targeting ABD-Fused Affibody® Molecules 177Lu-ABY-271 and 177Lu-ABY-027: Impact of DOTA Position on ABD Domain. Pharmaceutics 2021, 13, 839. [Google Scholar] [CrossRef] [PubMed]
- Westerlund, K.; Altai, M.; Mitran, B.; Konijnenberg, M.; Oroujeni, M.; Atterby, C.; de Jong, M.; Orlova, A.; Mattsson, J.; Micke, P.; et al. Radionuclide Therapy of HER2-Expressing Human Xenografts Using Affibody-Based Peptide Nucleic Acid-Mediated Pretargeting: In Vivo Proof of Principle. J. Nucl. Med. 2018, 59, 1092–1098. [Google Scholar] [CrossRef]
- Oroujeni, M.; Tano, H.; Vorobyeva, A.; Liu, Y.; Vorontsova, O.; Xu, T.; Westerlund, K.; Orlova, A.; Tolmachev, V.; Karlström, A.E. Affibody-Mediated PNA-Based Pretargeted Cotreatment Improves Survival of Trastuzumab-Treated Mice Bearing HER2-Expressing Xenografts. J. Nucl. Med. 2022, 63, 1046–1051. [Google Scholar] [CrossRef]
- Tolmachev, V.; Mume, E.; Sjöberg, S.; Frejd, F.Y.; Orlova, A. Influence of valency and labelling chemistry on in vivo targeting using radioiodinated HER2-binding Affibody molecules. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 692–701. [Google Scholar] [CrossRef]
- Liu, Y.; Vorobyeva, A.; Orlova, A.; Konijnenberg, M.W.; Xu, T.; Bragina, O.; Loftenius, A.; Rosander, E.; Frejd, F.Y.; Tolmachev, V. Experimental Therapy of HER2-Expressing Xenografts Using the Second-Generation HER2-Targeting Affibody Molecule 188Re-ZHER2:41071. Pharmaceutics 2022, 14, 1092. [Google Scholar] [CrossRef]
- Garousi, J.; von Witting, E.; Borin, J.; Vorobyeva, A.; Altai, M.; Vorontsova, O.; Konijnenberg, M.W.; Oroujeni, M.; Orlova, A.; Tolmachev, V.; et al. Radionuclide therapy using ABD-fused ADAPT scaffold protein: Proof of Principle. Biomaterials 2021, 266, 120381. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Ding, H.; Vorobyeva, A.; Oroujeni, M.; Orlova, A.; Tolmachev, V.; Gräslund, T. Drug Conjugates Based on a Monovalent Affibody Targeting Vector Can Efficiently Eradicate HER2 Positive Human Tumors in an Experimental Mouse Model. Cancers 2020, 13, 85. [Google Scholar] [CrossRef]
- Orlova, A.; Bass, T.Z.; Rinne, S.S.; Leitao, C.D.; Rosestedt, M.; Atterby, C.; Gudmundsdotter, L.; Frejd, F.Y.; Löfblom, J.; Tolmachev, V.; et al. Evaluation of the Therapeutic Potential of a HER3-Binding Affibody Construct TAM-HER3 in Comparison with a Monoclonal Antibody, Seribantumab. Mol. Pharm. 2018, 15, 3394–3403. [Google Scholar] [CrossRef] [PubMed]
- Honarvar, H.; Strand, J.; Perols, A.; Orlova, A.; Selvaraju, R.K.; Eriksson Karlström, A.; Tolmachev, V. Position for site-specific attachment of a DOTA chelator to synthetic affibody molecules has a different influence on the targeting properties of 68Ga- compared to 111In-labeled conjugates. Mol. Imaging 2014, 13, 10. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Güler, R.; Liao, Y.; Vorobyeva, A.; Widmark, O.; Meuleman, T.J.; Koijen, A.; van den Bos, L.J.; Naasz, R.; Bodenko, V.; et al. Biologic Evaluation of a Heterodimeric HER2-Albumin Targeted Affibody Molecule Produced by Chemo-Enzymatic Peptide Synthesis. Pharmaceutics 2022, 14, 2519. [Google Scholar] [CrossRef] [PubMed]
- Giordano, S.H.; Temin, S.; Chandarlapaty, S.; Crews, R.; Esteva, F.J.; Kirshner, J.J.; Krop, I.E.; Levinson, J.; Lin, N.U.; Modi, S. Systemic therapy for patients with advanced human epidermal growth factor receptor 2-positive breast cancer: ASCO clinical practice guideline update. J. Clin. Oncol. 2018, 36, 2736–2740. [Google Scholar] [CrossRef]
- Saputra, E.C.; Huang, L.; Chen, Y.; Tucker-Kellogg, L. Combination therapy and the evolution of resistance: The theoretical merits of synergism and antagonism in cancer. Cancer Res. 2018, 78, 2419–2431. [Google Scholar] [CrossRef] [PubMed]
- Chavez-MacGregor, M.; Zhang, N.; Buchholz, T.A.; Zhang, Y.; Niu, J.; Elting, L.; Smith, B.D.; Hortobagyi, G.N.; Giordano, S.H. Trastuzumab-related cardiotoxicity among older patients with breast cancer. J. Clin. Oncol. 2013, 31, 4222–4228. [Google Scholar] [CrossRef]
- Levillain, H.; Bagni, O.; Dieudonné, D.; Gnesin, S.; Grosser, O.S.; Kappadath, S.C.; Kennedy, A.; Kokabi, N.; Liu, D.M.; Madoff, D.C.; et al. International recommendations for personalised selective internal radiation therapy of primary and metastatic liver diseases with yttrium-90 resin microspheres. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1570–1584. [Google Scholar] [CrossRef]
- Orlova, A.; Tolmachev, V.; Pehrson, R.; Lindborg, M.; Tran, T.; Sandstrom, M.; Nilsson, F.Y.; Wennborg, A.; Abrahmse, L.; Feldwisch, J.; et al. Synthetic affibody molecules: A novel class of affinity ligands for molecular imaging of HER2-expressing malignant tumors. Cancer Res. 2007, 67, 2178–2186. [Google Scholar] [CrossRef]
- Perez, E.A.; Barrios, C.; Eiermann, W.; Toi, M.; Im, Y.H.; Conte, P.; Martin, M.; Pienkowski, T.; Pivot, X.; Burris, H., 3rd; et al. Trastuzumab Emtansine with or without Pertuzumab versus Trastuzumab Plus Taxane for Human Epidermal Growth Factor Receptor 2-Positive, Advanced Breast Cancer: Primary Results From the Phase III MARIANNE Study. J. Clin. Oncol. 2017, 35, 141–148. [Google Scholar] [CrossRef]
- Modi, S.; Jacot, W.; Yamashita, T.; Sohn, J.; Vidal, M.; Tokunaga, E.; Tsurutani, J.; Ueno, N.T.; Prat, A.; Chae, Y.S.; et al. DESTINY-Breast04 Trial Investigators. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. N. Engl. J. Med. 2022, 387, 9–20. [Google Scholar] [CrossRef]
- Kratochwil, C.; Bruchertseifer, F.; Giesel, F.L.; Weis, M.; Verburg, F.A.; Mottaghy, F.; Kopka, K.; Apostolidis, C.; Haberkorn, U.; Morgenstern, A. 225Ac-PSMA-617 for PSMA-targeted α-radiation therapy of metastatic castration-resistant prostate cancer. J. Nucl. Med. 2016, 57, 1941–1944. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Kim, Y.I. Effects of 225Ac-Labeled Prostate-Specific Membrane Antigen Radioligand Therapy in Metastatic Castration-Resistant Prostate Cancer: A Meta-Analysis. J. Nucl. Med. 2022, 63, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wilson, J.J.; Orvig, C.; Li, Y.; Wilbur, D.S.; Ramogida, C.F.; Radchenko, V.; Schaffer, P. Harnessing α-Emitting Radionuclides for Therapy: Radiolabeling Method Review. J. Nucl. Med. 2022, 63, 5–13. [Google Scholar] [CrossRef] [PubMed]
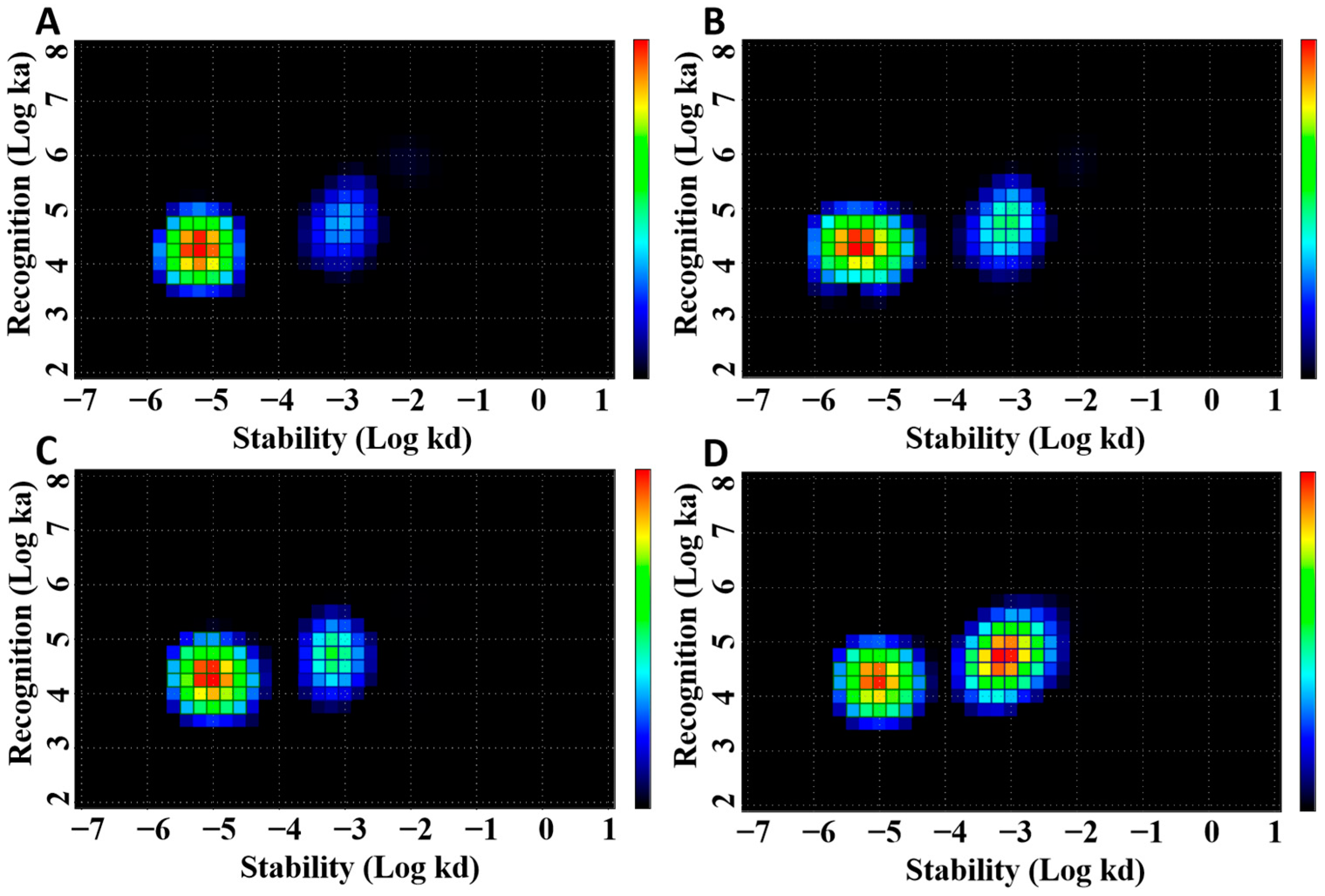
| Parameter | KD1 (pM) | KD2 (nM) |
|---|---|---|
| [177Lu]Lu-ABY-027 only | 382.5 ± 53.0 | 15.7 ± 2.5 |
| [177Lu]Lu-ABY-027 with HSA | 324.5 ± 75.7 | 16.5 ± 2.0 |
| [177Lu]Lu-ABY-027 with trastuzumab | 502.5 ± 46.0 | 14.5 ± 0.1 |
| [177Lu]Lu-ABY-027 with trastuzumab and HSA | 533.5 ± 43.1 | 14.2 ± 2.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Xu, T.; Vorobyeva, A.; Loftenius, A.; Bodenko, V.; Orlova, A.; Frejd, F.Y.; Tolmachev, V. Radionuclide Therapy of HER2-Expressing Xenografts Using [177Lu]Lu-ABY-027 Affibody Molecule Alone and in Combination with Trastuzumab. Cancers 2023, 15, 2409. https://doi.org/10.3390/cancers15092409
Liu Y, Xu T, Vorobyeva A, Loftenius A, Bodenko V, Orlova A, Frejd FY, Tolmachev V. Radionuclide Therapy of HER2-Expressing Xenografts Using [177Lu]Lu-ABY-027 Affibody Molecule Alone and in Combination with Trastuzumab. Cancers. 2023; 15(9):2409. https://doi.org/10.3390/cancers15092409
Chicago/Turabian StyleLiu, Yongsheng, Tianqi Xu, Anzhelika Vorobyeva, Annika Loftenius, Vitalina Bodenko, Anna Orlova, Fredrik Y. Frejd, and Vladimir Tolmachev. 2023. "Radionuclide Therapy of HER2-Expressing Xenografts Using [177Lu]Lu-ABY-027 Affibody Molecule Alone and in Combination with Trastuzumab" Cancers 15, no. 9: 2409. https://doi.org/10.3390/cancers15092409
APA StyleLiu, Y., Xu, T., Vorobyeva, A., Loftenius, A., Bodenko, V., Orlova, A., Frejd, F. Y., & Tolmachev, V. (2023). Radionuclide Therapy of HER2-Expressing Xenografts Using [177Lu]Lu-ABY-027 Affibody Molecule Alone and in Combination with Trastuzumab. Cancers, 15(9), 2409. https://doi.org/10.3390/cancers15092409








