Lung Adenocarcinoma Diagnosed at a Younger Age Is Associated with Advanced Stage, Female Sex, and Ever-Smoker Status, in Patients Treated with Lung Resection
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Study Design and Statistical Analyses
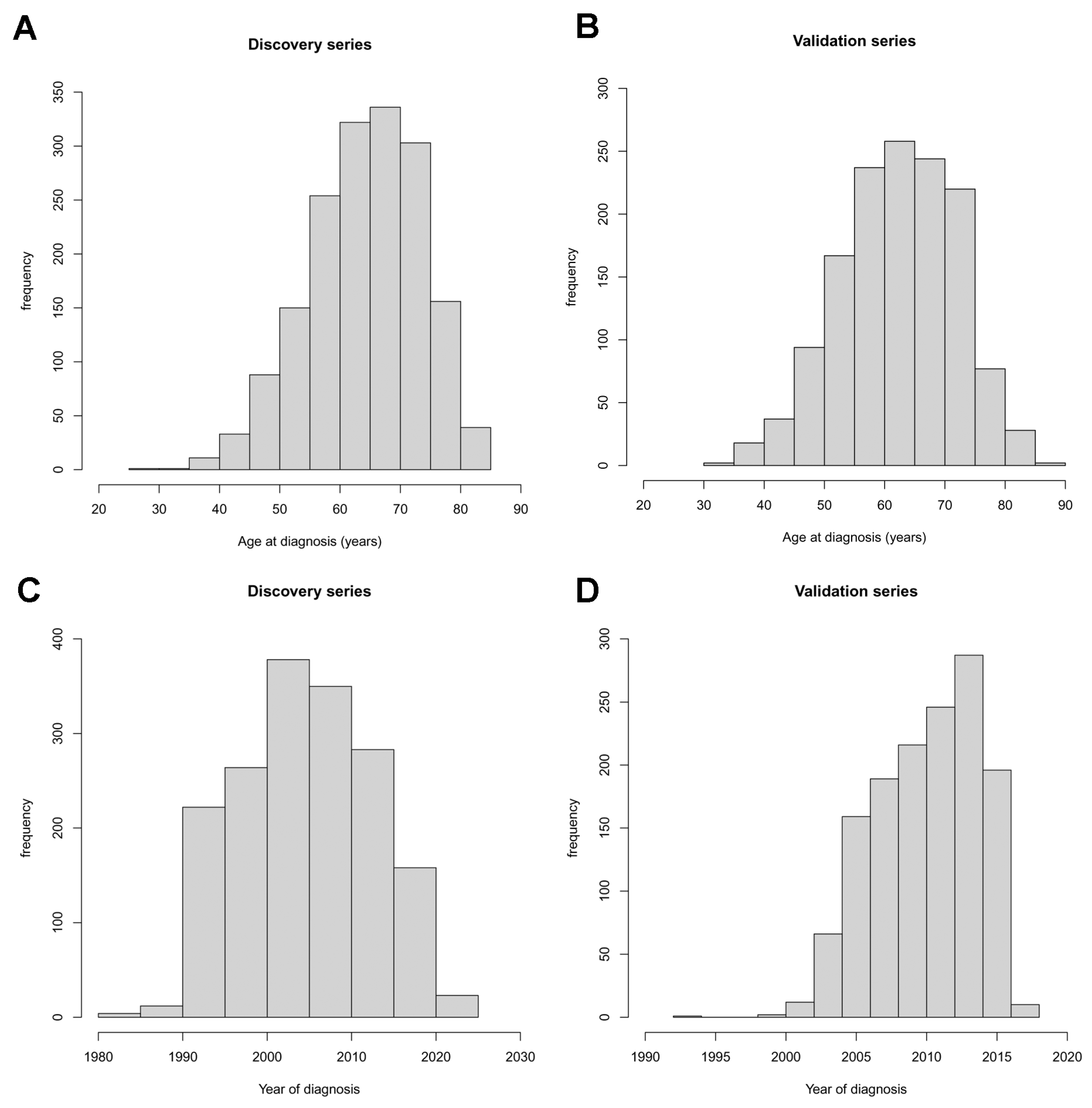
3. Results
Effects of Smoking Status, Pathological Stage, and Sex on Age at Diagnosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rudin, C.M.; Avila-Tang, E.; Samet, J.M. Lung Cancer in Never Smokers: A Call to Action. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2009, 15, 5622–5625. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, L.; Cheng, M. Identification of Genes and Pathways Associated with Sex in Non-Smoking Lung Cancer Population. Gene 2022, 831, 146566. [Google Scholar] [CrossRef] [PubMed]
- Debieuvre, D.; Molinier, O.; Falchero, L.; Locher, C.; Templement-Grangerat, D.; Meyer, N.; Morel, H.; Duval, Y.; Asselain, B.; Letierce, A.; et al. Lung Cancer Trends and Tumor Characteristic Changes over 20 Years (2000–2020): Results of Three French Consecutive Nationwide Prospective Cohorts’ Studies. Lancet Reg. Health. Eur. 2022, 22, 100492. [Google Scholar] [CrossRef]
- Doll, R.; Peto, R. Cigarette Smoking and Bronchial Carcinoma: Dose and Time Relationships among Regular Smokers and Lifelong Non-Smokers. J. Epidemiol. Community Health 1978, 32, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Freedman, N.D.; Leitzmann, M.F.; Hollenbeck, A.R.; Schatzkin, A.; Abnet, C.C. Cigarette Smoking and Subsequent Risk of Lung Cancer in Men and Women: Analysis of a Prospective Cohort Study. Lancet. Oncol. 2008, 9, 649–656. [Google Scholar] [CrossRef]
- Liu, M.; Cai, X.; Yu, W.; Lv, C.; Fu, X. Clinical Significance of Age at Diagnosis among Young Non-Small Cell Lung Cancer Patients under 40 Years Old: A Population-Based Study. Oncotarget 2015, 6, 44963–44970. [Google Scholar] [CrossRef]
- Nagy-Mignotte, H.; Guillem, P.; Vesin, A.; Toffart, A.C.; Colonna, M.; Bonneterre, V.; Brichon, P.Y.; Brambilla, C.; Brambilla, E.; Lantuejoul, S.; et al. Primary Lung Adenocarcinoma: Characteristics by Smoking Habit and Sex. Eur. Respir. J. 2011, 38, 1412–1419. [Google Scholar] [CrossRef]
- Tanaka, K.; Hida, T.; Oya, Y.; Yoshida, T.; Shimizu, J.; Mizuno, T.; Kuroda, H.; Sakakura, N.; Yoshimura, K.; Horio, Y.; et al. Unique Prevalence of Oncogenic Genetic Alterations in Young Patients with Lung Adenocarcinoma. Cancer 2017, 123, 1731–1740. [Google Scholar] [CrossRef]
- Jakubek, Y.; Lang, W.; Vattathil, S.; Garcia, M.; Xu, L.; Huang, L.; Yoo, S.-Y.; Shen, L.; Lu, W.; Chow, C.-W.; et al. Genomic Landscape Established by Allelic Imbalance in the Cancerization Field of a Normal Appearing Airway. Cancer Res. 2016, 76, 3676–3683. [Google Scholar] [CrossRef]
- Ding, L.; Getz, G.; Wheeler, D.A.; Mardis, E.R.; McLellan, M.D.; Cibulskis, K.; Sougnez, C.; Greulich, H.; Muzny, D.M.; Morgan, M.B.; et al. Somatic Mutations Affect Key Pathways in Lung Adenocarcinoma. Nature 2008, 455, 1069–1075. [Google Scholar] [CrossRef]
- Niller, H.H.; Wolf, H.; Minarovits, J. Viral Hit and Run-Oncogenesis: Genetic and Epigenetic Scenarios. Cancer Lett. 2011, 305, 200–217. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Gowers, K.H.C.; Lee-Six, H.; Chandrasekharan, D.P.; Coorens, T.; Maughan, E.F.; Beal, K.; Menzies, A.; Millar, F.R.; Anderson, E.; et al. Tobacco Smoking and Somatic Mutations in Human Bronchial Epithelium. Nature 2020, 578, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Saaoud, F.; Shao, Y.; Cornwell, W.; Wang, H.; Rogers, T.; Yang, X. Cigarette Smoke Modulates Inflammation and Immunity via ROS-Regulated Trained Immunity and Trained Tolerance Mechanisms. Antioxid. Redox Signal. 2022. [Google Scholar] [CrossRef]
- Galvan, A.; Colombo, F.; Frullanti, E.; Dassano, A.; Noci, S.; Wang, Y.; Eisen, T.; Matakidou, A.; Tomasello, L.; Vezzalini, M.; et al. Germline Polymorphisms and Survival of Lung Adenocarcinoma Patients: A Genome-Wide Study in Two European Patient Series. Int. J. Cancer. J. Int. Du Cancer 2015, 136, E262–E271. [Google Scholar] [CrossRef]
- Galvan, A.; Falvella, F.S.; Frullanti, E.; Spinola, M.; Incarbone, M.; Nosotti, M.; Santambrogio, L.; Conti, B.; Pastorino, U.; Gonzalez-Neira, A.; et al. Genome-Wide Association Study in Discordant Sibships Identifies Multiple Inherited Susceptibility Alleles Linked to Lung Cancer. Carcinogenesis 2010, 31, 462–465. [Google Scholar] [CrossRef] [PubMed]
- Galvan, A.; Frullanti, E.; Anderlini, M.; Manenti, G.; Noci, S.; Dugo, M.; Ambrogi, F.; De Cecco, L.; Spinelli, R.; Piazza, R.; et al. Gene Expression Signature of Non-Involved Lung Tissue Associated with Survival in Lung Adenocarcinoma Patients. Carcinogenesis 2013, 34, 2767–2773. [Google Scholar] [CrossRef]
- Groome, P.A.; Bolejack, V.; Crowley, J.J.; Kennedy, C.; Krasnik, M.; Sobin, L.H.; Goldstraw, P. The IASLC Lung Cancer Staging Project: Validation of the Proposals for Revision of the T, N, and M Descriptors and Consequent Stage Groupings in the Forthcoming (Seventh) Edition of the TNM Classification of Malignant Tumours. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2007, 2, 694–705. [Google Scholar] [CrossRef]
- Mirsadraee, S.; Oswal, D.; Alizadeh, Y.; Caulo, A.; van Beek, E.J. The 7th Lung Cancer TNM Classification and Staging System: Review of the Changes and Implications. World J. Radiol. 2012, 4, 128–134. [Google Scholar] [CrossRef]
- Rami-Porta, R.; Bolejack, V.; Giroux, D.J.; Chansky, K.; Crowley, J.; Asamura, H.; Goldstraw, P. The IASLC Lung Cancer Staging Project: The New Database to Inform the Eighth Edition of the TNM Classification of Lung Cancer. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2014, 9, 1618–1624. [Google Scholar] [CrossRef]
- Lüdecke, D.; Ben-Shachar, M.; Patil, I.; Waggoner, P.; Makowski, D. Performance: An R Package for Assessment, Comparison and Testing of Statistical Models. J. Open Source Softw. 2021, 6, 3139. [Google Scholar] [CrossRef]
- Hazelton, W.D.; Clements, M.S.; Moolgavkar, S.H. Multistage Carcinogenesis and Lung Cancer Mortality in Three Cohorts. Cancer Epidemiol. Biomark. Prev. A Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2005, 14, 1171–1181. [Google Scholar] [CrossRef] [PubMed]
- Pirie, K.; Peto, R.; Reeves, G.K.; Green, J.; Beral, V. The 21st Century Hazards of Smoking and Benefits of Stopping: A Prospective Study of One Million Women in the UK. Lancet 2013, 381, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Gou, L.-Y.; Niu, F.-Y.; Wu, Y.-L.; Zhong, W.-Z. Differences in Driver Genes between Smoking-Related and Non-Smoking-Related Lung Cancer in the Chinese Population. Cancer 2015, 121 (Suppl. 17), 3069–3079. [Google Scholar] [CrossRef] [PubMed]
- Mack, P.C.; Klein, M.I.; Ayers, K.L.; Zhou, X.; Guin, S.; Fink, M.; Rossi, M.; Ai-Kateb, H.; O’Connell, T.; Hantash, F.M.; et al. Targeted Next-Generation Sequencing Reveals Exceptionally High Rates of Molecular Driver Mutations in Never-Smokers With Lung Adenocarcinoma. Oncologist 2022, 27, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Schiller, J.H.; Gazdar, A.F. Lung Cancer in Never Smokers--a Different Disease. Nat. Rev. Cancer 2007, 7, 778–790. [Google Scholar] [CrossRef]
- Zhang, T.; Joubert, P.; Ansari-Pour, N.; Zhao, W.; Hoang, P.H.; Lokanga, R.; Moye, A.L.; Rosenbaum, J.; Gonzalez-Perez, A.; Martínez-Jiménez, F.; et al. Genomic and Evolutionary Classification of Lung Cancer in Never Smokers. Nat. Genet. 2021, 53, 1348–1359. [Google Scholar] [CrossRef]
- Tomasetti, C.; Marchionni, L.; Nowak, M.A.; Parmigiani, G.; Vogelstein, B. Only Three Driver Gene Mutations Are Required for the Development of Lung and Colorectal Cancers. Proc. Natl. Acad. Sci. USA 2015, 112, 118–123. [Google Scholar] [CrossRef]
- Rozhok, A.I.; DeGregori, J. Toward an Evolutionary Model of Cancer: Considering the Mechanisms That Govern the Fate of Somatic Mutations. Proc. Natl. Acad. Sci. USA 2015, 112, 8914–8921. [Google Scholar] [CrossRef]
- Jamal-Hanjani, M.; Wilson, G.A.; McGranahan, N.; Birkbak, N.J.; Watkins, T.B.K.; Veeriah, S.; Shafi, S.; Johnson, D.H.; Mitter, R.; Rosenthal, R.; et al. Tracking the Evolution of Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 376, 2109–2121. [Google Scholar] [CrossRef]
- Chen, Z.; Teng, X.; Zhang, J.; Huang, K.; Shen, Q.; Cao, H.; Luo, H.; Yuan, Y.; Teng, X. Molecular Features of Lung Adenocarcinoma in Young Patients. BMC Cancer 2019, 19, 777. [Google Scholar] [CrossRef]
- Patel, M.I.; McKinley, M.; Cheng, I.; Haile, R.; Wakelee, H.; Gomez, S.L. Lung Cancer Incidence Trends in California by Race/Ethnicity, Histology, Sex, and Neighborhood Socioeconomic Status: An Analysis Spanning 28 Years. Lung Cancer 2017, 108, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Lara, V.; Avila-Costa, M.R. An Overview of Lung Cancer in Women and the Impact of Estrogen in Lung Carcinogenesis and Lung Cancer Treatment. Front. Med. 2021, 8, 600121. [Google Scholar] [CrossRef] [PubMed]
| Factor | Discovery Series (n = 1694) | Validation Series (n = 1384) | p-Value |
|---|---|---|---|
| Age at diagnosis, years a | 65 (59–72), (29–85) | 63 (56–70), (32–89) | <0.001 § |
| Age group, n (%) | <0.001 # | ||
| <55 | 251 (14.8) | 280 (20.2) | |
| 55–64 | 549 (32.4) | 471 (34.0) | |
| 65–74 | 665 (39.3) | 498 (36.0) | |
| ≥75 | 229 (13.5) | 135 (9.75) | |
| Sex, n (%) | <0.001 # | ||
| Female | 557 (32.9) | 572 (41.3) | |
| Male | 1137 (67.1) | 812 (58.7) | |
| Smoking habit, n (%) | 0.065 # | ||
| Never | 290 (17.1) | 203 (14.7) | |
| Ever | 1404 (82.9) | 1181 (85.3) | |
| Pathological stage, n (%) | <0.001 # | ||
| I | 942 (55.6) | 478 (34.5) | |
| II | 292 (17.2) | 310 (22.4) | |
| III | 341 (20.1) | 491 (35.5) | |
| IV | 119 (7.02) | 105 (7.59) | |
| Year of diagnosis a | 2005 (2000–2011), (1981–2022) | 2011 (1008–2014), (1992–2018) | <0.001 § |
| Factor | Age at Diagnosis, Median (Q1–Q3), (Range) | Multivariable Glm a | |
|---|---|---|---|
| Beta (95% CI) | p-Value | ||
| Smoking habit | |||
| Never | 68 (59–73), (29–85) | 1.0 | |
| Ever | 65 (59–71), (36–84) | −1.58 (−2.8–−0.39) | 0.0091 |
| Pathological stage | |||
| I | 66 (60–72), (36–85) | 1.0 | |
| II | 66 (59–72), (29–84) | −1.17 (−2.3–−0.010) | 0.048 |
| III | 64 (56–70), (38–84) | −2.27 (−3.4–−1.2) | <0.001 |
| IV | 61 (55–69), (36–84) | −2.68 (−4.4–−0.98) | 0.0020 |
| Sex | |||
| Female | 64 (56–71), (33–85) | 1.0 | |
| Male | 66 (60–72), (29–84) | 3.27 (2.3–4.2) | <0.001 |
| Year of diagnosis | 0.29 (0.24–0.35) | <0.001 | |
| Factor | OR | 95% CI | p-Value | |
|---|---|---|---|---|
| Smoking habit | never | 1.0 | ||
| ever | 1.5 | 1.2–2.0 | 0.0035 | |
| Stage | I | 1.0 | ||
| II | 1.2 | 0.91–1.6 | 0.21 | |
| III | 1.4 | 1.1–1.8 | 0.0067 | |
| IV | 1.7 | 1.2–2.6 | 0.0080 | |
| Sex | female | 1.0 | ||
| male | 0.54 | 0.43–0.93 | <0.001 | |
| Year of diagnosis | 0.95 | 0.94–0.96 | <0.001 |
| Factor | Age at Diagnosis, Median (Q1–Q3), (Range) | Multivariable Glm a | |
|---|---|---|---|
| Beta (95% CI) | p-Value | ||
| Smoking habit | |||
| Never | 69 (62–74), (32–89) | 1.0 | |
| Ever | 62 (56–69), (33–85) | −5.90 (−7.3–−4.5) | <0.001 |
| Pathological stage | |||
| I | 64 (57–71), (38–89) | 1.0 | |
| II | 64 (56–70), (40–88) | −0.879 (−2.2–0.46) | 0.20 |
| III | 62 (55–69), (32–82) | −2.51 (−3.7–−1.3) | <0.001 |
| IV | 60 (55–69), (33–81) | −3.42 (−5.4–−1.4) | <0.001 |
| Sex | |||
| Female | 63 (55–70), (36–88) | 1.0 | |
| Male | 63 (57–70), (32–89) | 1.60 (0.56–2.6) | 0.0027 |
| Year of diagnosis | 0.106 (−0.029–0.24) | 0.12 | |
| Factor | OR | 95% CI | p-Value | |
|---|---|---|---|---|
| Smoking habit | never | 1.0 | ||
| ever | 2.9 | 2.1–4.0 | <0.001 | |
| Stage | I | 1.0 | ||
| II | 1.0 | 0.75–1.3 | 0.97 | |
| III | 1.4 | 1.1–1.8 | 0.0066 | |
| IV | 2.0 | 1.3–3.1 | 0.0022 | |
| Sex | female | 1.0 | ||
| male | 0.78 | 0.62–0.98 | 0.032 | |
| Year of diagnosis | 1.0 | 0.97–1.0 | 0.93 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dragani, T.A.; Muley, T.; Schneider, M.A.; Kobinger, S.; Eichhorn, M.; Winter, H.; Hoffmann, H.; Kriegsmann, M.; Noci, S.; Incarbone, M.; et al. Lung Adenocarcinoma Diagnosed at a Younger Age Is Associated with Advanced Stage, Female Sex, and Ever-Smoker Status, in Patients Treated with Lung Resection. Cancers 2023, 15, 2395. https://doi.org/10.3390/cancers15082395
Dragani TA, Muley T, Schneider MA, Kobinger S, Eichhorn M, Winter H, Hoffmann H, Kriegsmann M, Noci S, Incarbone M, et al. Lung Adenocarcinoma Diagnosed at a Younger Age Is Associated with Advanced Stage, Female Sex, and Ever-Smoker Status, in Patients Treated with Lung Resection. Cancers. 2023; 15(8):2395. https://doi.org/10.3390/cancers15082395
Chicago/Turabian StyleDragani, Tommaso A., Thomas Muley, Marc A. Schneider, Sonja Kobinger, Martin Eichhorn, Hauke Winter, Hans Hoffmann, Mark Kriegsmann, Sara Noci, Matteo Incarbone, and et al. 2023. "Lung Adenocarcinoma Diagnosed at a Younger Age Is Associated with Advanced Stage, Female Sex, and Ever-Smoker Status, in Patients Treated with Lung Resection" Cancers 15, no. 8: 2395. https://doi.org/10.3390/cancers15082395
APA StyleDragani, T. A., Muley, T., Schneider, M. A., Kobinger, S., Eichhorn, M., Winter, H., Hoffmann, H., Kriegsmann, M., Noci, S., Incarbone, M., Tosi, D., Franzi, S., & Colombo, F. (2023). Lung Adenocarcinoma Diagnosed at a Younger Age Is Associated with Advanced Stage, Female Sex, and Ever-Smoker Status, in Patients Treated with Lung Resection. Cancers, 15(8), 2395. https://doi.org/10.3390/cancers15082395








