Focal Cryo-Immunotherapy with Intratumoral IL-12 Prevents Recurrence of Large Murine Tumors
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Cell Lines
2.2. Immunotherapy
2.3. Transcriptome Sequencing
2.4. Statistics
3. Results
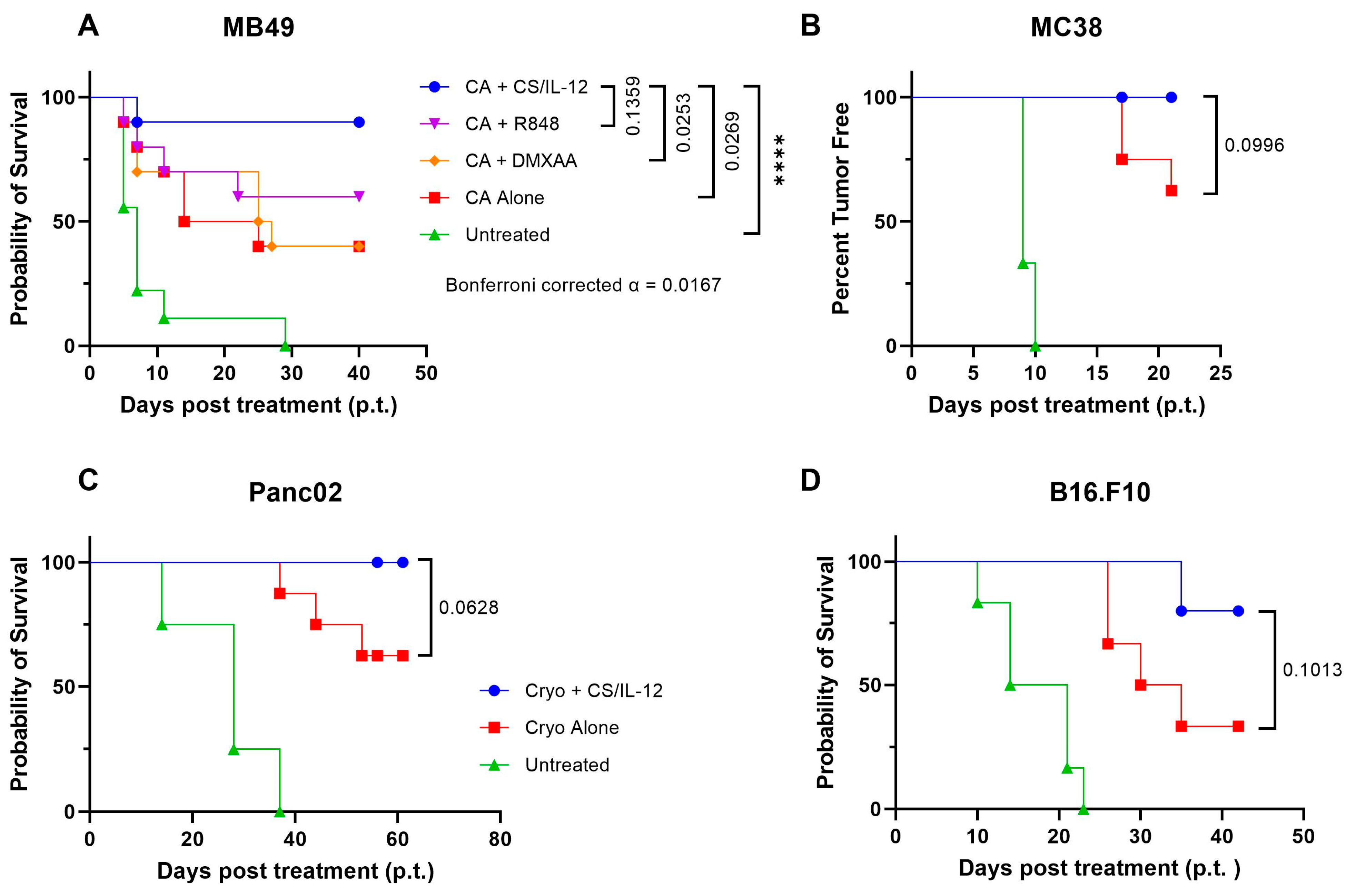
3.1. Localized IL-12, but Not TLR or STING Agonists, Improves Primary Tumor Elimination and Prevents Recurrence after Ablation in Multiple Tumor Models
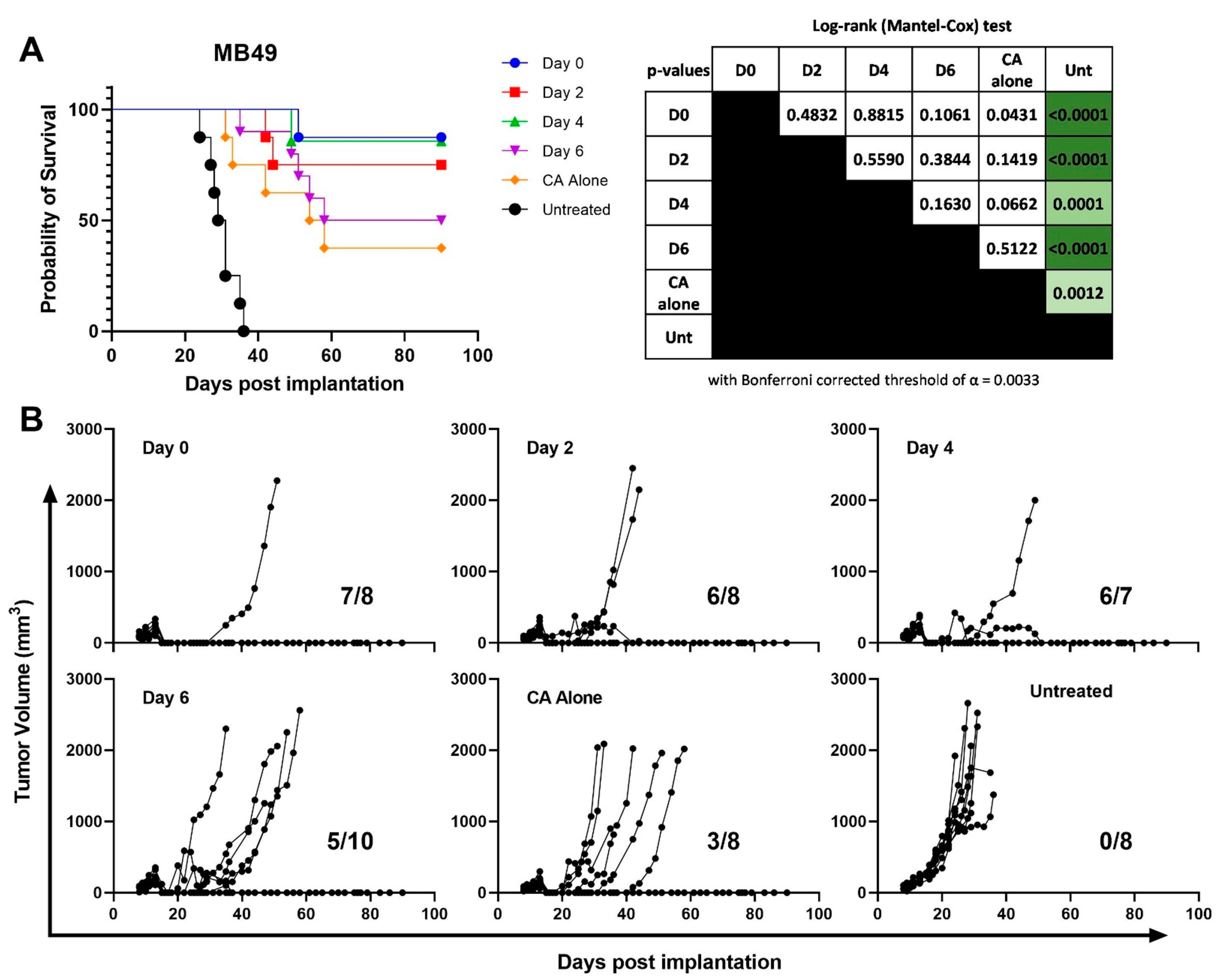
3.2. CS/IL-12 Delivered within 4 Days after CA Is the Most Effective Therapeutic Window
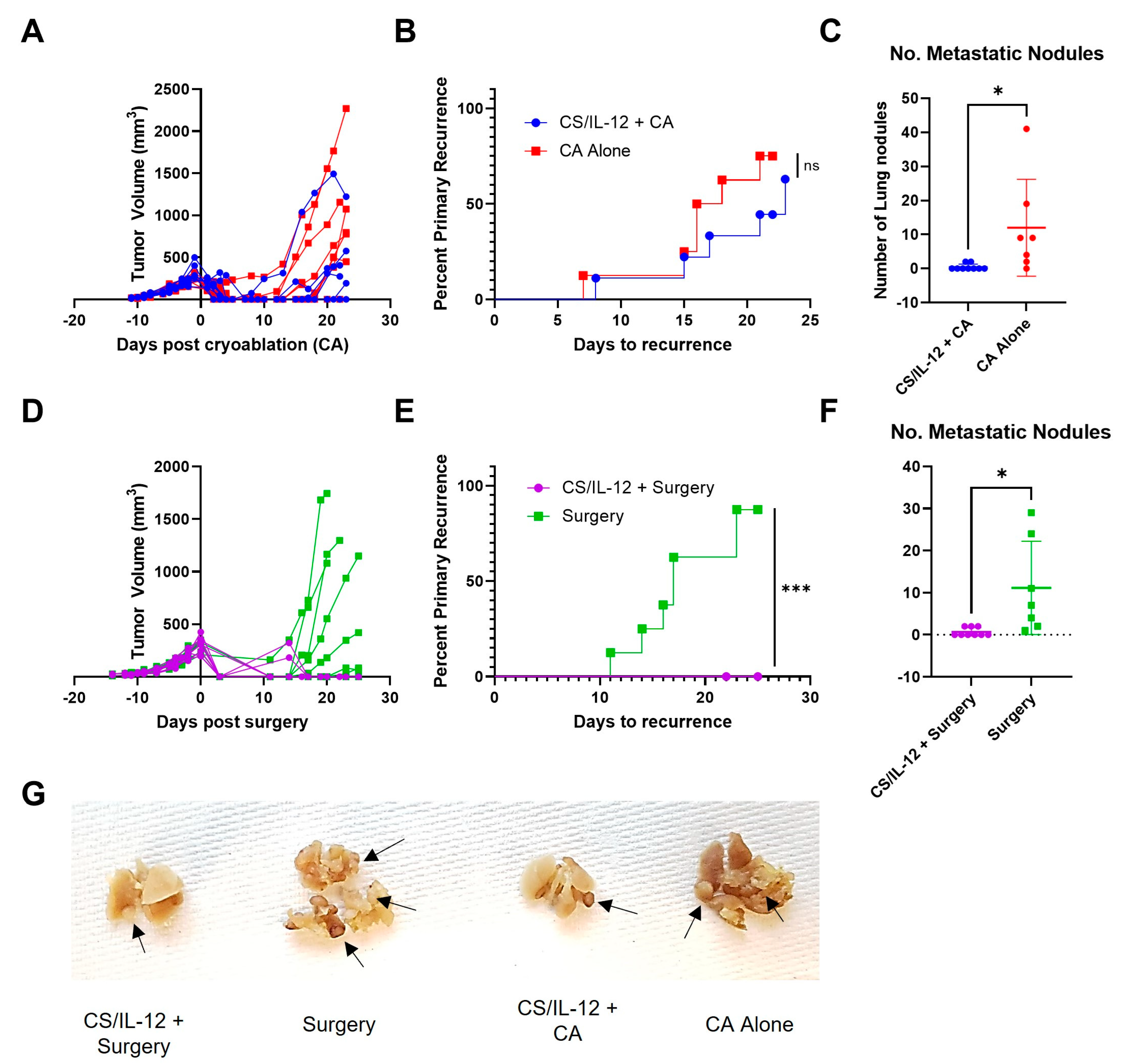
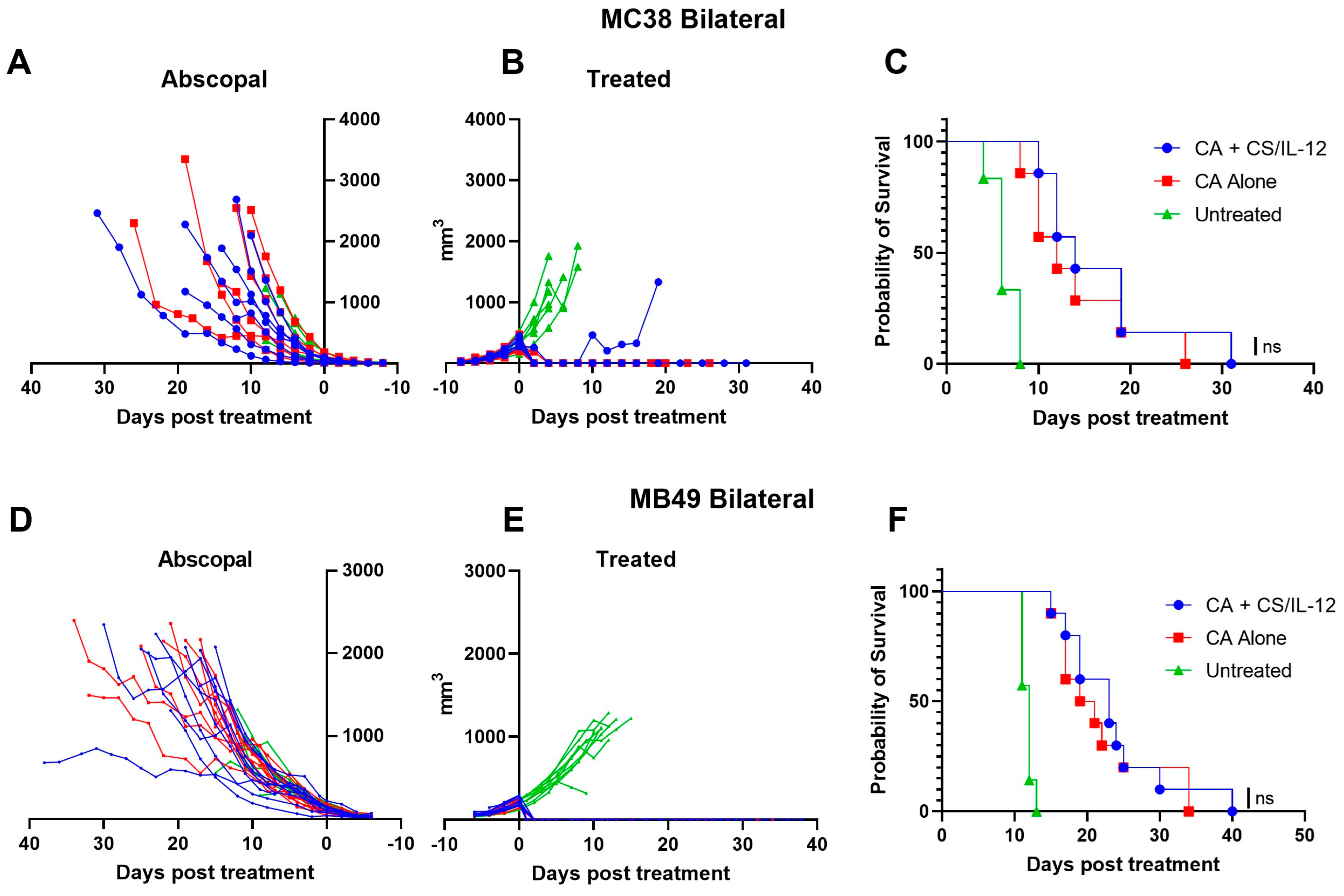
3.3. Addition of CS/IL-12 to CA Inhibits Lung Metastases but Not Established Abscopal Tumors
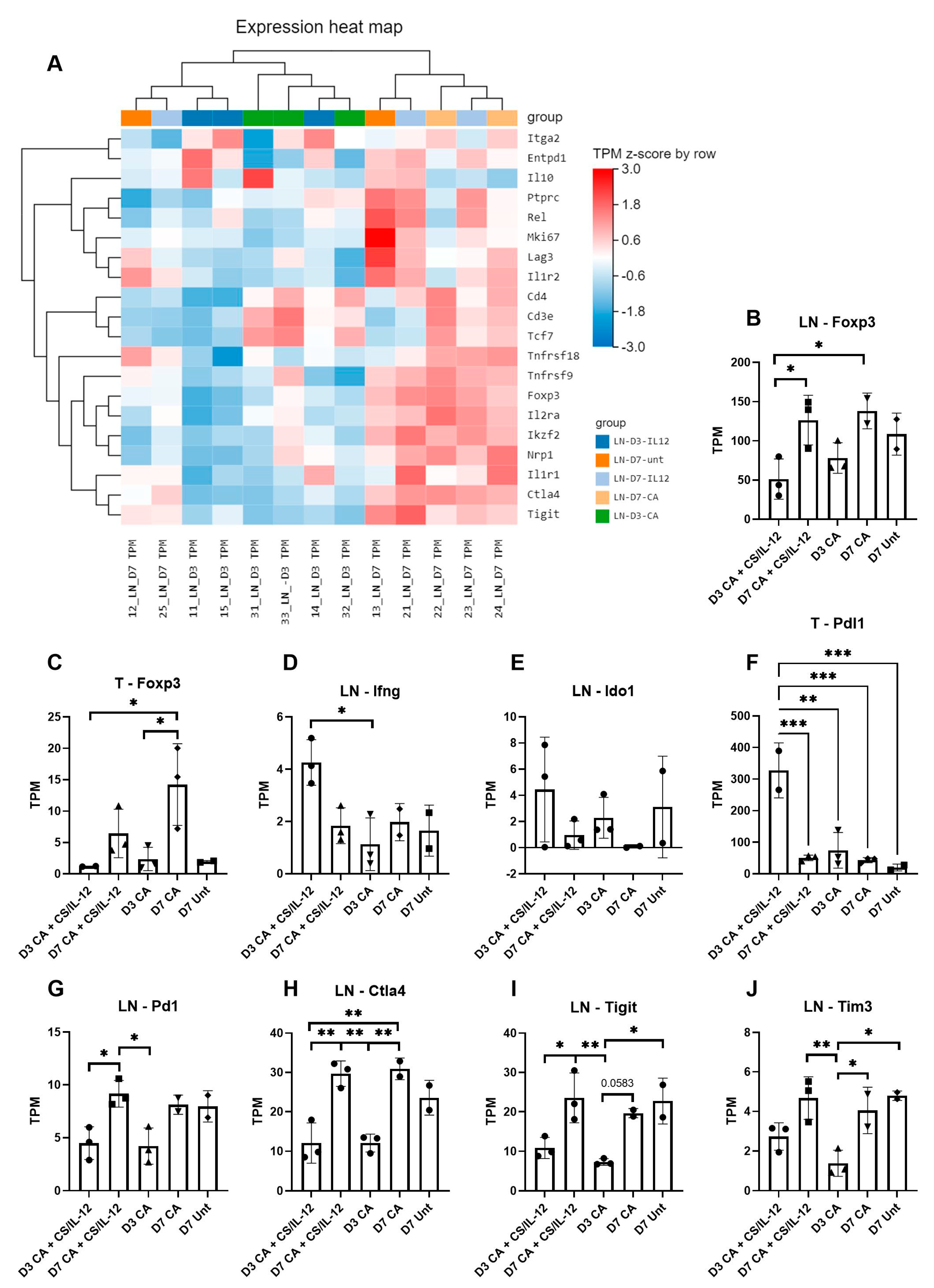
3.4. CS/IL-12 Alters Differential Gene Expression in the Tumor Draining Lymph Node
3.5. CS/IL-12 Increases Gene Expression of Markers for Cross Presentation and Costimulation Early after CA
3.6. Immune Suppression Increases over Time after CA Treatment
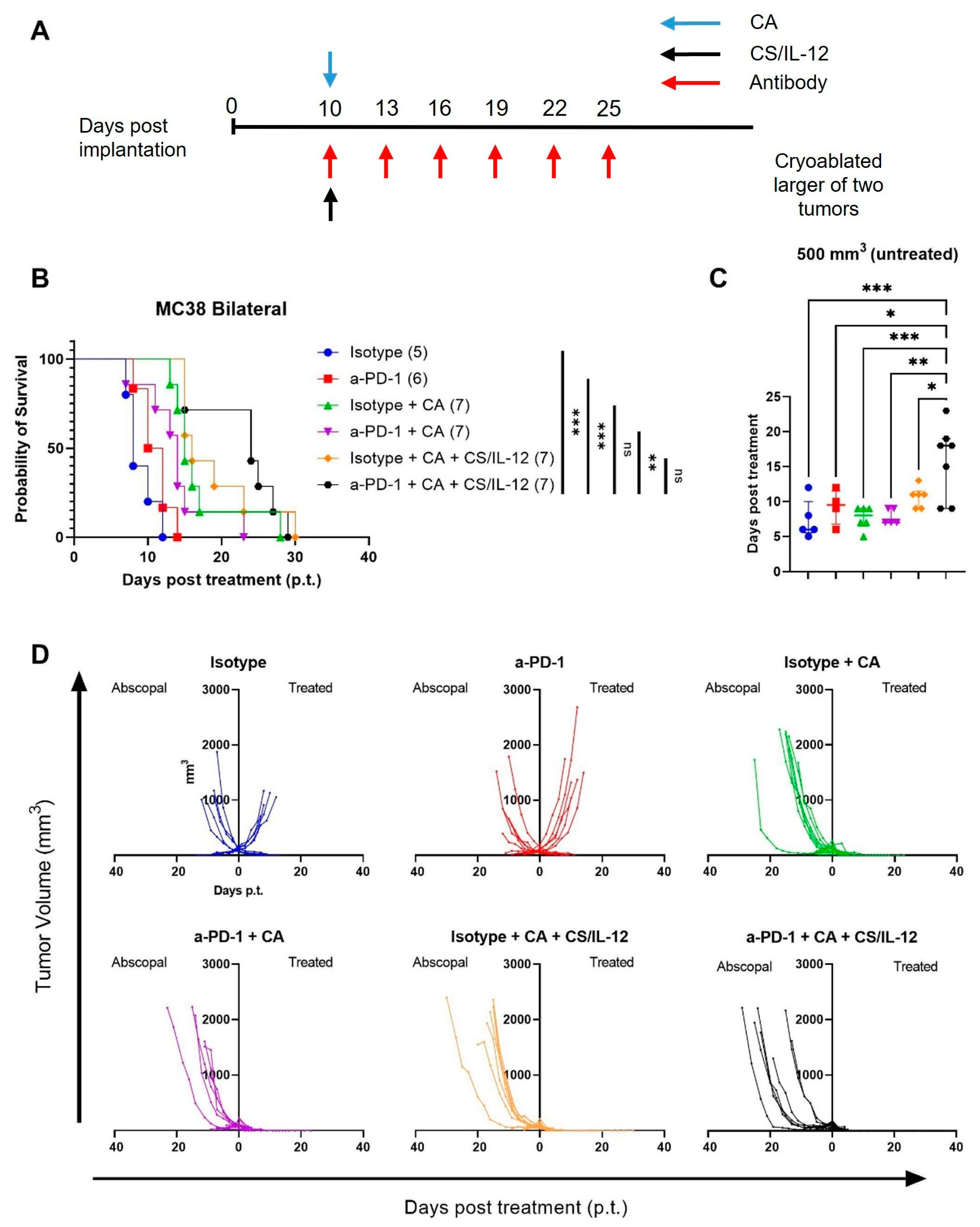
3.7. Addition of Checkpoint Inhibitors to CA plus CS/IL-12 Delays Abscopal Tumor Growth
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gao, F.; Bai, Y.; Ma, S.-R.; Liu, F.; Li, Z.-S. Systematic review: Photodynamic therapy for unresectable cholangiocarcinoma. J. Hepato-Biliary-Pancreat. Sci. 2010, 17, 125–131. [Google Scholar] [CrossRef] [PubMed]
- van der Geest, L.G.M.; van Eijck, C.H.J.; Groot Koerkamp, B.; Lemmens, V.E.P.P.; Busch, O.R.; Vissers, P.A.J.; Wilmink, J.W.; Besselink, M.G. the Dutch Pancreatic Cancer Group Trends in treatment and survival of patients with nonresected, nonmetastatic pancreatic cancer: A population-based study. Cancer Med. 2018, 7, 4943–4951. [Google Scholar] [CrossRef] [PubMed]
- Chari, S.T. Detecting Early Pancreatic Cancer: Problems and Prospects. Semin. Oncol. 2007, 34, 284–294. [Google Scholar] [CrossRef]
- Gholam, P.M.; Iyer, R.; Johnson, M.S. Multidisciplinary Management of Patients with Unresectable Hepatocellular Carcinoma: A Critical Appraisal of Current Evidence. Cancers 2019, 11, 873. [Google Scholar] [CrossRef]
- Moghissi, K.; Dixon, K.; Gibbins, S. A Surgical View of Photodynamic Therapy in Oncology: A Review. Surg. J. 2015, 01, e1–e15. [Google Scholar] [CrossRef]
- Yoon, S.M.; Shaikh, T.; Hallman, M. Therapeutic management options for stage III non-small cell lung cancer. World J. Clin. Oncol. 2017, 8, 1–20. [Google Scholar] [CrossRef]
- Curigliano, G.; Criscitiello, C.; Esposito, A.; Fumagalli, L.; Gelao, L.; Locatelli, M.; Minchella, I.; Goldhirsch, A. Best management of locally advanced inoperable breast cancer. Eur. J. Cancer Suppl. 2013, 11, 289–290. [Google Scholar] [CrossRef]
- Fujihara, A.; Ukimura, O. Focal therapy of localized prostate cancer. J. Urol. 2022, 29, 1254–1263. [Google Scholar] [CrossRef]
- Carriero, S.; Lanza, C.; Pellegrino, G.; Ascenti, V.; Sattin, C.; Pizzi, C.; Angileri, S.A.; Biondetti, P.; Ianniello, A.A.; Piacentino, F.; et al. Ablative Therapies for Breast Cancer: State of Art. Technol. Cancer Res. Treat. 2023, 22, 15330338231157192. [Google Scholar] [CrossRef]
- Pandolfo, S.D.; Carbonara, U.; Beksac, A.T.; Derweesh, I.; Celia, A.; Schiavina, R.; Elbich, J.; Basile, G.; Hampton, L.J.; Cerrato, C.; et al. Microwave versus cryoablation and radiofrequency ablation for small renal mass: A multicenter comparative analysis. Minerva Urol. Nephrol. 2023, 75, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenhuizen, S.; Dijkstra, M.; Puijk, R.S.; Geboers, B.; Ruarus, A.H.; Schouten, E.A.; Nielsen, K.; de Vries, J.J.J.; Bruynzeel, A.M.E.; Scheffer, H.J.; et al. Microwave Ablation, Radiofrequency Ablation, Irreversible Electroporation, and Stereotactic Ablative Body Radiotherapy for Intermediate Size (3–5 cm) Unresectable Colorectal Liver Metastases: A Systematic Review and Meta-analysis. Curr. Oncol. Rep. 2022, 24, 793–808. [Google Scholar] [CrossRef]
- Tomita, K.; Matsui, Y.; Uka, M.; Umakoshi, N.; Kawabata, T.; Munetomo, K.; Nagata, S.; Iguchi, T.; Hiraki, T. Evidence on percutaneous radiofrequency and microwave ablation for liver metastases over the last decade. Jpn. J. Radiol. 2022, 40, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Selvaggio, O.; Falagario, U.G.; Bruno, S.M.; Recchia, M.; Sighinolfi, M.C.; Sanguedolce, F.; Milillo, P.; Macarini, L.; Rastinehad, A.R.; Sanchez-Salas, R.; et al. Intraoperative Digital Analysis of Ablation Margins (DAAM) by Fluorescent Confocal Microscopy to Improve Partial Prostate Gland Cryoablation Outcomes. Cancers 2021, 13, 4382. [Google Scholar] [CrossRef] [PubMed]
- Tay, K.J.; Polascik, T.J.; Elshafei, A.; Cher, M.L.; Given, R.W.; Mouraviev, V.; Ross, A.E.; Jones, J.S. Primary Cryotherapy for High-Grade Clinically Localized Prostate Cancer: Oncologic and Functional Outcomes from the COLD Registry. J. Endourol. 2016, 30, 43–48. [Google Scholar] [CrossRef]
- de Marini, P.; Cazzato, R.L.; Garnon, J.; Shaygi, B.; Koch, G.; Auloge, P.; Tricard, T.; Lang, H.; Gangi, A. Percutaneous MR-guided prostate cancer cryoablation technical updates and literature review. BJR Open 2019, 1, 20180043. [Google Scholar] [CrossRef]
- Seager, M.; Kumar, S.; Lim, E.; Munneke, G.; Bandula, S.; Walkden, M. Renal cryoablation—A practical guide for interventional radiologists. Br. J. Radiol. 2021, 94, 20200854. [Google Scholar] [CrossRef] [PubMed]
- Uemura, T.; Kato, T.; Nagahara, A.; Kawashima, A.; Hatano, K.; Ujike, T.; Ono, Y.; Higashihara, H.; Fujita, K.; Fukuhara, S.; et al. Therapeutic and Clinical Outcomes of Robot-assisted Partial Nephrectomy Versus Cryoablation for T1 Renal Cell Carcinoma. In Vivo 2021, 35, 1573–1579. [Google Scholar] [CrossRef]
- Gobara, H.; Hiraki, T.; Iguchi, T.; Matsui, Y.; Sakurai, J.; Uka, M.; Tomita, K.; Komaki, T.; Kobayasi, Y.; Araki, M.; et al. Oncologic outcomes and safety of percutaneous cryoablation for biopsy-proven renal cell carcinoma up to 4 cm in diameter: A prospective observational study. Int. J. Clin. Oncol. 2021, 26, 562–568. [Google Scholar] [CrossRef]
- Rong, G.; Bai, W.; Dong, Z.; Wang, C.; Lu, Y.; Zeng, Z.; Qu, J.; Lou, M.; Wang, H.; Gao, X.; et al. Long-Term Outcomes of Percutaneous Cryoablation for Patients with Hepatocellular Carcinoma within Milan Criteria. PLoS ONE 2015, 10, e0123065. [Google Scholar] [CrossRef]
- Pandolfo, S.D.; Loizzo, D.; Beksac, A.T.; Derweesh, I.; Celia, A.; Bianchi, L.; Elbich, J.; Costa, G.; Carbonara, U.; Lucarelli, G.; et al. Percutaneous thermal ablation for cT1 renal mass in solitary kidney: A multicenter trifecta comparative analysis versus robot-assisted partial nephrectomy. Eur. J. Surg. Oncol. 2023, 49, 486–490. [Google Scholar] [CrossRef]
- Pandolfo, S.D.; Beksac, A.T.; Derweesh, I.; Celia, A.; Schiavina, R.; Bianchi, L.; Costa, G.; Carbonara, U.; Loizzo, D.; Lucarelli, G.; et al. Percutaneous Ablation vs Robot-Assisted Partial Nephrectomy for Completely Endophytic Renal Masses: A Multicenter Trifecta Analysis with a Minimum 3-Year Follow-Up. J. Endourol. 2023, 37, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Pickersgill, N.A.; Vetter, J.M.; Kim, E.H.; Cope, S.J.; Du, K.; Venkatesh, R.; Giardina, J.D.; Saad, N.E.S.; Bhayani, S.B.; Figenshau, R.S. Ten-Year Experience with Percutaneous Cryoablation of Renal Tumors: Tumor Size Predicts Disease Progression. J. Endourol. 2020, 34, 1211–1217. [Google Scholar] [CrossRef]
- Spiliopoulos, S.; Marzoug, A.; Ra, H.; Arcot Ragupathy, S.K. Long-term outcomes of CT-guided percutaneous cryoablation of T1a and T1b renal cell carcinoma. Diagn. Interv. Radiol. 2021, 27, 524–528. [Google Scholar] [CrossRef] [PubMed]
- Morkos, J.; Porosnicu Rodriguez, K.A.; Zhou, A.; Kolarich, A.R.; Frangakis, C.; Rodriguez, R.; Georgiades, C.S. Percutaneous Cryoablation for Stage 1 Renal Cell Carcinoma: Outcomes from a 10-year Prospective Study and Comparison with Matched Cohorts from the National Cancer Database. Radiology 2020, 296, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.H.; Atwell, T.; Schmit, G.; Lohse, C.M.; Kurup, A.N.; Weisbrod, A.; Psutka, S.P.; Stewart, S.B.; Callstrom, M.R.; Cheville, J.C.; et al. Comparison of partial nephrectomy and percutaneous ablation for cT1 renal masses. Eur. Urol. 2015, 67, 252–259. [Google Scholar] [CrossRef]
- Breen, D.J.; King, A.J.; Patel, N.; Lockyer, R.; Hayes, M. Image-guided Cryoablation for Sporadic Renal Cell Carcinoma: Three- and 5-year Outcomes in 220 Patients with Biopsy-proven Renal Cell Carcinoma. Radiology 2018, 289, 554–561. [Google Scholar] [CrossRef]
- Caputo, P.A.; Zargar, H.; Ramirez, D.; Andrade, H.S.; Akca, O.; Gao, T.; Kaouk, J.H. Cryoablation versus Partial Nephrectomy for Clinical T1b Renal Tumors: A Matched Group Comparative Analysis. Eur. Urol. 2017, 71, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Moynagh, M.R.; Schmit, G.D.; Thompson, R.H.; Boorjian, S.A.; Woodrum, D.A.; Curry, T.B.; Atwell, T.D. Percutaneous Cryoablation of Clinical T2 (>7 cm) Renal Masses: Technical Considerations, Complications, and Short-Term Outcomes. J. Vasc. Interv. Radiol. 2015, 26, 800–806. [Google Scholar] [CrossRef]
- Zaharoff, D.A.; Rogers, C.J.; Hance, K.W.; Schlom, J.; Greiner, J.W. Chitosan solution enhances the immunoadjuvant properties of GM-CSF. Vaccine 2007, 25, 8673–8686. [Google Scholar] [CrossRef]
- Zaharoff, D.A.; Hance, K.W.; Rogers, C.J.; Schlom, J.; Greiner, J.W. Intratumoral immunotherapy of established solid tumors with chitosan/IL-12. J. Immunother. 2010, 33, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Zaharoff, D.A.; Rogers, C.J.; Hance, K.W.; Schlom, J.; Greiner, J.W. Chitosan solution enhances both humoral and cell-mediated immune responses to subcutaneous vaccination. Vaccine 2007, 25, 2085–2094. [Google Scholar] [CrossRef]
- Jayanthi, S.; prasanth Koppolu, B.; Smith, S.G.; Jalah, R.; Bear, J.; Rosati, M.; Pavlakis, G.N.; Felber, B.K.; Zaharoff, D.A.; Kumar, T.K.S. Efficient production and purification of recombinant human interleukin-12 (IL-12) overexpressed in mammalian cells without affinity tag. Protein Expr. Purif. 2014, 102, 76–84. [Google Scholar] [CrossRef]
- Yang, L.; Zaharoff, D.A. Role of chitosan co-formulation in enhancing interleukin-12 delivery and antitumor activity. Biomaterials 2013, 34, 3828–3836. [Google Scholar] [CrossRef]
- Vo, J.L.; Yang, L.; Kurtz, S.L.; Smith, S.G.; Koppolu, B.P.; Ravindranathan, S.; Zaharoff, D.A. Neoadjuvant immunotherapy with chitosan and interleukin-12 to control breast cancer metastasis. Oncoimmunology 2014, 3, e968001. [Google Scholar] [CrossRef]
- Silvestrini, M.T.; Ingham, E.S.; Mahakian, L.M.; Kheirolomoom, A.; Liu, Y.; Fite, B.Z.; Tam, S.M.; Tucci, S.T.; Watson, K.D.; Wong, A.W.; et al. Priming is key to effective incorporation of image-guided thermal ablation into immunotherapy protocols. JCI Insight 2017, 2, e90521. [Google Scholar] [CrossRef]
- Ho, W.J.; Zhu, Q.; Durham, J.; Popovic, A.; Xavier, S.; Leatherman, J.; Mohan, A.; Mo, G.; Zhang, S.; Gross, N.; et al. Neoadjuvant cabozantinib and nivolumab convert locally advanced hepatocellular carcinoma into resectable disease with enhanced antitumor immunity. Nat. Cancer 2021, 2, 891–903. [Google Scholar] [CrossRef] [PubMed]
- Kaseb, A.O.; Hasanov, E.; Cao, H.S.T.; Xiao, L.; Vauthey, J.-N.; Lee, S.S.; Yavuz, B.G.; Mohamed, Y.I.; Qayyum, A.; Jindal, S.; et al. Perioperative nivolumab monotherapy versus nivolumab plus ipilimumab in resectable hepatocellular carcinoma: A randomised, open-label, phase 2 trial. Lancet Gastroenterol. Hepatol. 2022, 7, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Burton, E.M.; Amaria, R.N.; Cascone, T.; Chalabi, M.; Gross, N.D.; Mittendorf, E.A.; Scolyer, R.A.; Sharma, P.; Ascierto, P.A. Neoadjuvant immunotherapy across cancers: Meeting report from the Immunotherapy Bridge—December 1st–2nd, 2021. J. Transl. Med. 2022, 20, 271. [Google Scholar] [CrossRef]
- Gu, T.; Rowswell-Turner, R.B.; Kilinc, M.O.; Egilmez, N.K. Central Role of IFNγ–Indoleamine 2,3-Dioxygenase Axis in Regulation of Interleukin-12–Mediated Antitumor Immunity. Cancer Res. 2010, 70, 129–138. [Google Scholar] [CrossRef]
- Li, Q.; Virtuoso, L.P.; Anderson, C.D.; Egilmez, N.K. Regulatory Rebound in IL-12–Treated Tumors Is Driven by Uncommitted Peripheral Regulatory T Cells. J. Immunol. 2015, 195, 1293–1300. [Google Scholar] [CrossRef] [PubMed]
- Beg, A.A.; Baldwin, A.S. Activation of multiple NF-kappa B/Rel DNA-binding complexes by tumor necrosis factor. Oncogene 1994, 9, 1487–1492. [Google Scholar]
- Tchilian, E.Z.; Wallace, D.L.; Wells, R.S.; Flower, D.R.; Morgan, G.; Beverley, P.C.L. A Deletion in the Gene Encoding the CD45 Antigen in a Patient with SCID. J. Immunol. 2001, 166, 1308–1313. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Sun, X.; Csizmadia, E.; Han, L.; Bian, S.; Murakami, T.; Wang, X.; Robson, S.C.; Wu, Y. Vascular CD39/ENTPD1 directly promotes tumor cell growth by scavenging extracellular adenosine triphosphate. Neoplasia 2011, 13, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Waitz, R.; Solomon, S.B.; Petre, E.N.; Trumble, A.E.; Fassò, M.; Norton, L.; Allison, J.P. Potent Induction of Tumor Immunity by Combining Tumor Cryoablation with Anti–CTLA-4 Therapy. Cancer Res. 2012, 72, 430–439. [Google Scholar] [CrossRef] [PubMed]
- den Brok, M.H.M.G.M.; Sutmuller, R.P.M.; Nierkens, S.; Bennink, E.J.; Frielink, C.; Toonen, L.W.J.; Boerman, O.C.; Figdor, C.G.; Ruers, T.J.M.; Adema, G.J. Efficient loading of dendritic cells following cryo and radiofrequency ablation in combination with immune modulation induces anti-tumour immunity. Br. J. Cancer 2006, 95, 896–905. [Google Scholar] [CrossRef]
- Homet Moreno, B.; Zaretsky, J.M.; Garcia-Diaz, A.; Tsoi, J.; Parisi, G.; Robert, L.; Meeth, K.; Ndoye, A.; Bosenberg, M.; Weeraratna, A.T.; et al. Response to Programmed Cell Death-1 Blockade in a Murine Melanoma Syngeneic Model Requires Costimulation, CD4, and CD8 T Cells. Cancer Immunol. Res. 2016, 4, 845–857. [Google Scholar] [CrossRef]
- Nguyen, K.G.; Vrabel, M.R.; Mantooth, S.M.; Hopkins, J.J.; Wagner, E.S.; Gabaldon, T.A.; Zaharoff, D.A. Localized Interleukin-12 for Cancer Immunotherapy. Front. Immunol. 2020, 11, 2510. [Google Scholar] [CrossRef] [PubMed]
- Algazi, A.P.; Twitty, C.G.; Tsai, K.K.; Le, M.; Pierce, R.; Browning, E.; Hermiz, R.; Canton, D.A.; Bannavong, D.; Oglesby, A.; et al. Phase II Trial of IL-12 Plasmid Transfection and PD-1 Blockade in Immunologically Quiescent Melanoma. Clin. Cancer Res. 2020, 26, 2827–2837. [Google Scholar] [CrossRef] [PubMed]
- Chiocca, E.A.; Yu, J.S.; Lukas, R.V.; Solomon, I.H.; Ligon, K.L.; Nakashima, H.; Triggs, D.A.; Reardon, D.A.; Wen, P.; Stopa, B.M.; et al. Regulatable interleukin-12 gene therapy in patients with recurrent high-grade glioma: Results of a phase 1 trial. Sci. Transl. Med. 2019, 11, eaaw5680. [Google Scholar] [CrossRef]
- Greaney, S.K.; Algazi, A.P.; Tsai, K.K.; Takamura, K.T.; Chen, L.; Twitty, C.G.; Zhang, L.; Paciorek, A.; Pierce, R.H.; Le, M.H.; et al. Intratumoral Plasmid IL12 Electroporation Therapy in Patients with Advanced Melanoma Induces Systemic and Intratumoral T-cell Responses. Cancer Immunol. Res. 2020, 8, 246–254. [Google Scholar] [CrossRef]
- Wallace, A.; LaRosa, D.F.; Kapoor, V.; Sun, J.; Cheng, G.; Jassar, A.; Blouin, A.; Ching, L.-M.; Albelda, S.M. The Vascular Disrupting Agent, DMXAA, Directly Activates Dendritic Cells through a MyD88-Independent Mechanism and Generates Antitumor Cytotoxic T Lymphocytes. Cancer Res. 2007, 67, 7011–7019. [Google Scholar] [CrossRef]
- Weiss, J.M.; Guérin, M.V.; Regnier, F.; Renault, G.; Galy-Fauroux, I.; Vimeux, L.; Feuillet, V.; Peranzoni, E.; Thoreau, M.; Trautmann, A.; et al. The STING agonist DMXAA triggers a cooperation between T lymphocytes and myeloid cells that leads to tumor regression. OncoImmunology 2017, 6, e1346765. [Google Scholar] [CrossRef]
- Bourquin, C.; Hotz, C.; Noerenberg, D.; Voelkl, A.; Heidegger, S.; Roetzer, L.C.; Storch, B.; Sandholzer, N.; Wurzenberger, C.; Anz, D.; et al. Systemic Cancer Therapy with a Small Molecule Agonist of Toll-like Receptor 7 Can Be Improved by Circumventing TLR Tolerance. Cancer Res. 2011, 71, 5123–5133. [Google Scholar] [CrossRef] [PubMed]
- Michaelis, K.A.; Norgard, M.A.; Zhu, X.; Levasseur, P.R.; Sivagnanam, S.; Liudahl, S.M.; Burfeind, K.G.; Olson, B.; Pelz, K.R.; Angeles Ramos, D.M.; et al. The TLR7/8 agonist R848 remodels tumor and host responses to promote survival in pancreatic cancer. Nat. Commun. 2019, 10, 4682. [Google Scholar] [CrossRef]
- Nguyen, K.G.; Wagner, E.S.; Vrabel, M.R.; Mantooth, S.M.; Meritet, D.M.; Zaharoff, D.A. Safety and Pharmacokinetics of Intravesical Chitosan/Interleukin-12 Immunotherapy in Murine Bladders. Bl. Cancer 2021, 7, 427–437. [Google Scholar] [CrossRef]
- Smith, S.G.; Koppolu, B.P.; Ravindranathan, S.; Kurtz, S.L.; Yang, L.; Katz, M.D.; Zaharoff, D.A. Intravesical chitosan/interleukin-12 immunotherapy induces tumor-specific systemic immunity against murine bladder cancer. Cancer Immunol. Immunother. 2015, 64, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Alteber, Z.; Azulay, M.; Cafri, G.; Vadai, E.; Tzehoval, E.; Eisenbach, L. Cryoimmunotherapy with local co-administration of ex vivo generated dendritic cells and CpG-ODN immune adjuvant, elicits a specific antitumor immunity. Cancer Immunol. Immunother. 2014, 63, 369–380. [Google Scholar] [CrossRef]
- Zhang, M.; Yin, T.; Lu, Y.; Feng, H. The Application of Cytidyl Guanosyl Oligodeoxynucleotide Can Affect the Antitumor Immune Response Induced by a Combined Protocol of Cryoablation and Dendritic Cells in Lewis Lung Cancer Model. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2016, 22, 1309. [Google Scholar] [CrossRef]
- Udagawa, M.; Kudo-Saito, C.; Hasegawa, G.; Yano, K.; Yamamoto, A.; Yaguchi, M.; Toda, M.; Azuma, I.; Iwai, T.; Kawakami, Y. Enhancement of Immunologic Tumor Regression by Intratumoral Administration of Dendritic Cells in Combination with Cryoablative Tumor Pretreatment and Bacillus Calmette-Guerin Cell Wall Skeleton Stimulation. Clin. Cancer Res. 2006, 12, 7465–7475. [Google Scholar] [CrossRef]
- Pidugu, V.K.; Pidugu, H.B.; Wu, M.-M.; Liu, C.-J.; Lee, T.-C. Emerging Functions of Human IFIT Proteins in Cancer. Front. Mol. Biosci. 2019, 6, 148. [Google Scholar] [CrossRef]
- Smyth, M.J.; Taniguchi, M.; Street, S.E.A. The Anti-Tumor Activity of IL-12: Mechanisms of Innate Immunity That Are Model and Dose Dependent. J. Immunol. 2000, 165, 2665–2670. [Google Scholar] [CrossRef]
- Abel, S.; Hundhausen, C.; Mentlein, R.; Schulte, A.; Berkhout, T.A.; Broadway, N.; Hartmann, D.; Sedlacek, R.; Dietrich, S.; Muetze, B.; et al. The transmembrane CXC-chemokine ligand 16 is induced by IFN-gamma and TNF-alpha and shed by the activity of the disintegrin-like metalloproteinase ADAM10. J. Immunol. 2004, 172, 6362–6372. [Google Scholar] [CrossRef] [PubMed]
- Fanelli, G.; Romano, M.; Nova-Lamperti, E.; Werner Sunderland, M.; Nerviani, A.; Scottà, C.; Bombardieri, M.; Quezada, S.A.; Sacks, S.H.; Noelle, R.J.; et al. PD-L1 signaling on human memory CD4+ T cells induces a regulatory phenotype. PLoS Biol. 2021, 19, e3001199. [Google Scholar] [CrossRef] [PubMed]
- Kazanova, A.; Rudd, C.E. Programmed cell death 1 ligand (PD-L1) on T cells generates Treg suppression from memory. PLoS Biol. 2021, 19, e3001272. [Google Scholar] [CrossRef] [PubMed]
- Diskin, B.; Adam, S.; Cassini, M.F.; Sanchez, G.; Liria, M.; Aykut, B.; Buttar, C.; Li, E.; Sundberg, B.; Salas, R.D.; et al. PD-L1 engagement on T cells promotes self-tolerance and suppression of neighboring macrophages and effector T cells in cancer. Nat. Immunol. 2020, 21, 442–454. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vrabel, M.R.; Schulman, J.A.; Gillam, F.B.; Mantooth, S.M.; Nguyen, K.G.; Zaharoff, D.A. Focal Cryo-Immunotherapy with Intratumoral IL-12 Prevents Recurrence of Large Murine Tumors. Cancers 2023, 15, 2210. https://doi.org/10.3390/cancers15082210
Vrabel MR, Schulman JA, Gillam FB, Mantooth SM, Nguyen KG, Zaharoff DA. Focal Cryo-Immunotherapy with Intratumoral IL-12 Prevents Recurrence of Large Murine Tumors. Cancers. 2023; 15(8):2210. https://doi.org/10.3390/cancers15082210
Chicago/Turabian StyleVrabel, Maura R., Jacob A. Schulman, Francis B. Gillam, Siena M. Mantooth, Khue G. Nguyen, and David A. Zaharoff. 2023. "Focal Cryo-Immunotherapy with Intratumoral IL-12 Prevents Recurrence of Large Murine Tumors" Cancers 15, no. 8: 2210. https://doi.org/10.3390/cancers15082210
APA StyleVrabel, M. R., Schulman, J. A., Gillam, F. B., Mantooth, S. M., Nguyen, K. G., & Zaharoff, D. A. (2023). Focal Cryo-Immunotherapy with Intratumoral IL-12 Prevents Recurrence of Large Murine Tumors. Cancers, 15(8), 2210. https://doi.org/10.3390/cancers15082210




