Epidemiology of Oral Cancer in Taiwan: A Population-Based Cancer Registry Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources
2.2. Statistical Analysis
3. Results
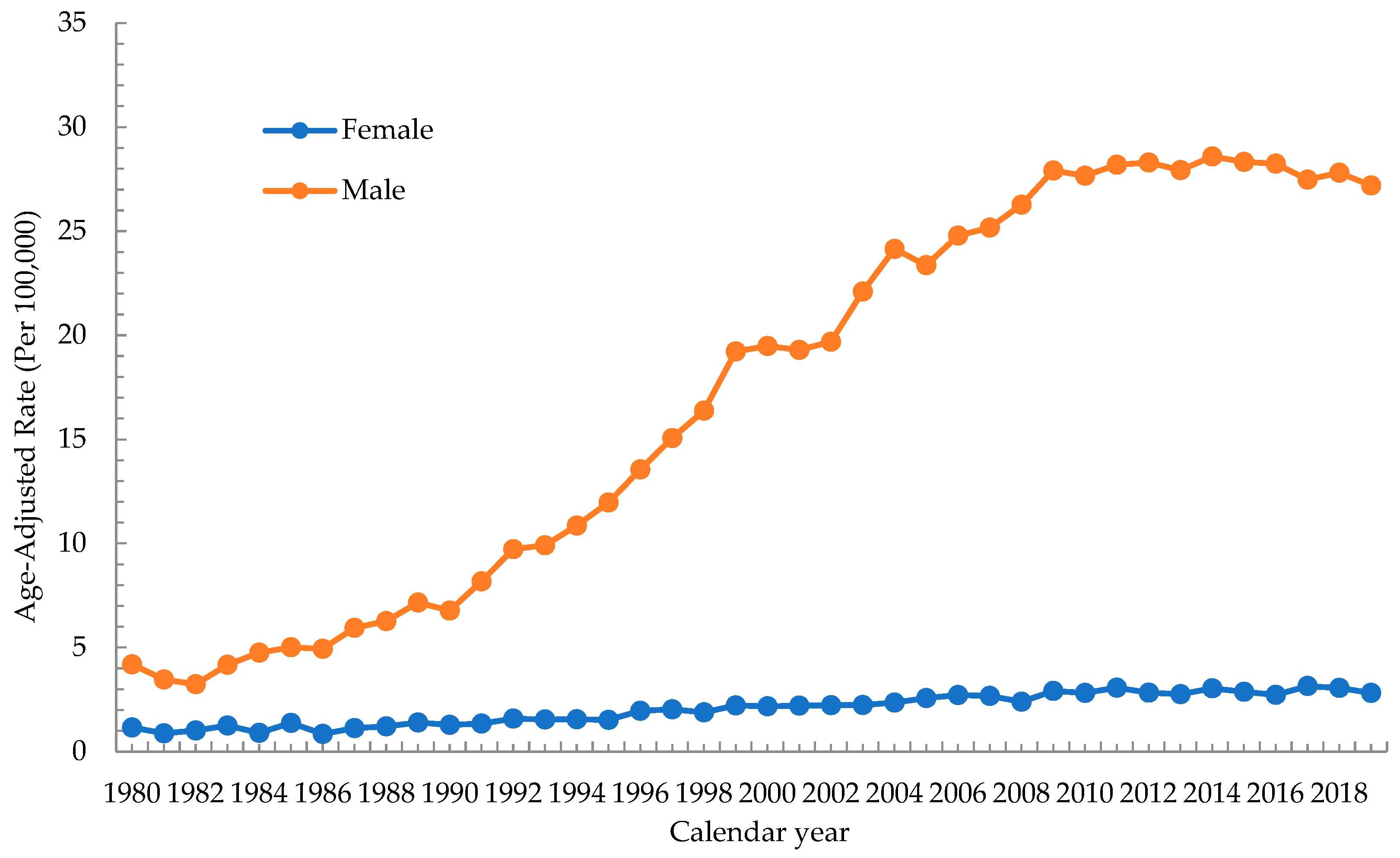
3.1. Trend of Incidence Rate of Oral Cancer
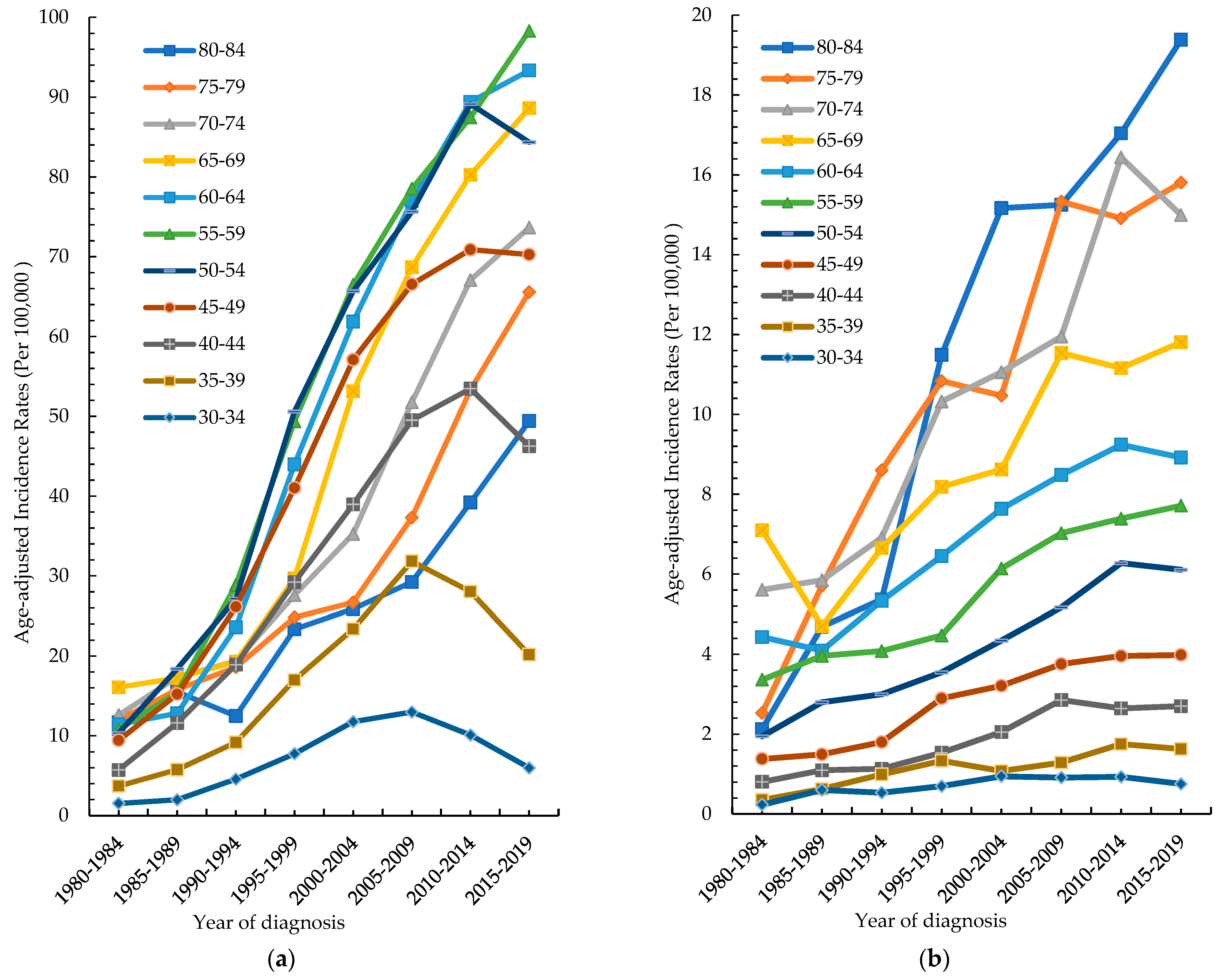
3.2. Age-Specific Incidence Rate by Different Periods
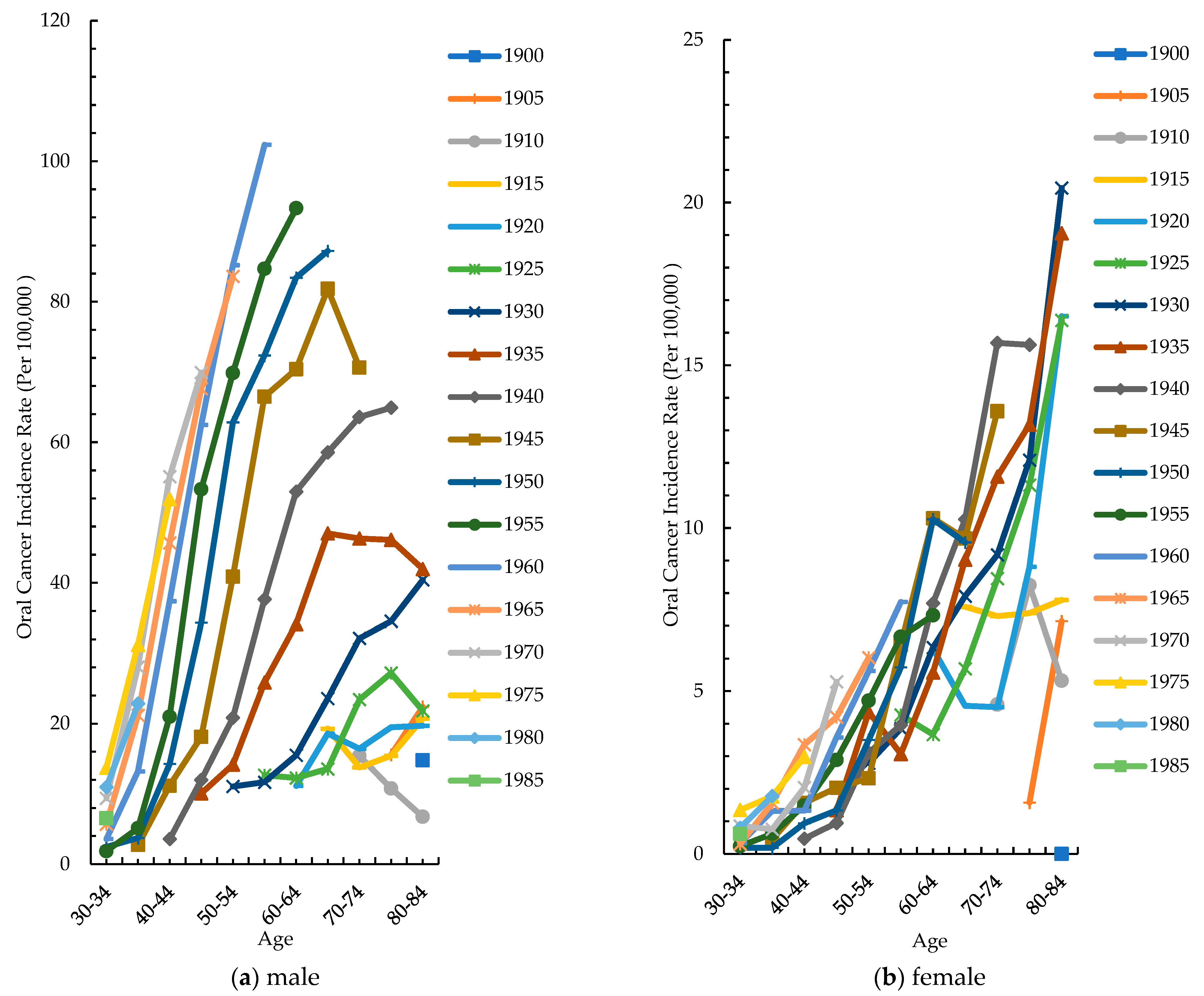
3.3. Cohort-Specific Incidence Rate by Different Age Groups
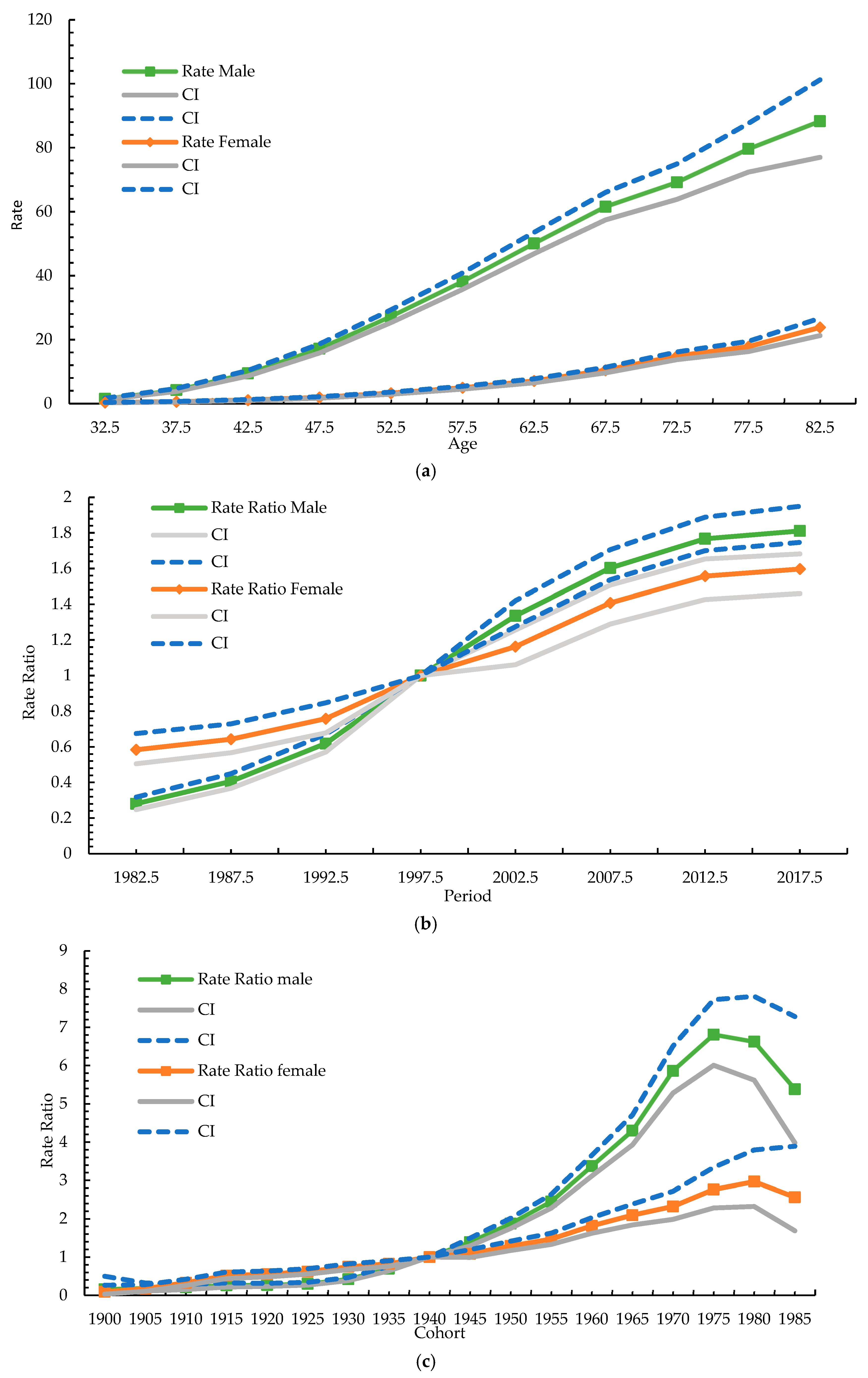
3.4. Age–Period–Cohort Model
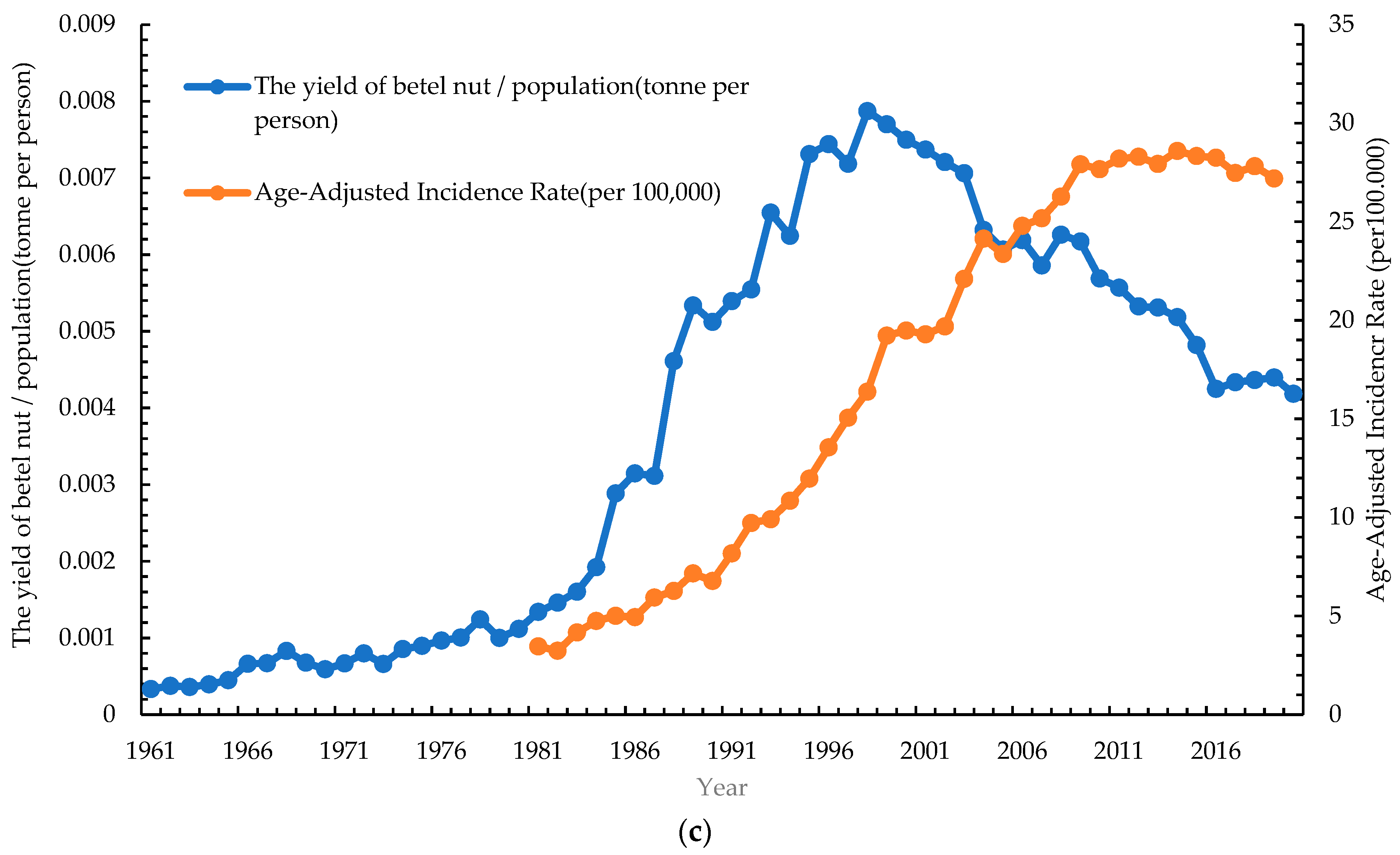
3.5. Correlation of Risk Factors with Age-Standardized Incidence Rate of Oral Cancer
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Rivera, C. Essentials of oral cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 11884–11894. [Google Scholar] [PubMed]
- Warnakulasuriya, S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009, 45, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Montero, P.H.; Patel, S.G. Cancer of the oral cavity. Surg. Oncol. Clin. N. Am. 2015, 24, 491–508. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.P.; Tsai, M.K.; Chung, W.S.; Hsu, H.L.; Chang, Y.C.; Chan, H.T.; Chiang, P.H.; Cheng, T.Y.; Tsai, S.P. Cancer risks from betel quid chewing beyond oral cancer: A multiple-site carcinogen when acting with smoking. Cancer Causes Control 2010, 21, 1427–1435. [Google Scholar] [CrossRef]
- Ren, Z.H.; Hu, C.Y.; He, H.R.; Li, Y.J.; Lyu, J. Global and regional burdens of oral cancer from 1990 to 2017: Results from the global burden of disease study. Cancer Commun. 2020, 40, 81–92. [Google Scholar] [CrossRef]
- Warnakulasuriya, S.; Kerr, A.R. Oral Cancer Screening: Past, Present, and Future. J. Dent. Res. 2021, 100, 1313–1320. [Google Scholar] [CrossRef]
- Chuang, S.L.; Su, W.W.; Chen, S.L.; Yen, A.M.; Wang, C.P.; Fann, J.C.; Chiu, S.Y.; Lee, Y.C.; Chiu, H.M.; Chang, D.C.; et al. Population-based screening program for reducing oral cancer mortality in 2,334,299 Taiwanese cigarette smokers and/or betel quid chewers. Cancer 2017, 123, 1597–1609. [Google Scholar] [CrossRef]
- Cohen, N.; Fedewa, S.; Chen, A.Y. Epidemiology and Demographics of the Head and Neck Cancer Population. Oral Maxillofac. Surg. Clin. N. Am. 2018, 30, 381–395. [Google Scholar] [CrossRef]
- Health Promotion Administration Taiwan Cancer Registry Database. Available online: https://cris.hpa.gov.tw/pagepub/Home.aspx (accessed on 11 November 2022).
- Chiang, C.J.; Wang, Y.W.; Lee, W.C. Taiwan’s Nationwide Cancer Registry System of 40 years: Past, present, and future. J. Formos. Med. Assoc. 2019, 118, 856–858. [Google Scholar] [CrossRef]
- Taiwan Tobacco and Liquor Corporation Taiwan Tobacco and Wine Statistical Yearbook. Available online: https://www.ttl.com.tw/ (accessed on 11 November 2022).
- Finance, M.O. Yearbook of Financial Statistics. Available online: https://www.mof.gov.tw/ (accessed on 11 November 2022).
- Council of Agriculture. Agricultural Statistics Yearbook. Available online: https://www.coa.gov.tw/ (accessed on 11 November 2022).
- Ahmad, O.B.; Boschi-Pinto, C.; Lopez, A.D.; Murray, C.J.; Lozano, R.; Inoue, M. Age standardization of rates: A new WHO standard. Geneva World Health Organ. 2001, 9, 1–14. [Google Scholar]
- Dhanuthai, K.; Rojanawatsirivej, S.; Thosaporn, W.; Kintarak, S.; Subarnbhesaj, A.; Darling, M.; Kryshtalskyj, E.; Chiang, C.P.; Shin, H.I.; Choi, S.Y.; et al. Oral cancer: A multicenter study. Med. Oral Patol. Oral Cir. Bucal 2018, 23, e23–e29. [Google Scholar] [CrossRef] [PubMed]
- Neville, B.W.; Day, T.A. Oral cancer and precancerous lesions. CA Cancer J. Clin. 2002, 52, 195–215. [Google Scholar] [CrossRef] [PubMed]
- Feller, L.L.; Khammissa, R.R.; Kramer, B.B.; Lemmer, J.J. Oral squamous cell carcinoma in relation to field precancerisation: Pathobiology. Cancer Cell Int. 2013, 13, 31. [Google Scholar] [CrossRef]
- Kumar, M.; Nanavati, R.; Modi, T.G.; Dobariya, C. Oral cancer: Etiology and risk factors: A review. J. Cancer Res. Ther. 2016, 12, 458–463. [Google Scholar] [CrossRef]
- Yang, C.H.; Lin, Y.D.; Yen, C.Y.; Chuang, L.Y.; Chang, H.W. A systematic gene-gene and gene-environment interaction analysis of DNA repair genes XRCC1, XRCC2, XRCC3, XRCC4, and oral cancer risk. Omics 2015, 19, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Dissanayaka, W.L.; Pitiyage, G.; Kumarasiri, P.V.; Liyanage, R.L.; Dias, K.D.; Tilakaratne, W.M. Clinical and histopathologic parameters in survival of oral squamous cell carcinoma. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 113, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Koontongkaew, S. The tumor microenvironment contribution to development, growth, invasion and metastasis of head and neck squamous cell carcinomas. J. Cancer 2013, 4, 66–83. [Google Scholar] [CrossRef] [PubMed]
- Gandini, S.; Botteri, E.; Iodice, S.; Boniol, M.; Lowenfels, A.B.; Maisonneuve, P.; Boyle, P. Tobacco smoking and cancer: A meta-analysis. Int. J. Cancer 2008, 122, 155–164. [Google Scholar] [CrossRef]
- Marron, M.; Boffetta, P.; Zhang, Z.F.; Zaridze, D.; Wünsch-Filho, V.; Winn, D.M.; Wei, Q.; Talamini, R.; Szeszenia-Dabrowska, N.; Sturgis, E.M.; et al. Cessation of alcohol drinking, tobacco smoking and the reversal of head and neck cancer risk. Int. J. Epidemiol. 2010, 39, 182–196. [Google Scholar] [CrossRef]
- Choudhari, S.K.; Chaudhary, M.; Gadbail, A.R.; Sharma, A.; Tekade, S. Oxidative and antioxidative mechanisms in oral cancer and precancer: A review. Oral Oncol. 2014, 50, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.P.; Tsai, S.P.; Chen, C.J.; Cheng, T.Y.; Tsai, M.C.; Levy, D.T. Smoking attributable mortality for Taiwan and its projection to 2020 under different smoking scenarios. Tob. Control 2005, 14, i76–i80. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.P.; Levy, D.T.; Cheng, T.Y.; Hsu, C.C.; Tsai, S.P. Smoking behaviour in Taiwan, 2001. Tob. Control 2005, 14, i51–i55. [Google Scholar] [CrossRef] [PubMed]
- Wu, I.; Sansgiry, S.; Peters, R.; Yang, M.; Abughosh, S. Cigarette smoking among Taiwanese adults. Epidemiology 2011, 1, 107. [Google Scholar]
- Tramacere, I.; Negri, E.; Bagnardi, V.; Garavello, W.; Rota, M.; Scotti, L.; Islami, F.; Corrao, G.; Boffetta, P.; La Vecchia, C. A meta-analysis of alcohol drinking and oral and pharyngeal cancers. Part 1: Overall results and dose-risk relation. Oral Oncol. 2010, 46, 497–503. [Google Scholar] [CrossRef]
- Reidy, J.; McHugh, E.; Stassen, L.F. A review of the relationship between alcohol and oral cancer. Surgeon 2011, 9, 278–283. [Google Scholar] [CrossRef]
- Seitz, H.K.; Stickel, F. Molecular mechanisms of alcohol-mediated carcinogenesis. Nat. Rev. Cancer 2007, 7, 599–612. [Google Scholar] [CrossRef]
- Wang, L.J.; Lin, C.L.; Chen, Y.C.; Lin, C.; Shyu, Y.C.; Chen, C.K. Sex Differences in the Relationship between Excessive Alcohol Consumption and Metabolic Abnormalities: A Community-Based Study in Taiwan. Nutrients 2022, 14, 2957. [Google Scholar] [CrossRef]
- Huang, Y.C.; Wu, S.C.; Hsiao, P.C.; Chen, L.Y.; Ting, T.T.; Chen, C.Y.; Kuan, C.C.; Tu, Y.K.; Huang, J.H.; Yen, C.F.; et al. Men’s decrease and women’s increase in harmful alcohol use from the 2014 to 2018 national surveys in Taiwan: A harbinger for an emerging national trend in East Asia? Int. J. Drug Policy 2022, 99, 103441. [Google Scholar] [CrossRef]
- Warnakulasuriya, S.; Trivedy, C.; Peters, T.J. Areca nut use: An independent risk factor for oral cancer. BMJ 2002, 324, 799–800. [Google Scholar] [CrossRef]
- Guha, N.; Warnakulasuriya, S.; Vlaanderen, J.; Straif, K. Betel quid chewing and the risk of oral and oropharyngeal cancers: A meta-analysis with implications for cancer control. Int. J. Cancer 2014, 135, 1433–1443. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.C.; Ho, Y.S.; Lee, P.H.; Chan, C.P.; Lee, J.J.; Hahn, L.J.; Wang, Y.J.; Jeng, J.H. Areca nut extract and arecoline induced the cell cycle arrest but not apoptosis of cultured oral KB epithelial cells: Association of glutathione, reactive oxygen species and mitochondrial membrane potential. Carcinogenesis 2001, 22, 1527–1535. [Google Scholar] [CrossRef]
- Tsai, Y.S.; Lee, K.W.; Huang, J.L.; Liu, Y.S.; Juo, S.H.; Kuo, W.R.; Chang, J.G.; Lin, C.S.; Jong, Y.J. Arecoline, a major alkaloid of areca nut, inhibits p53, represses DNA repair, and triggers DNA damage response in human epithelial cells. Toxicology 2008, 249, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.J.; Chang, M.L.; Chiang, C.P.; Hahn, L.J.; Hsieh, L.L.; Chen, C.J. Interaction of collagen-related genes and susceptibility to betel quid-induced oral submucous fibrosis. Cancer Epidemiol. Biomark. Prev. 2002, 11, 646–653. [Google Scholar]
- Chiba, I. Prevention of Betel Quid Chewers’ Oral Cancer in the Asian-Pacific Area. Asian Pac. J. Cancer Prev. 2001, 2, 263–269. [Google Scholar] [PubMed]
- Betel-quid and areca-nut chewing. IARC Monogr. Eval. Carcinog. Risk Chem. Hum. 1985, 37, 137–202.
- Gupta, B.; Johnson, N.W. Systematic review and meta-analysis of association of smokeless tobacco and of betel quid without tobacco with incidence of oral cancer in South Asia and the Pacific. PLoS ONE 2014, 9, e113385. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.S.; Jen, Y.M.; Wang, B.B.; Lee, J.C.; Kang, B.H. Epidemiology of oral cavity cancer in taiwan with emphasis on the role of betel nut chewing. ORL J. Oto-Rhino-Laryngol. Relat. Spec. 2005, 67, 230–236. [Google Scholar] [CrossRef]
- Hsu, W.-L.; Yu, K.; Chiang, C.-J.; Chen, T.-C.; Wang, C.-P. Head and Neck Cancer Incidence Trends in Taiwan, 1980~2014. Int. J. Head Neck Sci. 2017, 1, 180–189. [Google Scholar]
- Lee, Y.A.; Li, S.; Chen, Y.; Li, Q.; Chen, C.J.; Hsu, W.L.; Lou, P.J.; Zhu, C.; Pan, J.; Shen, H.; et al. Tobacco smoking, alcohol drinking, betel quid chewing, and the risk of head and neck cancer in an East Asian population. Head Neck 2019, 41, 92–102. [Google Scholar] [CrossRef]
- Su, S.Y. Evaluation of Nationwide Oral Mucosal Screening Program for Oral Cancer Mortality among Men in Taiwan. Int. J. Environ. Res. Public Health 2022, 19, 14329. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health and Welfare Tobacco Hazards Prevention Act. Available online: https://law.moj.gov.tw/ENG/LawClass/LawAll.aspx?pcode=L0070021 (accessed on 22 March 2022).
- Ministry of Finance Tobacco and Alcohol Tax Act. Available online: https://law.moj.gov.tw/ENG/LawClass/LawAll.aspx?pcode=G0330010 (accessed on 22 March 2022).
- Tham, J.; Sem, G.; Sit, E.; Tai, M.C. The ethics of betel nut consumption in Taiwan. J. Med. Ethics 2017, 43, 739–740. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.Y.; Ho, L.M.; Lee, J.M.; Hwang, J.Y. The possible impact of an alcohol welfare surcharge on consumption of alcoholic beverages in Taiwan. BMC Public Health 2013, 13, 810. [Google Scholar] [CrossRef]
- Huang, C.C.; Ou, C.Y.; Lee, W.T.; Hsiao, J.R.; Tsai, S.T.; Wang, J.D. Life expectancy and expected years of life lost to oral cancer in Taiwan: A nation-wide analysis of 22,024 cases followed for 10 years. Oral Oncol. 2015, 51, 349–354. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chou, C.-W.; Lin, C.-R.; Chung, Y.-T.; Tang, C.-S. Epidemiology of Oral Cancer in Taiwan: A Population-Based Cancer Registry Study. Cancers 2023, 15, 2175. https://doi.org/10.3390/cancers15072175
Chou C-W, Lin C-R, Chung Y-T, Tang C-S. Epidemiology of Oral Cancer in Taiwan: A Population-Based Cancer Registry Study. Cancers. 2023; 15(7):2175. https://doi.org/10.3390/cancers15072175
Chicago/Turabian StyleChou, Chao-Wei, Chun-Ru Lin, Yi-Ting Chung, and Chin-Sheng Tang. 2023. "Epidemiology of Oral Cancer in Taiwan: A Population-Based Cancer Registry Study" Cancers 15, no. 7: 2175. https://doi.org/10.3390/cancers15072175
APA StyleChou, C.-W., Lin, C.-R., Chung, Y.-T., & Tang, C.-S. (2023). Epidemiology of Oral Cancer in Taiwan: A Population-Based Cancer Registry Study. Cancers, 15(7), 2175. https://doi.org/10.3390/cancers15072175




