Increased Local Testosterone Levels Alter Human Fallopian Tube mRNA Profile and Signaling
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Human Fallopian Tissue
2.3. RNA Isolation, cDNA Synthesis and RT-PCR
2.4. Spheroid Formation
2.5. Wound Healing Assay
2.6. Boyden Chamber Invasion Assay
2.7. Dynamic Invasion in the PREDICT-MOS Microfluidic System
2.8. Ex Vivo Colonization Assay
2.9. Immunohistochemistry (IHC)
2.10. Statistical Analyses
3. Results
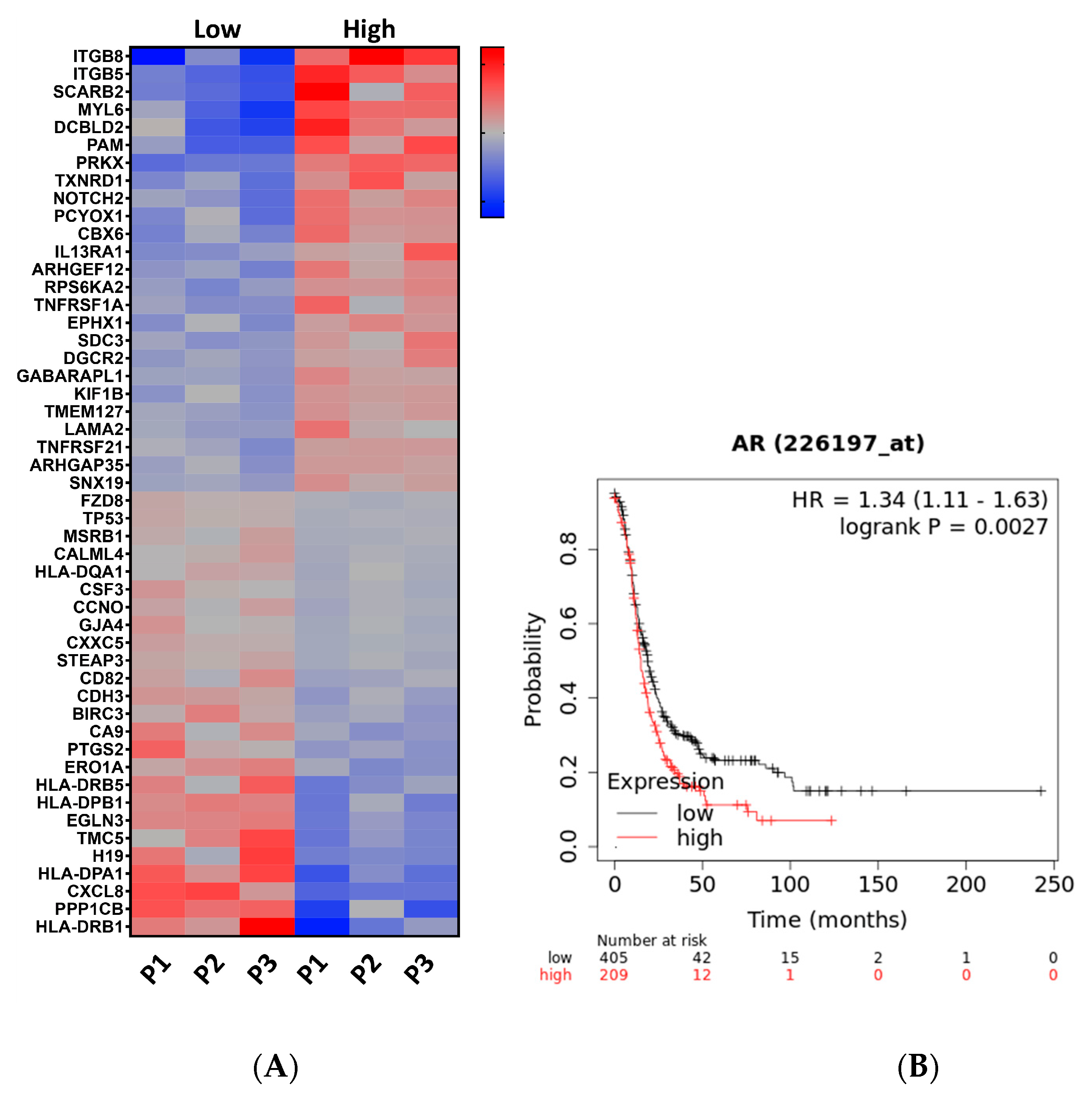
3.1. RNAseq Analysis Revealed That High Testosterone Regulates Genes Involved in Early Tumorigenesis
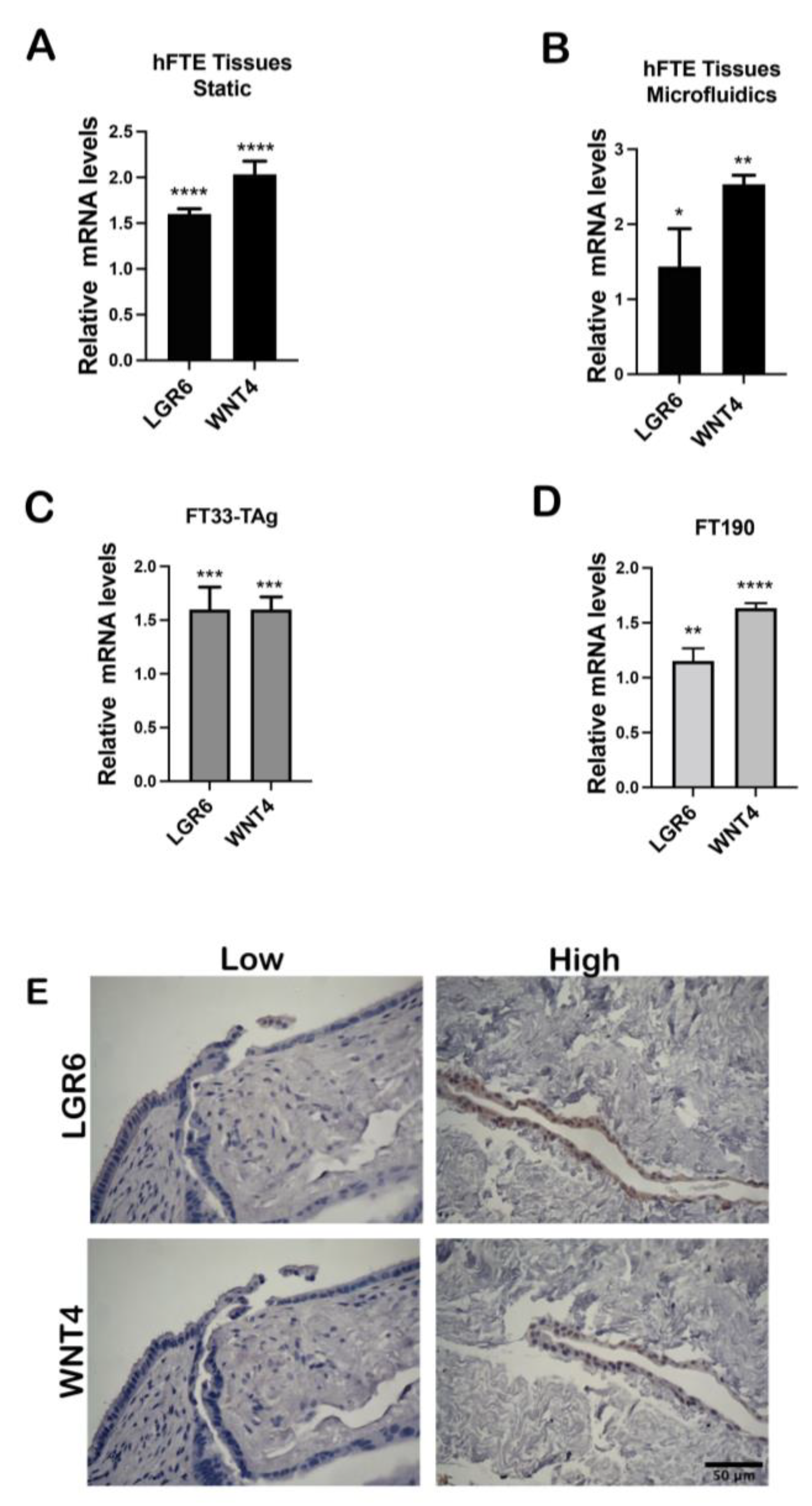
3.2. High Testosterone Induces mRNA Expression of Cancer Stem Cell Markers
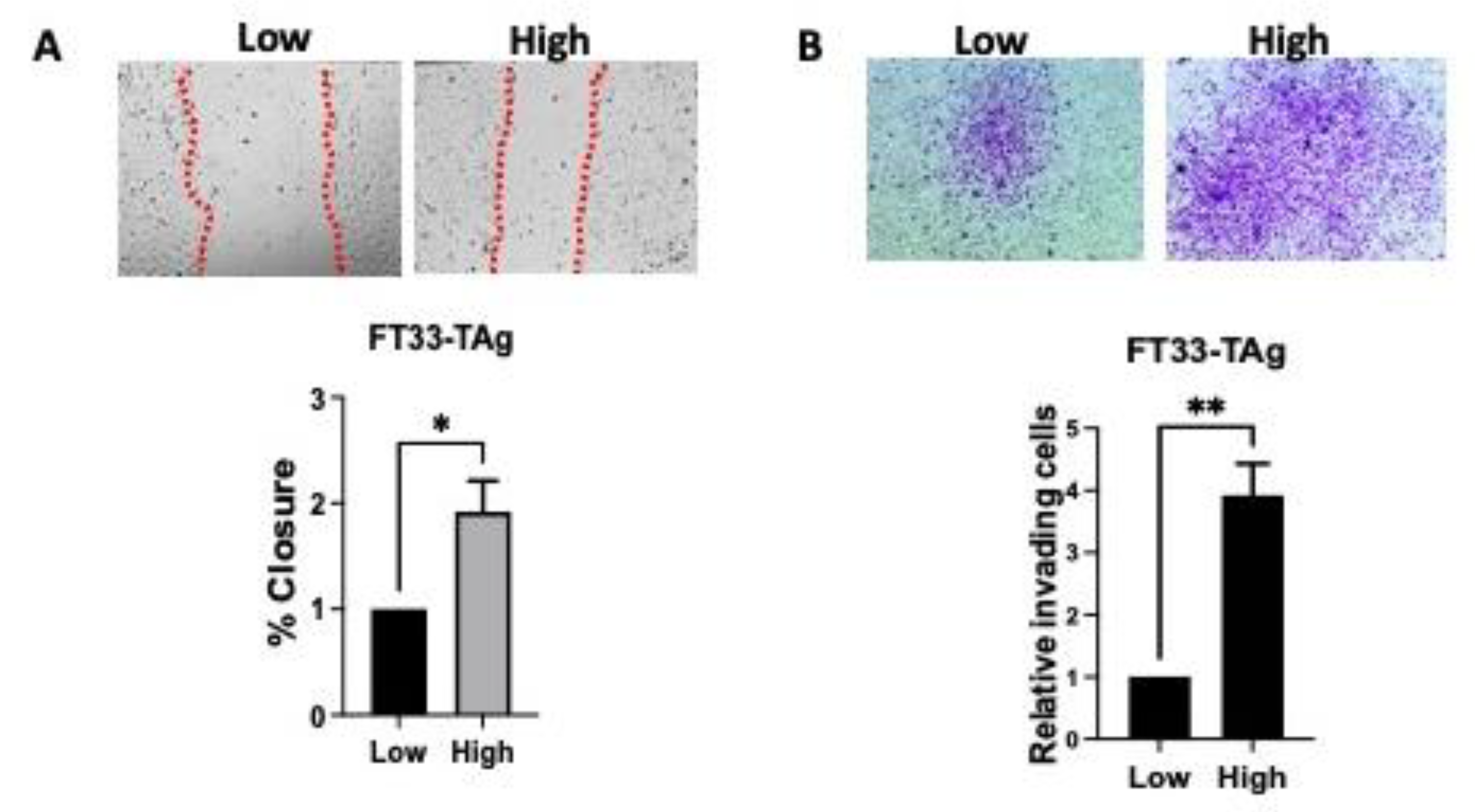
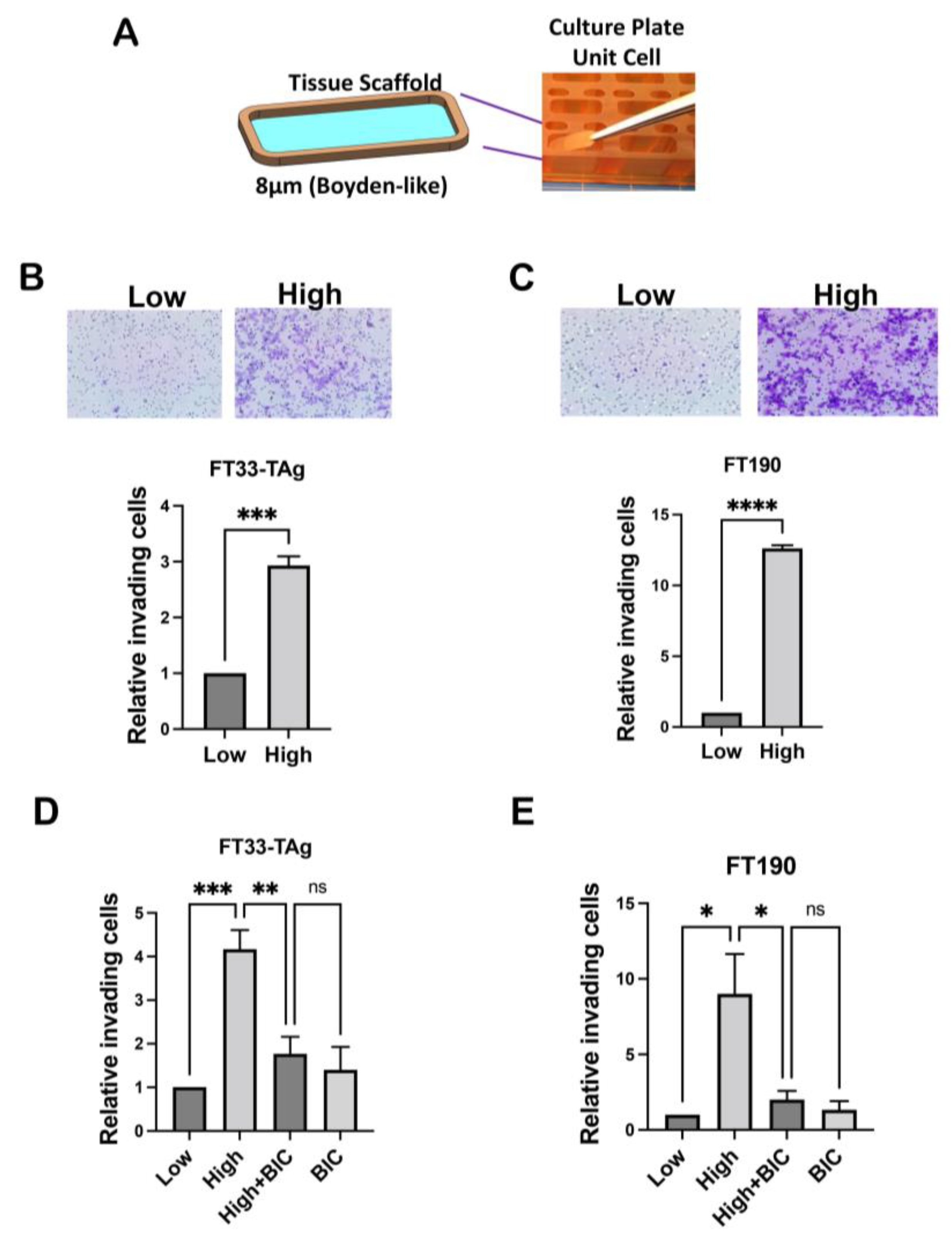
3.3. High Testosterone Increased the Migratory and Invasive Ability of Human Fallopian Tube Cells through AR in a Microfluidic Platform
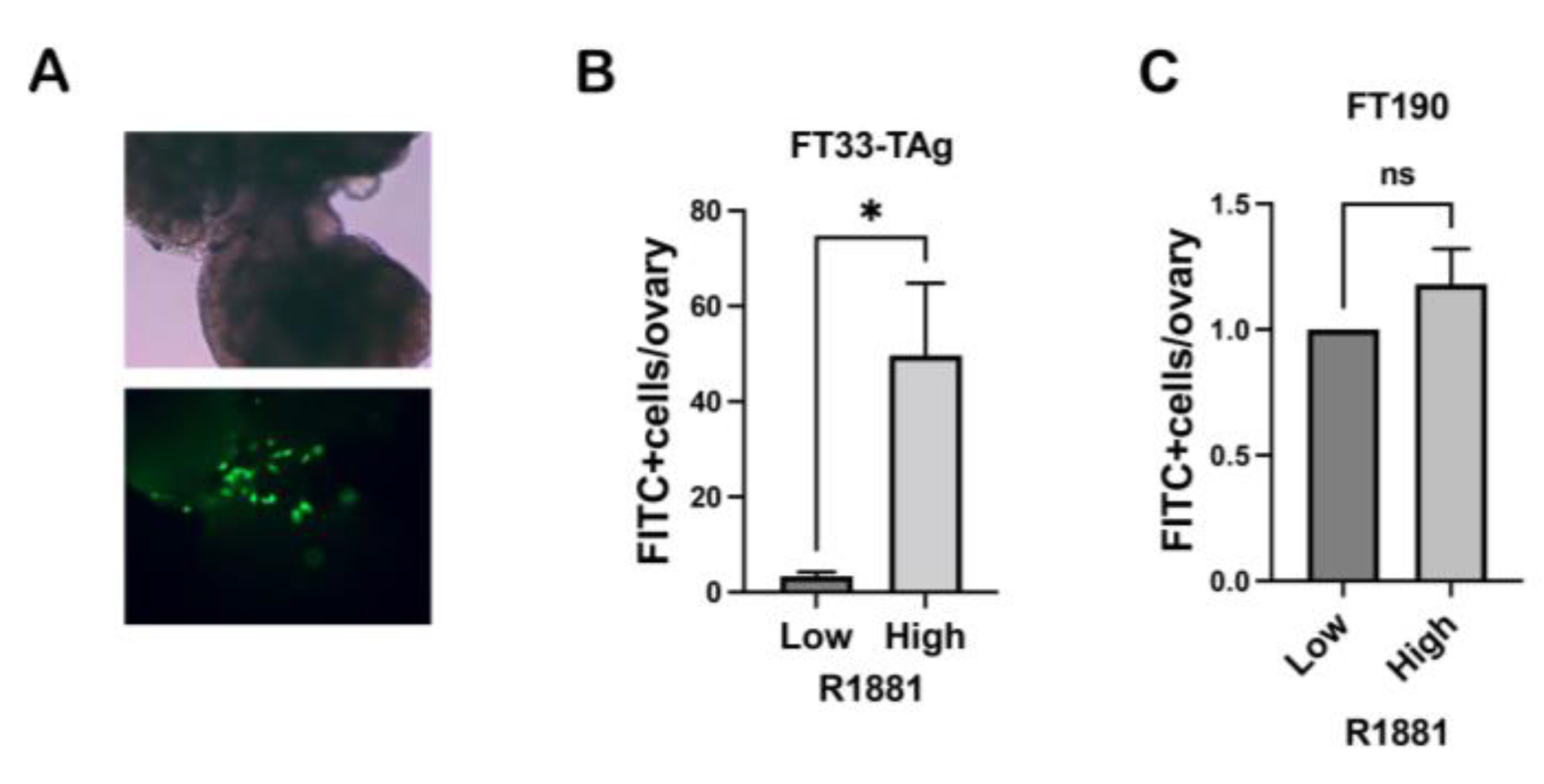
3.4. High Testosterone Increased the Ability of Human Fallopian Tube Cells to Attach to Ovarian Stroma
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ose, J.; Poole, E.M.; Schock, H.; Lehtinen, M.; Arslan, A.A.; Zeleniuch-Jacquotte, A.; Visvanathan, K.; Helzlsouer, K.; Buring, J.E.; Lee, I.M.; et al. Androgens Are Differentially Associated with Ovarian Cancer Subtypes in the Ovarian Cancer Cohort Consortium. Cancer Res. 2017, 77, 3951–3960. [Google Scholar] [CrossRef] [PubMed]
- Navaratnarajah, R.; Pillay, O.C.; Hardiman, P. Polycystic ovary syndrome and endometrial cancer. Semin. Reprod. Med. 2008, 26, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Jackson-Bey, T.; Colina, J.; Isenberg, B.C.; Coppeta, J.; Urbanek, M.; Kim, J.J.; Woodruff, T.K.; Burdette, J.E.; Russo, A. Exposure of human fallopian tube epithelium to elevated testosterone results in alteration of cilia gene expression and beating. Hum. Reprod. 2020, 35, 2086–2096. [Google Scholar] [CrossRef] [PubMed]
- Colina, J.A.; Zink, K.E.; Eliadis, K.; Salehi, R.; Gargus, E.S.; Wagner, S.R.; Moss, K.J.; Baligod, S.; Li, K.; Kirkpatrick, B.J.; et al. Fallopian Tube-Derived Tumor Cells Induce Testosterone Secretion from the Ovary, Increasing Epithelial Proliferation and Invasion. Cancers 2021, 13, 1925. [Google Scholar] [CrossRef]
- Erickson, B.K.; Conner, M.G.; Landen, C.N., Jr. The role of the fallopian tube in the origin of ovarian cancer. Am. J. Obstet. Gynecol. 2013, 209, 409–414. [Google Scholar] [CrossRef]
- Dulohery, K.; Trottmann, M.; Bour, S.; Liedl, B.; Alba-Alejandre, I.; Reese, S.; Hughes, B.; Stief, C.G.; Kölle, S. How do elevated levels of testosterone affect the function of the human fallopian tube and fertility?—New insights. Mol. Reprod. Dev. 2020, 87, 30–44. [Google Scholar] [CrossRef]
- Maclean, A.; Bunni, E.; Makrydima, S.; Withington, A.; Kamal, A.M.; Valentijn, A.J.; Hapangama, D.K. Fallopian tube epithelial cells express androgen receptor and have a distinct hormonal responsiveness when compared with endometrial epithelium. Hum. Reprod. 2020, 35, 2097–2106. [Google Scholar] [CrossRef]
- Gaspard, U.J.; Romus, M.A.; Gillain, D.; Duvivier, J.; Demey-Ponsart, E.; Franchimont, P. Plasma hormone levels in women receiving new oral contraceptives containing ethinyl estradiol plus levonorgestrel or desogestrel. Contraception 1983, 27, 577–590. [Google Scholar] [CrossRef]
- Murphy, A.; Cropp, C.S.; Smith, B.S.; Burkman, R.T.; Zacur, H.A. Effect of low-dose oral contraceptive on gonadotropins, androgens, and sex hormone binding globulin in nonhirsute women. Fertil. Steril. 1990, 53, 35–39. [Google Scholar] [CrossRef]
- Laughlin, G.A.; Barrett-Connor, E.; Kritz-Silverstein, D.; von Muhlen, D. Hysterectomy, oophorectomy, and endogenous sex hormone levels in older women: The Rancho Bernardo Study. J. Clin. Endocrinol. Metab. 2000, 85, 645–651. [Google Scholar]
- Danforth, K.N.; Eliassen, A.H.; Tworoger, S.S.; Missmer, S.A.; Barbieri, R.L.; Rosner, B.A.; Colditz, G.A.; Hankinson, S.E. The association of plasma androgen levels with breast, ovarian and endometrial cancer risk factors among postmenopausal women. Int. J. Cancer 2010, 126, 199–207. [Google Scholar] [CrossRef]
- Parchwani, D.; Dholariya, S.J.; Takodara, S.; Singh, R.; Sharma, V.K.; Saxena, A.; Patel, D.D.; Radadiya, M. Analysis of Prediagnostic Circulating Levels of Gonadotropins and Androgens with Risk of Epithelial Ovarian Cancer. J. Lab. Physicians 2022, 14, 47–56. [Google Scholar] [CrossRef]
- Mizushima, T.; Miyamoto, H. The Role of Androgen Receptor Signaling in Ovarian Cancer. Cells 2019, 8, 176. [Google Scholar] [CrossRef]
- Harris, H.R.; Babic, A.; Webb, P.M.; Nagle, C.M.; Jordan, S.J.; Risch, H.A.; Rossing, M.A.; Doherty, J.A.; Goodman, M.T.; Modugno, F.; et al. Polycystic Ovary Syndrome, Oligomenorrhea, and Risk of Ovarian Cancer Histotypes: Evidence from the Ovarian Cancer Association Consortium. Cancer Epidemiol. Biomark. Prev. 2017, 27, 174–182. [Google Scholar] [CrossRef]
- Nantermet, P.V.; Xu, J.; Yu, Y.; Hodor, P.; Holder, D.; Adamski, S.; Gentile, M.A.; Kimmel, D.B.; Harada, S.; Gerhold, D.; et al. Identification of genetic pathways activated by the androgen receptor during the induction of proliferation in the ventral prostate gland. J. Biol. Chem. 2004, 279, 1310–1322. [Google Scholar] [CrossRef] [PubMed]
- Pozzobon, A.; Schneider, L.; Brum, I.S. Androgen-modulated p21 and p53 gene expression in human non-transformed epithelial prostatic cells in primary cultures. Int. J. Mol. Med. 2012, 30, 967–973. [Google Scholar] [CrossRef]
- Rokhlin, O.W.; Taghiyev, A.F.; Guseva, N.V.; Glover, R.A.; Chumakov, P.M.; Kravchenko, J.E.; Cohen, M.B. Androgen regulates apoptosis induced by TNFR family ligands via multiple signaling pathways in LNCaP. Oncogene 2005, 24, 6773–6784. [Google Scholar] [CrossRef] [PubMed]
- Cronauer, M.V.; Schulz, W.A.; Burchardt, T.; Ackermann, R.; Burchardt, M. Inhibition of p53 function diminishes androgen receptor-mediated signaling in prostate cancer cell lines. Oncogene 2004, 23, 3541–3549. [Google Scholar] [CrossRef]
- Alimirah, F.; Panchanathan, R.; Chen, J.; Zhang, X.; Ho, S.M.; Choubey, D. Expression of androgen receptor is negatively regulated by p53. Neoplasia 2007, 9, 1152–1159. [Google Scholar] [CrossRef]
- Kobayashi, H.; Iwai, K.; Niiro, E.; Morioka, S.; Yamada, Y.; Ogawa, K.; Kawahara, N. The conceptual advances of carcinogenic sequence model in high-grade serous ovarian cancer. Biomed. Rep. 2017, 7, 209–213. [Google Scholar] [CrossRef]
- George, S.H.; Milea, A.; Sowamber, R.; Chehade, R.; Tone, A.; Shaw, P.A. Loss of LKB1 and p53 synergizes to alter fallopian tube epithelial phenotype and high-grade serous tumorigenesis. Oncogene 2016, 35, 59–68. [Google Scholar] [CrossRef]
- McConnell, A.M.; Yao, C.; Yeckes, A.R.; Wang, Y.; Selvaggio, A.S.; Tang, J.; Kirsch, D.G.; Stripp, B.R. p53 Regulates Progenitor Cell Quiescence and Differentiation in the Airway. Cell Rep. 2016, 17, 2173–2182. [Google Scholar] [CrossRef]
- Azizgolshani, H.; Coppeta, J.R.; Vedula, E.M.; Marr, E.E.; Cain, B.P.; Luu, R.J.; Lech, M.P.; Kann, S.H.; Mulhern, T.J.; Tandon, V.; et al. High-throughput organ-on-chip platform with integrated programmable fluid flow and real-time sensing for complex tissue models in drug development workflows. Lab Chip 2021, 21, 1454–1474. [Google Scholar] [CrossRef] [PubMed]
- Karst, A.M.; Drapkin, R. Primary culture and immortalization of human fallopian tube secretory epithelial cells. Nat. Protoc. 2012, 7, 1755–1764. [Google Scholar] [CrossRef] [PubMed]
- Karst, A.M.; Levanon, K.; Drapkin, R. Modeling high-grade serous ovarian carcinogenesis from the fallopian tube. Proc. Natl. Acad. Sci. USA 2011, 108, 7547–7552. [Google Scholar] [CrossRef]
- King, S.M.; Quartuccio, S.M.; Vanderhyden, B.C.; Burdette, J.E. Early transformative changes in normal ovarian surface epithelium induced by oxidative stress require Akt upregulation, DNA damage and epithelial-stromal interaction. Carcinogenesis 2013, 34, 1125–1133. [Google Scholar] [CrossRef] [PubMed]
- Haley, J.; Tomar, S.; Pulliam, N.; Xiong, S.; Perkins, S.M.; Karpf, A.R.; Mitra, S.; Nephew, K.P.; Mitra, A.K. Functional characterization of a panel of high-grade serous ovarian cancer cell lines as representative experimental models of the disease. Oncotarget 2016, 7, 32810–32820. [Google Scholar] [CrossRef] [PubMed]
- Quartuccio, S.M.; Karthikeyan, S.; Eddie, S.L.; Lantvit, D.D.; Modi, D.A.; Wei, J.J.; Burdette, J.E. Mutant p53 expression in fallopian tube epithelium drives cell migration. Int. J. Cancer 2015, 137, 1528–1538. [Google Scholar] [CrossRef]
- Russo, A.; Czarnecki, A.A.; Dean, M.; Modi, D.A.; Lantvit, D.D.; Hardy, L.; Baligod, S.; Davis, D.A.; Wei, J.J.; Burdette, J.E. PTEN loss in the fallopian tube induces hyperplasia and ovarian tumor formation. Oncogene 2018, 37, 1976–1990. [Google Scholar] [CrossRef]
- Dean, M.; Jin, V.; Russo, A.; Lantvit, D.D.; Burdette, J.E. Exposure of the extracellular matrix and colonization of the ovary in metastasis of fallopian-tube-derived cancer. Carcinogenesis 2019, 40, 41–51. [Google Scholar] [CrossRef]
- Eddie, S.L.; Quartuccio, S.M.; Moyle-Heyrman, G.; Lantvit, D.D.; Wei, J.J.; Vanderhyden, B.C.; Burdette, J.E. Tumorigenesis and peritoneal colonization from fallopian tube epithelium. Oncotarget 2015, 14, 94–106. [Google Scholar] [CrossRef] [PubMed]
- King, S.M.; Hilliard, T.S.; Wu, L.Y.; Jaffe, R.C.; Fazleabas, A.T.; Burdette, J.E. The impact of ovulation on fallopian tube epithelial cells: Evaluating three hypotheses connecting ovulation and serous ovarian cancer. Endocr. Relat. Cancer 2011, 18, 627–642. [Google Scholar] [CrossRef]
- Cermik, D.; Selam, B.; Taylor, H.S. Regulation of HOXA-10 expression by testosterone in vitro and in the endometrium of patients with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2003, 88, 238–243. [Google Scholar] [CrossRef]
- Braunstein, G.D.; Reitz, R.E.; Buch, A.; Schnell, D.; Caulfield, M.P. Testosterone reference ranges in normally cycling healthy premenopausal women. J. Sex. Med. 2011, 8, 2924–2934. [Google Scholar] [CrossRef]
- Waldstreicher, J.; Santoro, N.F.; Hall, J.E.; Filicori, M.; Crowley, W.F., Jr. Hyperfunction of the hypothalamic-pituitary axis in women with polycystic ovarian disease: Indirect evidence for partial gonadotroph desensitization. J. Clin. Endocrinol. Metab. 1988, 66, 165–172. [Google Scholar] [CrossRef]
- Modi, D.A.; Tagare, R.D.; Karthikeyan, S.; Russo, A.; Dean, M.; Davis, D.A.; Lantvit, D.D.; Burdette, J.E. PAX2 function, regulation and targeting in fallopian tube-derived high-grade serous ovarian cancer. Oncogene 2017, 36, 3015–3024. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, S.; Lantvit, D.D.; Chae, D.H.; Burdette, J.E. Cadherin-6 type 2, K-cadherin (CDH6) is regulated by mutant p53 in the fallopian tube but is not expressed in the ovarian surface. Oncotarget 2016, 7, 69871–69882. [Google Scholar] [CrossRef] [PubMed]
- Noh, K.H.; Kim, B.W.; Song, K.H.; Cho, H.; Lee, Y.H.; Kim, J.H.; Chung, J.Y.; Kim, J.H.; Hewitt, S.M.; Seong, S.Y.; et al. Nanog signaling in cancer promotes stem-like phenotype and immune evasion. J. Clin. Invest. 2012, 122, 4077–4093. [Google Scholar] [CrossRef] [PubMed]
- Ling, K.; Jiang, L.; Liang, S.; Kwong, J.; Yang, L.; Li, Y.; Ping, Y.; Deng, Q.; Liang, Z. Nanog interaction with the androgen receptor signaling axis induce ovarian cancer stem cell regulation: Studies based on the CRISPR/Cas9 system. J. Ovarian Res. 2018, 11, 36. [Google Scholar] [CrossRef]
- Ruan, X.; Liu, A.; Zhong, M.; Wei, J.; Zhang, W.; Rong, Y.; Liu, W.; Li, M.; Qing, X.; Chen, G.; et al. Silencing LGR6 Attenuates Stemness and Chemoresistance via Inhibiting Wnt/beta-Catenin Signaling in Ovarian Cancer. Mol. Ther. Oncolytics 2019, 14, 94–106. [Google Scholar] [CrossRef]
- Ng, A.; Tan, S.; Singh, G.; Rizk, P.; Swathi, Y.; Tan, T.Z.; Huang, R.Y.; Leushacke, M.; Barker, N. Lgr5 marks stem/progenitor cells in ovary and tubal epithelia. Nat. Cell Biol. 2014, 16, 745–757. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Yang, Z.; Heyrman, G.M.; Cain, B.P.; Lopez Carrero, A.; Isenberg, B.C.; Dean, M.J.; Coppeta, J.; Burdette, J.E. Versican secreted by the ovary links ovulation and migration in fallopian tube derived serous cancer. Cancer Lett. 2022, 543, 215779. [Google Scholar] [CrossRef]
- Fathalla, M.F. Incessant ovulation and ovarian cancer—A hypothesis re-visited. Facts. Views Vis. Obgyn. 2013, 5, 292–297. [Google Scholar] [PubMed]
- Shao, R.; Ljungstrom, K.; Weijdegard, B.; Egecioglu, E.; Fernandez-Rodriguez, J.; Zhang, F.P.; Thurin-Kjellberg, A.; Bergh, C.; Billig, H. Estrogen-induced upregulation of AR expression and enhancement of AR nuclear translocation in mouse fallopian tubes in vivo. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E604–E614. [Google Scholar] [CrossRef] [PubMed]
- Morelli, M.; Venturella, R.; Mocciaro, R.; Di Cello, A.; Rania, E.; Lico, D.; D’Alessandro, P.; Zullo, F. Prophylactic salpingectomy in premenopausal low-risk women for ovarian cancer: Primum non nocere. Gynecol. Oncol. 2013, 129, 448–451. [Google Scholar] [CrossRef]
- Russo, A.; Colina, J.A.; Moy, J.; Baligod, S.; Czarnecki, A.A.; Varughese, P.; Lantvit, D.D.; Dean, M.J.; Burdette, J.E. Silencing PTEN in the fallopian tube promotes enrichment of cancer stem cell-like function through loss of PAX2. Cell Death Dis. 2021, 12, 375. [Google Scholar] [CrossRef]
- Alwosaibai, K.; Abedini, A.; Al-Hujaily, E.M.; Tang, Y.; Garson, K.; Collins, O.; Vanderhyden, B.C. PAX2 maintains the differentiation of mouse oviductal epithelium and inhibits the transition to a stem cell-like state. Oncotarget 2017, 8, 76881–76897. [Google Scholar] [CrossRef]
- Shih, I.M.; Wang, Y.; Wang, T.L. The Origin of Ovarian Cancer Species and Precancerous Landscape. Am. J. Pathol. 2021, 191, 26–39. [Google Scholar] [CrossRef]
- Elattar, A.; Warburton, K.G.; Mukhopadhyay, A.; Freer, R.M.; Shaheen, F.; Cross, P.; Plummer, E.R.; Robson, C.N.; Edmondson, R.J. Androgen receptor expression is a biological marker for androgen sensitivity in high grade serous epithelial ovarian cancer. Gynecol. Oncol. 2012, 124, 142–147. [Google Scholar] [CrossRef]
- Levine, D.; Park, K.; Juretzka, M.; Esch, J.; Hensley, M.; Aghajanian, C.; Lewin, S.; Konner, J.; Derosa, F.; Spriggs, D.; et al. A phase II evaluation of goserelin and bicalutamide in patients with ovarian cancer in second or higher complete clinical disease remission. Cancer 2007, 110, 2448–2456. [Google Scholar] [CrossRef]
- Limaye, S.; Kumar, P.; Pragya, R.; Sambath, J.; Patil, D.; Srinivasan, A.; Apurva, S.; Srivastava, N.; Patil, S.; Patil, R.; et al. A case report of androgen receptor inhibitor therapy in recurrent high-grade serous ovarian cancer. Oncotarget 2020, 11, 4358–4363. [Google Scholar] [CrossRef] [PubMed]
- Ala-Fossi, S.L.; Maenpaa, J.; Aine, R.; Punnonen, R. Ovarian testosterone secretion during perimenopause. Maturitas 1998, 29, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Burdette, J.E. Isolation of Fallopian Tube Epithelium for Assessment of Cilia Beating Frequency (CBF). Methods Mol. Biol. 2022, 2424, 179–187. [Google Scholar]
- Xiao, S.; Coppeta, J.R.; Rogers, H.B.; Isenberg, B.C.; Zhu, J.; Olalekan, S.A.; McKinnon, K.E.; Dokic, D.; Rashedi, A.S.; Haisenleder, D.J.; et al. A microfluidic culture model of the human reproductive tract and 28-day menstrual cycle. Nat. Commun. 2017, 8, 14584. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russo, A.; Cain, B.P.; Jackson-Bey, T.; Lopez Carrero, A.; Miglo, J.; MacLaughlan, S.; Isenberg, B.C.; Coppeta, J.; Burdette, J.E. Increased Local Testosterone Levels Alter Human Fallopian Tube mRNA Profile and Signaling. Cancers 2023, 15, 2062. https://doi.org/10.3390/cancers15072062
Russo A, Cain BP, Jackson-Bey T, Lopez Carrero A, Miglo J, MacLaughlan S, Isenberg BC, Coppeta J, Burdette JE. Increased Local Testosterone Levels Alter Human Fallopian Tube mRNA Profile and Signaling. Cancers. 2023; 15(7):2062. https://doi.org/10.3390/cancers15072062
Chicago/Turabian StyleRusso, Angela, Brian P. Cain, Tia Jackson-Bey, Alfredo Lopez Carrero, Jane Miglo, Shannon MacLaughlan, Brett C. Isenberg, Jonathan Coppeta, and Joanna E. Burdette. 2023. "Increased Local Testosterone Levels Alter Human Fallopian Tube mRNA Profile and Signaling" Cancers 15, no. 7: 2062. https://doi.org/10.3390/cancers15072062
APA StyleRusso, A., Cain, B. P., Jackson-Bey, T., Lopez Carrero, A., Miglo, J., MacLaughlan, S., Isenberg, B. C., Coppeta, J., & Burdette, J. E. (2023). Increased Local Testosterone Levels Alter Human Fallopian Tube mRNA Profile and Signaling. Cancers, 15(7), 2062. https://doi.org/10.3390/cancers15072062





