Immune Checkpoint and EMT-Related Molecules in Circulating Tumor Cells (CTCs) from Triple Negative Breast Cancer Patients and Their Clinical Impact
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients’ Samples and Cytospins’ Preparation
2.2. Cell Cultures
2.3. Triple Immunofluorescence
2.4. Statistical Analysis
3. Results
3.1. Expression of Immune Checkpoints PD-L1 and CTLA-4 in the CTCs of TNBC Patients
3.2. Expression of Immune Checkpoints PD-L1 and CTLA-4 in the CTCs of Luminal BC Patients Compared to TNBC Patients
3.3. Expression of EMT-Related Molecules; GLU and VIM in the CTCs of TNBC Patients
3.4. PD-L1, CTLA-4, GLU, and VIM in the CTCs of BC Patients and Clinical Outcome
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA. Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Chantzara, E.; Xenidis, N.; Kallergi, G.; Georgoulias, V.; Kotsakis, A. Circulating Tumor Cells as Prognostic Biomarkers in Breast Cancer: Current Status and Future Prospects. Expert Rev. Mol. Diagn. 2021, 21, 1037–1048. [Google Scholar] [CrossRef] [PubMed]
- Mego, M.; Karaba, M.; Sedlackova, T.; Benca, J.; Repiska, G.; Krasnicanova, L.; Macuch, J.A.N.; Sieberova, G.; Jurisova, S.; Pindak, D.; et al. Circulating Tumor Cells and Breast Cancer-Specific Mutations in Primary Breast Cancer. Mol. Clin. Oncol. 2020, 12, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Stathopoulou, A.; Vlachonikolis, I.; Mavroudis, D.; Perraki, M.; Kouroussis, C.; Apostolaki, S.; Malamos, N.; Kakolyris, S.; Kotsakis, A.; Xenidis, N.; et al. Molecular Detection of Cytokeratin-19–Positive Cells in the Peripheral Blood of Patients With Operable Breast Cancer: Evaluation of Their Prognostic Significance. J. Clin. Oncol. 2002, 20, 3404–3412. [Google Scholar] [CrossRef] [PubMed]
- Chimonidou, M.; Kallergi, G.; Georgoulias, V.; Welch, D.R.; Lianidou, E.S. Breast Cancer Metastasis Suppressor-1 Promoter Methylation in Primary Breast Tumors and Corresponding Circulating Tumor Cells. Mol. Cancer Res. 2013, 11, 1248–1257. [Google Scholar] [CrossRef]
- Zong, Y.; Pegram, M.D. Research Advances and New Challenges in Overcoming Triple-Negative Breast Cancer. Cancer Drug Resist. 2021, 4, 517–542. [Google Scholar] [CrossRef] [PubMed]
- Burguin, A.; Diorio, C.; Durocher, F. Breast Cancer Treatments: Updates and New Challenges. J. Pers. Med. 2021, 11, 808. [Google Scholar] [CrossRef] [PubMed]
- Kallergi, G.; Aggouraki, D.; Zacharopoulou, N.; Stournaras, C.; Georgoulias, V.; Martin, S.S. Evaluation of α-Tubulin, Detyrosinated α-Tubulin, and Vimentin in CTCs: Identification of the Interaction between CTCs and Blood Cells through Cytoskeletal Elements. Breast Cancer Res. 2018, 20, 67. [Google Scholar] [CrossRef]
- Suhail, Y.; Cain, M.P.; Vanaja, K.; Kurywchak, P.A.; Levchenko, A.; Kalluri, R. Kshitiz Systems Biology of Cancer Metastasis. Cell Syst. 2019, 9, 109–127. [Google Scholar] [CrossRef]
- Pantazaka, E.; Vardas, V.; Roumeliotou, A.; Kakavogiannis, S.; Kallergi, G. Clinical Relevance of Mesenchymal- and Stem-associated Phenotypes in Circulating Tumor Cells Isolated from Lung Cancer Patients. Cancers 2021, 13, 2158. [Google Scholar] [CrossRef]
- Croker, A.K.; Allan, A.L. Inhibition of Aldehyde Dehydrogenase (ALDH) Activity Reduces Chemotherapy and Radiation Resistance of Stem-like ALDHhiCD44+ Human Breast Cancer Cells. Breast Cancer Res. Treat. 2012, 133, 75–87. [Google Scholar] [CrossRef]
- Liu, H.; Patel, M.R.; Prescher, J.A.; Patsialou, A.; Qian, D.; Lin, J.; Wen, S.; Chang, Y.-F.; Bachmann, M.H.; Shimono, Y.; et al. Cancer Stem Cells from Human Breast Tumors Are Involved in Spontaneous Metastases in Orthotopic Mouse Models. Proc. Natl. Acad. Sci. USA 2010, 107, 18115–18120. [Google Scholar] [CrossRef]
- Mani, S.A.; Guo, W.; Liao, M.-J.; Eaton, E.N.; Ayyanan, A.; Zhou, A.Y.; Brooks, M.; Reinhard, F.; Zhang, C.C.; Shipitsin, M.; et al. The Epithelial-Mesenchymal Transition Generates Cells with Properties of Stem Cells. Cell 2008, 133, 704–715. [Google Scholar] [CrossRef]
- Jelski, W.; Chrostek, L.; Szmitkowski, M.; Markiewicz, W. The Activity of Class I, II, III and IV Alcohol Dehydrogenase Isoenzymes and Aldehyde Dehydrogenase in Breast Cancer. Clin. Exp. Med. 2006, 6, 89–93. [Google Scholar] [CrossRef]
- Jelski, W.; Chrostek, L.; Markiewicz, W.; Szmitkowski, M. Activity of Alcohol Dehydrogenase (Adh) Isoenzymes and Aldehyde Dehydrogenase (ALDH) in the Sera of Patients with Breast Cancer. J. Clin. Lab. Anal. 2006, 20, 105–108. [Google Scholar] [CrossRef]
- Theodoropoulos, P.A.; Polioudaki, H.; Agelaki, S.; Kallergi, G.; Saridaki, Z.; Mavroudis, D.; Georgoulias, V. Circulating Tumor Cells with a Putative Stem Cell Phenotype in Peripheral Blood of Patients with Breast Cancer. Cancer Lett. 2010, 288, 99–106. [Google Scholar] [CrossRef]
- Kallergi, G.; Papadaki, M.A.; Politaki, E.; Mavroudis, D.; Georgoulias, V.; Agelaki, S. Epithelial to Mesenchymal Transition Markers Expressed in Circulating Tumour Cells of Early and Metastatic Breast Cancer Patients. Breast Cancer Res. 2011, 13, R59. [Google Scholar] [CrossRef]
- Yamashita, N.; Tokunaga, E.; Kitao, H.; Hisamatsu, Y.; Taketani, K.; Akiyoshi, S.; Okada, S.; Aishima, S.; Morita, M.; Maehara, Y. Vimentin as a Poor Prognostic Factor for Triple-Negative Breast Cancer. J. Cancer Res. Clin. Oncol. 2013, 139, 739–746. [Google Scholar] [CrossRef]
- Whipple, R.A.; Vitolo, M.I.; Boggs, A.E.; Charpentier, M.S.; Thompson, K.; Martin, S.S. Parthenolide and Costunolide Reduce Microtentacles and Tumor Cell Attachment by Selectively Targeting Detyrosinated Tubulin Independent from NF-ΚB Inhibition. Breast Cancer Res. 2013, 15, R83. [Google Scholar] [CrossRef]
- Whipple, R.A.; Cheung, A.M.; Martin, S.S. Detyrosinated Microtubule Protrusions in Suspended Mammary Epithelial Cells Promote Reattachment. Exp. Cell Res. 2007, 313, 1326–1336. [Google Scholar] [CrossRef]
- Wu, Q.; Jiang, L.; Li, S.C.; He, Q.J.; Yang, B.; Cao, J. Small Molecule Inhibitors Targeting the PD-1/PD-L1 Signaling Pathway. Acta Pharmacol. Sin. 2021, 42, 1–9. [Google Scholar] [CrossRef]
- Kallergi, G.; Vetsika, E.K.; Aggouraki, D.; Lagoudaki, E.; Koutsopoulos, A.; Koinis, F.; Katsarlinos, P.; Trypaki, M.; Messaritakis, I.; Stournaras, C.; et al. Evaluation of PD-L1/PD-1 on Circulating Tumor Cells in Patients with Advanced Non-Small Cell Lung Cancer. Ther. Adv. Med. Oncol. 2018, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sinoquet, L.; Jacot, W.; Gauthier, L.; Pouderoux, S.; Viala, M.; Cayrefourcq, L.; Quantin, X.; Alix-Panabières, C. Programmed Cell Death Ligand 1-Expressing Circulating Tumor Cells: A New Prognostic Biomarker in Non-Small Cell Lung Cancer. Clin. Chem. 2021, 67, 1503–1512. [Google Scholar] [CrossRef] [PubMed]
- Mazel, M.; Jacot, W.; Pantel, K.; Bartkowiak, K.; Topart, D.; Cayrefourcq, L.; Rossille, D.; Maudelonde, T.; Fest, T.; Alix-Panabières, C. Frequent Expression of PD-L1 on Circulating Breast Cancer Cells. Mol. Oncol. 2015, 9, 1773–1782. [Google Scholar] [CrossRef]
- Strati, A.; Koutsodontis, G.; Papaxoinis, G.; Angelidis, I.; Zavridou, M.; Economopoulou, P.; Kotsantis, I.; Avgeris, M.; Mazel, M.; Perisanidis, C.; et al. Prognostic Significance of PD-L1 Expression on Circulating Tumor Cells in Patients with Head and Neck Squamous Cell Carcinoma. Ann. Oncol. 2017, 28, 1923–1933. [Google Scholar] [CrossRef]
- Kulasinghe, A.; Perry, C.; Kenny, L.; Warkiani, M.E.; Nelson, C.; Punyadeera, C.; Kallergi, G.; Vetsika, E.K.; Aggouraki, D.; Lagoudaki, E.; et al. PD-L1 Expressing Circulating Tumour Cells in Head and Neck Cancers. Med. Hypotheses 2017, 28, 1923–1933. [Google Scholar] [CrossRef]
- Wang, X.; Sun, Q.; Liu, Q.; Wang, C.; Yao, R.; Wang, Y. CTC Immune Escape Mediated by PD-L1. Med. Hypotheses 2016, 93, 138–139. [Google Scholar] [CrossRef]
- Xu, Y.; Song, G.; Xie, S.; Jiang, W.; Chen, X.; Chu, M.; Hu, X.; Wang, Z.-W. The Roles of PD-1/PD-L1 in the Prognosis and Immunotherapy of Prostate Cancer. Mol. Ther. 2021, 29, 1958–1969. [Google Scholar] [CrossRef]
- Gu, L.; Chen, M.; Guo, D.; Zhu, H.; Zhang, W.; Pan, J.; Zhong, X.; Li, X.; Qian, H.; Wang, X. PD-L1 and Gastric Cancer Prognosis: A Systematic Review and Meta-Analysis. PLoS ONE 2017, 12, e0182692. [Google Scholar] [CrossRef]
- Rotte, A. Combination of CTLA-4 and PD-1 Blockers for Treatment of Cancer. J. Exp. Clin. Cancer Res. 2019, 38, 255. [Google Scholar] [CrossRef]
- Voena, C.; Chiarle, R. Advances in Cancer Immunology and Cancer Immunotherapy. Discov. Med. 2016, 21, 125–133. [Google Scholar]
- Kazandjian, D.; Suzman, D.L.; Blumenthal, G.; Mushti, S.; He, K.; Libeg, M.; Keegan, P.; Pazdur, R. FDA Approval Summary: Nivolumab for the Treatment of Metastatic Non-Small Cell Lung Cancer With Progression On or After Platinum-Based Chemotherapy. Oncologist 2016, 21, 634–642. [Google Scholar] [CrossRef]
- Dramatic Survival Benefit with Nivolumab in Melanoma. Cancer Discov. 2016, 6, OF7. [CrossRef]
- Yang, X.; Cheng, B.; Xiao, Y.; Xue, M.; Liu, T.; Cao, H.; Chen, J. Discovery of Novel CA-4 Analogs as Dual Inhibitors of Tubulin Polymerization and PD-1/PD-L1 Interaction for Cancer Treatment. Eur. J. Med. Chem. 2021, 213, 113058. [Google Scholar] [CrossRef]
- Kern, R.; Panis, C. CTLA-4 Expression and Its Clinical Significance in Breast Cancer. Arch. Immunol. Ther. Exp. 2021, 69, 16. [Google Scholar] [CrossRef]
- Agelaki, S.; Dragolia, M.; Markonanolaki, H.; Alkahtani, S.; Stournaras, C.; Georgoulias, V.; Kallergi, G. Phenotypic Characterization of Circulating Tumor Cells in Triple Negative Breast Cancer Patients. Oncotarget 2017, 8, 5309. [Google Scholar] [CrossRef]
- Sieuwerts, A.M.; Kraan, J.; Bolt, J.; Van Der Spoel, P.; Elstrodt, F.; Schutte, M.; Martens, J.W.M.; Gratama, J.W.; Sleijfer, S.; Foekens, J.A. Anti-Epithelial Cell Adhesion Molecule Antibodies and the Detection of Circulating Normal-like Breast Tumor Cells. J. Natl. Cancer Inst. 2009, 101, 61–66. [Google Scholar] [CrossRef]
- Kallergi, G.; Agelaki, S.; Kalykaki, A.; Stournaras, C.; Mavroudis, D.; Georgoulias, V. Phosphorylated EGFR and PI3K/Akt Signaling Kinases Are Expressed in Circulating Tumor Cells of Breast Cancer Patients. Breast Cancer Res. 2008, 10, R80. [Google Scholar] [CrossRef]
- Kallergi, G.; Mavroudis, D.; Georgoulias, V.; Stournaras, C. Phosphorylation of FAK, PI-3K, and Impaired Actin Organization in CK-Positive Micrometastatic Breast Cancer Cells. Mol. Med. 2007, 13, 79–88. [Google Scholar] [CrossRef]
- Kallergi, G.; Konstantinidis, G.; Markomanolaki, H.; Papadaki, M.A.; Mavroudis, D.; Stournaras, C.; Georgoulias, V.; Agelaki, S. Apoptotic Circulating Tumor Cells in Early and Metastatic Breast Cancer Patients. Mol. Cancer Ther. 2013, 12, 1886–1895. [Google Scholar] [CrossRef]
- Meng, S.; Tripathy, D.; Frenkel, E.P.; Shete, S.; Naftalis, E.Z.; Huth, J.F.; Beitsch, P.D.; Leitch, M.; Hoover, S.; Euhus, D.; et al. Circulating Tumor Cells in Patients with Breast Cancer Dormancy. Clin. Cancer Res. 2004, 10, 8152–8162. [Google Scholar] [CrossRef] [PubMed]
- Mialhe, A.; Lafanechère, L.; Treilleux, I.; Peloux, N.; Dumontet, C.; Brémond, A.; Panh, M.H.; Payan, R.; Wehland, J.; Margolis, R.L.; et al. Tubulin Detyrosination Is a Frequent Occurrence in Breast Cancers of Poor Prognosis. Cancer Res. 2001, 61, 5024–5027. [Google Scholar] [PubMed]
- Katsarou, S.D.; Messaritakis, I.; Voumvouraki, A.; Kakavogiannis, S.; Kotsakis, A.; Alkahtani, S.; Stournaras, C.; Martin, S.S.; Georgoulias, V.; Kallergi, G. Detyrosinated α-Tubulin, Vimentin and PD-L1 in Circulating Tumor Cells (CTCs) Isolated from Non-Small Cell Lung Cancer (NSCLC) Patients. J. Pers. Med. 2022, 12, 154. [Google Scholar] [CrossRef] [PubMed]
- Whipple, R.A.; Balzer, E.M.; Cho, E.H.; Matrone, M.A.; Yoon, J.R.; Martin, S.S. Vimentin Filaments Support Extension of Tubulin-Based Microtentacles in Detached Breast Tumor Cells. Cancer Res. 2008, 68, 5678–5688. [Google Scholar] [CrossRef]
- Peng, Z.; Su, P.; Yang, Y.; Yao, X.; Zhang, Y.; Jin, F.; Yang, B. Identification of CTLA-4 Associated with Tumor Microenvironment and Competing Interactions in Triple Negative Breast Cancer by Co-Expression Network Analysis. J. Cancer 2020, 11, 6365–6375. [Google Scholar] [CrossRef]
- Kwapisz, D. Pembrolizumab and Atezolizumab in Triple-Negative Breast Cancer. Cancer Immunol. Immunother. 2021, 70, 607–617. [Google Scholar] [CrossRef]
- Li, Z.; Dong, P.; Ren, M.; Song, Y.; Qian, X.; Yang, Y.; Li, S.; Zhang, X.; Liu, F. PD-L1 Expression Is Associated with Tumor FOXP3(+) Regulatory T-Cell Infiltration of Breast Cancer and Poor Prognosis of Patient. J. Cancer 2016, 7, 784–793. [Google Scholar] [CrossRef]
- Muenst, S.; Schaerli, A.R.; Gao, F.; Däster, S.; Trella, E.; Droeser, R.A.; Muraro, M.G.; Zajac, P.; Zanetti, R.; Gillanders, W.E.; et al. Expression of Programmed Death Ligand 1 (PD-L1) Is Associated with Poor Prognosis in Human Breast Cancer. Breast Cancer Res. Treat. 2014, 146, 15–24. [Google Scholar] [CrossRef]
- Qin, T.; Zeng, Y.; Qin, G.; Xu, F.; Lu, J.; Fang, W.; Xue, C.; Zhan, J.; Zhang, X.; Zheng, Q.; et al. High PD-L1 Expression Was Associated with Poor Prognosis in 870 Chinese Patients with Breast Cancer. Oncotarget 2015, 6, 33972–33981. [Google Scholar] [CrossRef]
- Jacot, W.; Mazel, M.; Mollevi, C.; Pouderoux, S.; D’Hondt, V.; Cayrefourcq, L.; Bourgier, C.; Boissiere-Michot, F.; Berrabah, F.; Lopez-Crapez, E.; et al. Clinical Correlations of Programmed Cell Death Ligand 1 Status in Liquid and Standard Biopsies in Breast Cancer. Clin. Chem. 2020, 66, 1093–1101. [Google Scholar] [CrossRef]
- Raghavendra, N.M.; Pingili, D.; Kadasi, S.; Mettu, A.; Prasad, S.V.U.M. Dual or Multi-Targeting Inhibitors: The next Generation Anticancer Agents. Eur. J. Med. Chem. 2018, 143, 1277–1300. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Dai, Z.; Wu, W.; Wang, Z.; Zhang, N.; Zhang, L.; Zeng, W.J.; Liu, Z.; Cheng, Q. Regulatory Mechanisms of Immune Checkpoints PD-L1 and CTLA-4 in Cancer. J. Exp. Clin. Cancer Res. 2021, 40, 184. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.S.; Ribas, A. The Evolution of Checkpoint Blockade as a Cancer Therapy: What’s Here, What’s Next? Curr. Opin. Immunol. 2015, 33, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Ichinohe, K.; Sugawara, A.; Kida, S.; Murase, S.; Zhang, J.; Yamada, O.; Hattori, T.; Oshima, Y.; Kikuchi, H. Development of Indole Alkaloid-Type Dual Immune Checkpoint Inhibitors Against CTLA-4 and PD-L1 Based on Diversity-Enhanced Extracts. Front. Chem. 2021, 9, 766107. [Google Scholar] [CrossRef] [PubMed]
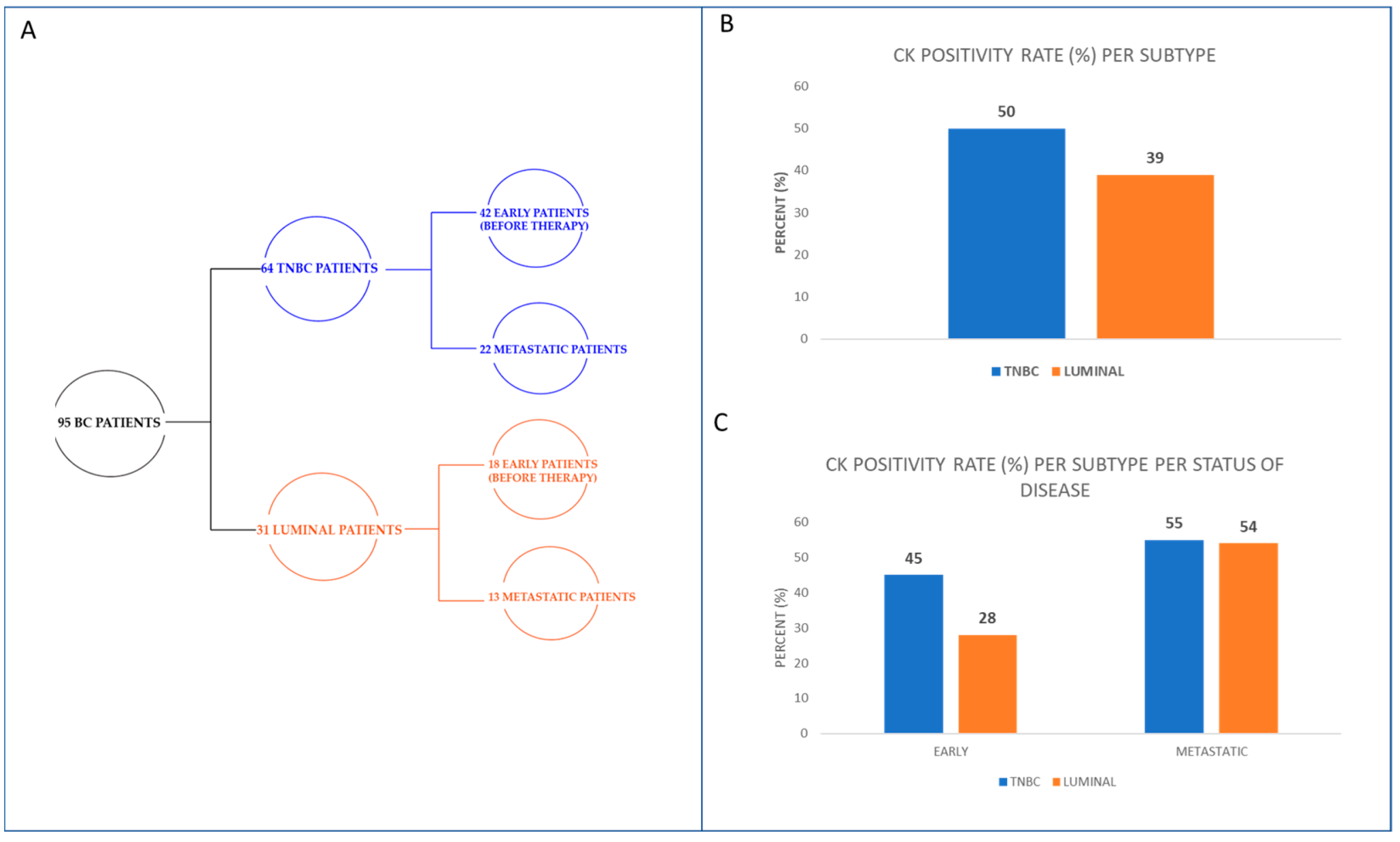


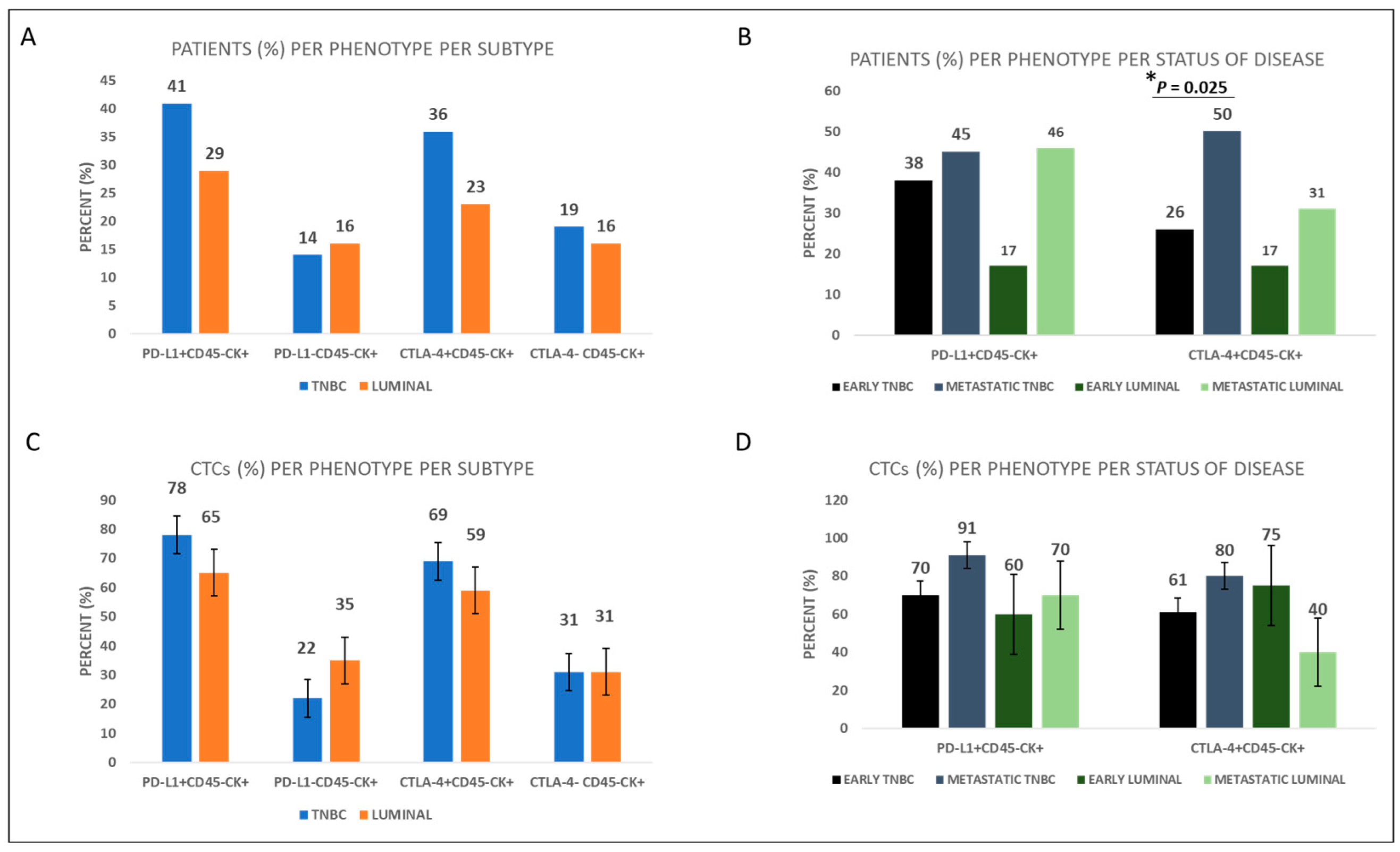

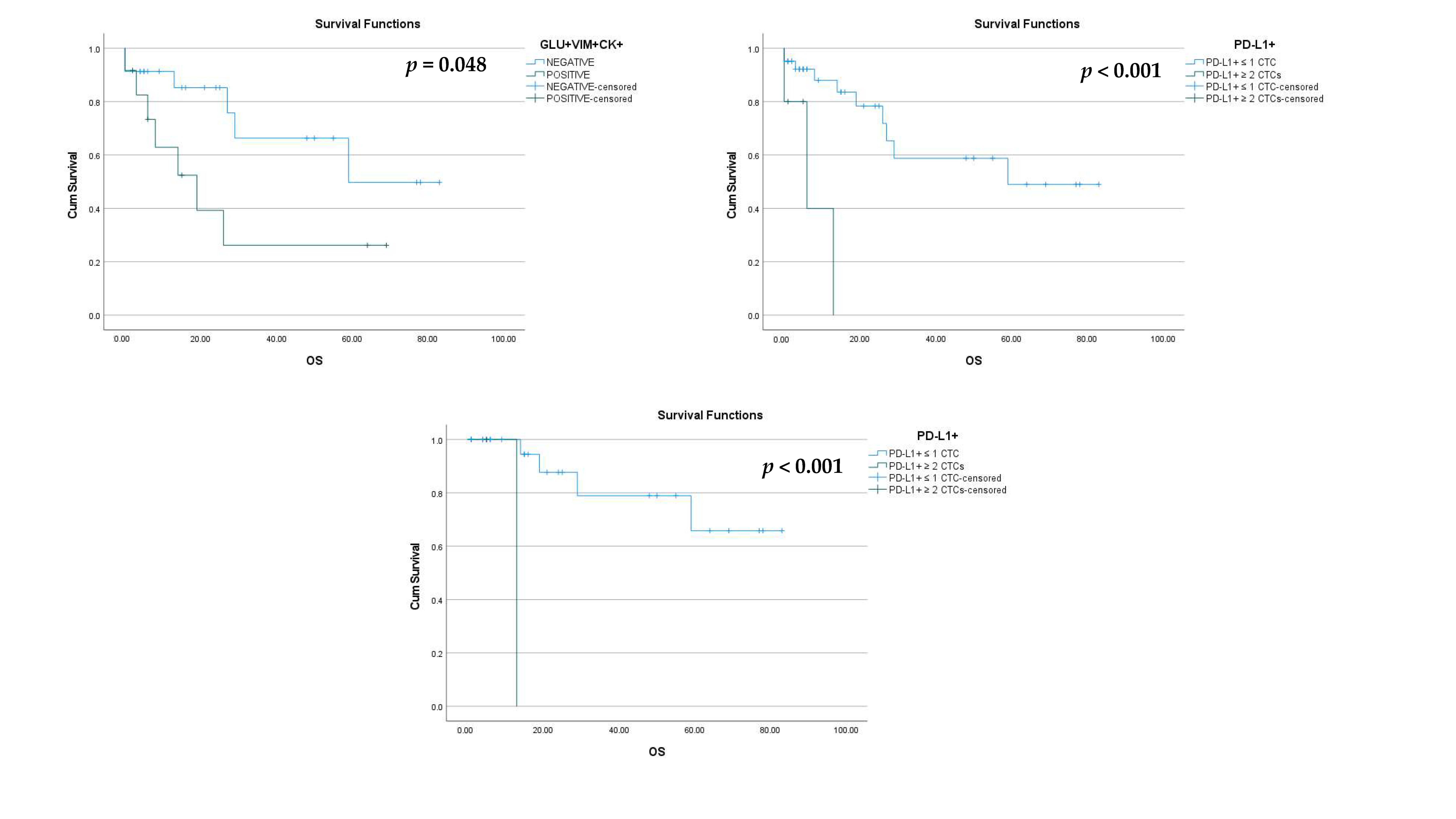
| Early Disease (42 TNBC Patients) | Early Disease (18 Luminal Patients) | Metastatic Disease (22 TNBC Patients) | Metastatic Disease (13 Luminal Patients) | ||||
|---|---|---|---|---|---|---|---|
| Age | Age | Age | Age | ||||
| Median, range | 58 (34–79) | Median, range | 44 (41–59) | Median, range | 58 (45–83) | Median, range | 67 (50–79) |
| Menopausal status | Menopausal status | Menopausal status | Menopausal status | ||||
| Premenopausal | 8 (19) | Premenopausal | 4 (22) | Premenopausal | 2 (9) | Premenopausal | 2 (15) |
| Postmenopausal | 18 (43) | Postmenopausal | 9 (50) | Postmenopausal | 9 (41) | Postmenopausal | 8 (62) |
| Unknown | 16 (38) | Unknown | 5 (28) | Unknown | 11 (50) | Unknown | 3 (23) |
| Tumor size | Tumor size | Disease sites | Disease sites | ||||
| pT1 | 6 (14) | pT1 | 3 (17) | 1 | 4 (18) | 1 | 2 (15) |
| pT2 | 12 (29) | pT2 | 3 (17) | 2 | 1 (4) | 2 | 0 (0) |
| pT3 | 1 (2) | pT3 | 2 (11) | 3 | 1 (5) | 3 | 0 (0) |
| Unknown | 23 (55) | Unknown | 10 (55) | ≥4 | 1 (5) | ≥4 | 0 (0) |
| Unknown | 15 (68) | Unknown | 11 (85) | ||||
| Histologic grade | Histologic grade | Primary breast cancer | Primary breast cancer | ||||
| Grade 1 | 2 (5) | Grade 1 | 0 (0) | Adjuvant | 2 (9) | Adjuvant | 5 (38) |
| Grade 2 | 5 (12) | Grade 2 | 3 (17) | Metastatic | 5 (23) | Metastatic | 1 (8) |
| Grade 3 | 12 (28) | Grade 3 | 4 (22) | Unknown | 15 (68) | Unknown | 7 (54) |
| Unknown | 23 (55) | Unknown | 11 (61) | ||||
| ER/PR tumor status | ER/PR tumor status | ER/PR tumor status | ER/PR tumor status | ||||
| Positive | 0 (0) | Positive | 8 (44) | Positive | 0 (0) | Positive | 5 (38) |
| Negative | 42 (100) | Negative | 0 (0) | Negative | 22 (100) | Negative | 0 (0) |
| Unknown | 0 (0) | Unknown | 10 (56) | Unknown | 0 (0) | Unknown | 8 (62) |
| HER2 tumor status | HER2 tumor status | HER2 tumor status | HER2 tumor status | ||||
| Positive | 0 (0) | Positive | 0 (0) | Positive | 0 (0) | Positive | 0 (0) |
| Negative | 42 (100) | Negative | 7 (39) | Negative | 22 (100) | Negative | 5 (38) |
| Unknown | 0 (0) | Unknown | 11 (61) | Unknown | 0 (0) | Unknown | 8 (62) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vardas, V.; Tolios, A.; Christopoulou, A.; Georgoulias, V.; Xagara, A.; Koinis, F.; Kotsakis, A.; Kallergi, G. Immune Checkpoint and EMT-Related Molecules in Circulating Tumor Cells (CTCs) from Triple Negative Breast Cancer Patients and Their Clinical Impact. Cancers 2023, 15, 1974. https://doi.org/10.3390/cancers15071974
Vardas V, Tolios A, Christopoulou A, Georgoulias V, Xagara A, Koinis F, Kotsakis A, Kallergi G. Immune Checkpoint and EMT-Related Molecules in Circulating Tumor Cells (CTCs) from Triple Negative Breast Cancer Patients and Their Clinical Impact. Cancers. 2023; 15(7):1974. https://doi.org/10.3390/cancers15071974
Chicago/Turabian StyleVardas, Vasileios, Anastasios Tolios, Athina Christopoulou, Vassilis Georgoulias, Anastasia Xagara, Filippos Koinis, Athanasios Kotsakis, and Galatea Kallergi. 2023. "Immune Checkpoint and EMT-Related Molecules in Circulating Tumor Cells (CTCs) from Triple Negative Breast Cancer Patients and Their Clinical Impact" Cancers 15, no. 7: 1974. https://doi.org/10.3390/cancers15071974
APA StyleVardas, V., Tolios, A., Christopoulou, A., Georgoulias, V., Xagara, A., Koinis, F., Kotsakis, A., & Kallergi, G. (2023). Immune Checkpoint and EMT-Related Molecules in Circulating Tumor Cells (CTCs) from Triple Negative Breast Cancer Patients and Their Clinical Impact. Cancers, 15(7), 1974. https://doi.org/10.3390/cancers15071974








