Novel Immunotherapeutic Approaches to Treating HPV-Related Head and Neck Cancer
Abstract
Simple Summary
Abstract
1. Introduction
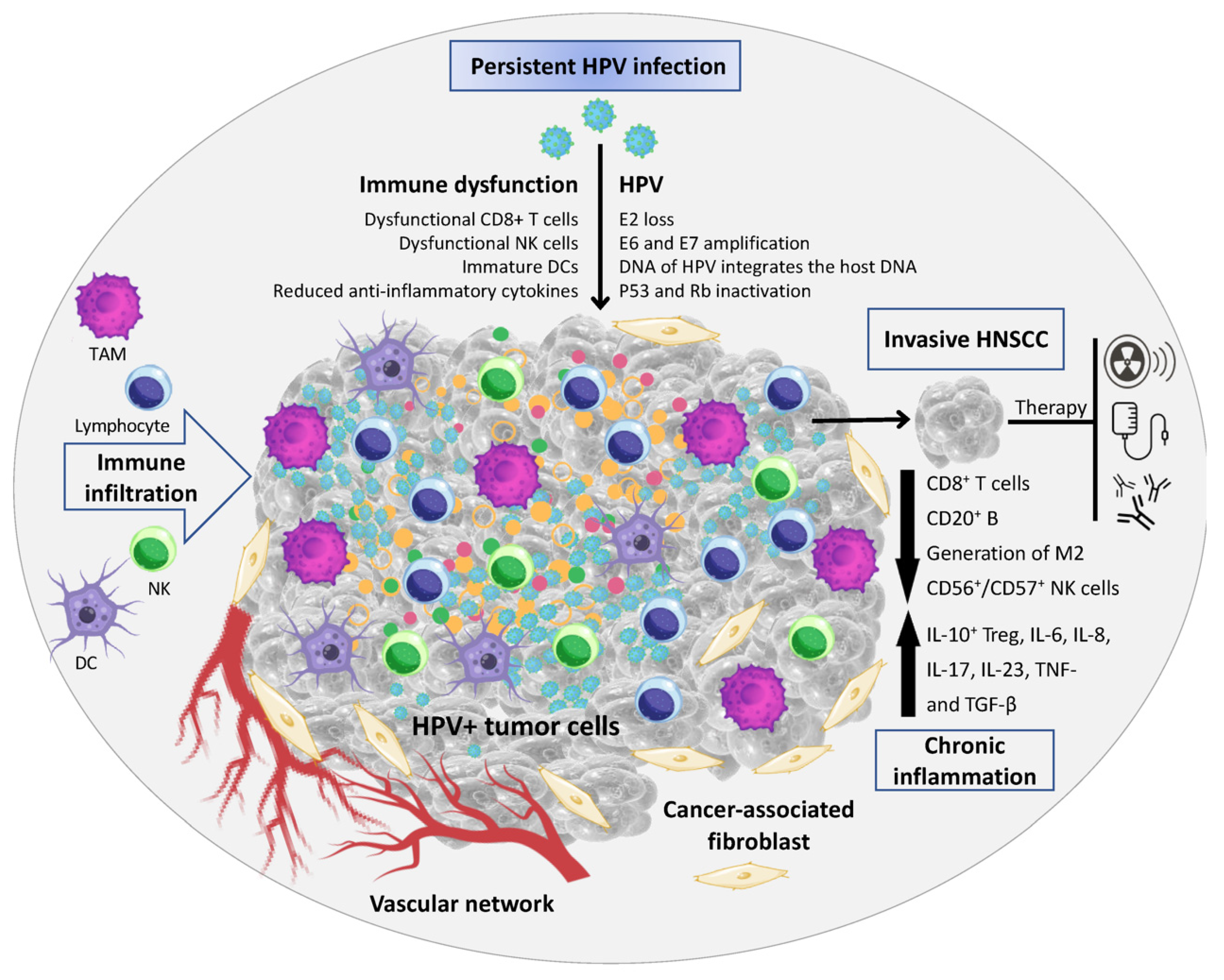
2. The Influence of HPV Status on the HNC Immune Microenvironment
3. Immunotherapy
3.1. Immune Checkpoint Inhibitors
3.2. T-Cell Associated Therapeutics
3.3. Immuno-STATs
3.4. B-Cell Associated Therapeutics
4. Therapeutic Vaccination for Personalized Cancer Treatment
5. Primary Prevention through Pre-Exposure Vaccination
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mody, M.D.; Rocco, J.W.; Yom, S.S.; Haddad, R.I.; Saba, N.F. Head and neck cancer. Lancet 2021, 398, 2289–2299. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, A.K.; Engels, E.A.; Pfeiffer, R.M.; Hernandez, B.Y.; Xiao, W.; Kim, E.; Jiang, B.; Goodman, M.T.; Sibug-Saber, M.; Cozen, W.; et al. Human Papillomavirus and Rising Oropharyngeal Cancer Incidence in the United States. J. Clin. Oncol. 2011, 29, 4294–4301. [Google Scholar] [CrossRef] [PubMed]
- Pike, L.R.; Royce, T.J.; Mahal, A.R.; Kim, D.W.; Hwang, W.L.; Mahal, B.A.; Sanford, N.N. Outcomes of HPV-Associated Squamous Cell Carcinoma of the Head and Neck: Impact of Race and Socioeconomic Status. J. Natl. Compr. Cancer Netw. 2020, 18, 177–184. [Google Scholar] [CrossRef]
- Carlander, A.F.; Jakobsen, K.K.; Bendtsen, S.K.; Garset-Zamani, M.; Lynggaard, C.D.; Jensen, J.S.; Grønhøj, C.; von Buchwald, C. A Contemporary Systematic Review on Repartition of HPV-Positivity in Oropharyngeal Cancer Worldwide. Viruses 2021, 13, 1326. [Google Scholar] [CrossRef]
- Doorbar, J.; Quint, W.; Banks, L.; Bravo, I.G.; Stoler, M.; Broker, T.R.; Stanley, M.A. The Biology and Life-Cycle of Human Papillomaviruses. Vaccine 2012, 30 (Suppl. 5), F55–F70. [Google Scholar] [CrossRef]
- Pytynia, K.B.; Dahlstrom, K.R.; Sturgis, E.M. Epidemiology of HPV-associated oropharyngeal cancer. Oral Oncol. 2014, 50, 380–386. [Google Scholar] [CrossRef]
- Rahimi, S. HPV-related squamous cell carcinoma of oropharynx: A review. J. Clin. Pathol. 2020, 73, 624–629. [Google Scholar] [CrossRef]
- Chaturvedi, A.K.; Engels, E.A.; Anderson, W.F.; Gillison, M.L. Incidence Trends for Human Papillomavirus–Related and –Unrelated Oral Squamous Cell Carcinomas in the United States. J. Clin. Oncol. 2008, 26, 612–619. [Google Scholar] [CrossRef]
- Mehta, V.; Yu, G.P.; Schantz, S.P. Population-based analysis of oral and oropharyngeal carcinoma: Changing trends of histopath-ologic differentiation, survival and patient demographics: Population-Based Analysis of Oral and Oropharyngeal Carcinoma. Laryngoscope 2010, 120, 2203–2212. [Google Scholar] [CrossRef]
- Zumsteg, Z.S.; Cook-Wiens, G.; Yoshida, E.; Shiao, S.L.; Lee, N.Y.; Mita, A. Incidence of Oropharyngeal Cancer Among Elderly Patients in the United States. JAMA Oncol. 2016, 2, 1617. [Google Scholar] [CrossRef]
- Marur, S.; Forastiere, A.A. Head and Neck Cancer: Changing Epidemiology, Diagnosis, and Treatment. Mayo Clin. Proc. 2008, 83, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Gillison, M.L.; Koch, W.M.; Capone, R.B.; Spafford, M.; Westra, W.H.; Wu, L.; Zahurak, M.L.; Daniel, R.W.; Viglione, M.; Symer, D.E.; et al. Evidence for a Causal Association Between Human Papillomavirus and a Subset of Head and Neck Cancers. J. Natl. Cancer Inst. 2000, 92, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Ang, K.K.; Harris, J.; Wheeler, R.; Weber, R.; Rosenthal, D.I.; Nguyen-Tân, P.F.; Westra, W.H.; Chung, C.H.; Jordan, R.C.; Lu, C.; et al. Human Papillomavirus and Survival of Patients with Oropharyngeal Cancer. N. Engl. J. Med. 2010, 363, 24–35. [Google Scholar] [CrossRef]
- Nguyen-Tan, P.F.; Zhang, Q.; Ang, K.K.; Weber, R.S.; Rosenthal, D.; Soulieres, D.; Kim, H.; Silverman, C.; Raben, A.; Galloway, T.J.; et al. Randomized Phase III Trial to Test Accelerated Versus Standard Fractionation in Combination with Concurrent Cisplatin for Head and Neck Carcinomas in the Radiation Therapy Oncology Group 0129 Trial: Long-Term Report of Efficacy and Toxicity. J. Clin. Oncol. 2014, 32, 3858–3867. [Google Scholar] [CrossRef]
- Kass, J.I.; Giraldez, L.; Gooding, W.; Choby, G.; Kim, S.; Miles, B. Oncologic outcomes of surgically treated early-stage oropha-ryngeal squamous cell carcinoma. Head Neck 2016, 38, 1467–1471. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Cipolla, M.J.; Old, M.O.; Brown, N.V.; Kang, S.Y.; Dziegielewski, P.T.; Durmus, K.; Ozer, E.; Agrawal, A.; Carrau, R.L.; et al. Surgical management of oropharyngeal squamous cell carcinoma: Survival and functional outcomes. Head Neck 2016, 38, E1794–E1802. [Google Scholar] [CrossRef] [PubMed]
- Ferris, R.L.; Flamand, Y.; Weinstein, G.S.; Li, S.; Quon, H.; Mehra, R.; Garcia, J.J.; Chung, C.H.; Gillison, M.L.; Duvvuri, U.; et al. Phase II Randomized Trial of Transoral Surgery and Low-Dose Intensity Modulated Radiation Therapy in Resectable p16+ Locally Advanced Oropharynx Cancer: An ECOG-ACRIN Cancer Research Group Trial (E3311). J. Clin. Oncol. 2022, 40, 138–149. [Google Scholar] [CrossRef]
- Feng, F.Y.; Kim, H.M.; Lyden, T.H.; Haxer, M.J.; Worden, F.P.; Feng, M.; Moyer, J.S.; Prince, M.E.; Carey, T.E.; Wolf, G.T.; et al. Intensity-Modulated Chemoradiotherapy Aiming to Reduce Dysphagia in Patients with Oropharyngeal Cancer: Clinical and Functional Results. J. Clin. Oncol. 2010, 28, 2732–2738. [Google Scholar] [CrossRef] [PubMed]
- Haughey, B.H.; Hinni, M.L.; Salassa, J.R.; Hayden, R.E.; Grant, D.G.; Rich, J.T. Transoral laser microsurgery as primary treatment for advanced-stage oropharyngeal cancer: A United States multicenter study. Head Neck 2011, 33, 1683–1694. [Google Scholar] [CrossRef]
- AJCC Cancer Staging Manual. Available online: https://link.springer.com/book/9783319406176 (accessed on 3 February 2022).
- Kimple, R.J.; Smith, M.A.; Blitzer, G.C.; Torres, A.D.; Martin, J.A.; Yang, R.Z.; Peet, C.R.; Lorenz, L.D.; Nickel, K.P.; Klingelhutz, A.J.; et al. Enhanced Radiation Sensitivity in HPV-Positive Head and Neck Cancer. Cancer Res. 2013, 73, 4791–4800. [Google Scholar] [CrossRef]
- Rietbergen, M.M.; Braakhuis, B.J.M.; Moukhtari, N.; Bloemena, E.; Brink, A.; Sie, D.; Ylstra, B.; de Jong, R.J.B.; Snijders, P.J.F.; Brakenhoff, R.H.; et al. No evidence for active human papillomavirus (HPV) in fields surrounding HPV-positive oropharyngeal tumors. J. Oral Pathol. Med. 2014, 43, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Peck, B.W.; Dahlstrom, K.R.; Gan, S.J.; Caywood, W.; Li, G.; Wei, Q.; Zafereo, M.E.; Sturgis, E.M. Low risk of second primary malignancies among never smokers with human papillomavirus-associated index oropharyngeal cancers. Head Neck 2013, 35, 794–799. [Google Scholar] [CrossRef] [PubMed]
- Samuels, S.E.; Eisbruch, A.; Beitler, J.J.; Corry, J.; Bradford, C.R.; Saba, N.F. Management of locally advanced HPV-related oro-pharyngeal squamous cell carcinoma: Where are we? Eur. Arch. Otorhinolaryngol. 2016, 273, 2877–2894. [Google Scholar] [CrossRef]
- Chen, D.S.; Mellman, I. Oncology Meets Immunology: The Cancer-Immunity Cycle. Immunity 2013, 39, 1–10. [Google Scholar] [CrossRef]
- Blankenstein, T.; Coulie, P.G.; Gilboa, E.; Jaffee, E.M. The determinants of tumour immunogenicity. Nat. Rev. Cancer 2012, 12, 307–313. [Google Scholar] [CrossRef]
- Lawrence, M.S.; Stojanov, P.; Polak, P.; Kryukov, G.V.; Cibulskis, K.; Sivachenko, A.; Carter, S.L.; Stewart, C.; Mermel, C.H.; Roberts, S.A.; et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 2013, 499, 214–218. [Google Scholar] [CrossRef]
- Campo, M.; Graham, S.; Cortese, M.; Ashrafi, G.; Araibi, E.; Dornan, E.; Miners, K.; Nunes, C.; Man, S. HPV-16 E5 down-regulates expression of surface HLA class I and reduces recognition by CD8 T cells. Virology 2010, 407, 137–142. [Google Scholar] [CrossRef] [PubMed]
- De Freitas, A.C.; de Oliveira, T.H.A.; Barros, M.R.; Venuti, A. hrHPV E5 oncoprotein: Immune evasion and related immunotherapies. J. Exp. Clin. Cancer Res. 2017, 36, 71. [Google Scholar] [CrossRef]
- Gildener-Leapman, N.; Ferris, R.L.; Bauman, J.E. Promising systemic immunotherapies in head and neck squamous cell carcinoma. Oral Oncol. 2013, 49, 1089–1096. [Google Scholar] [CrossRef]
- Grinnell, M.; Krishnan, M.; Ganti, A.K. HPV and the Immune System in Head and Neck Cancers: Therapeutic Considerations. Oncology 2020, 34, 139–143. [Google Scholar]
- Solomon, B.; Young, R.J.; Bressel, M.; Urban, D.; Hendry, S.; Thai, A.; Angel, C.; Haddad, A.; Kowanetz, M.; Fua, T.; et al. Prognostic Significance of PD-L1+ and CD8+ Immune Cells in HPV+ Oropharyngeal Squamous Cell Carcinoma. Cancer Immunol. Res. 2018, 6, 295–304. [Google Scholar] [CrossRef]
- Mandal, R.; Şenbabaoğlu, Y.; Desrichard, A.; Havel, J.J.; Dalin, M.G.; Riaz, N.; Lee, K.-W.; Ganly, I.; Hakimi, A.A.; Chan, T.A.; et al. The head and neck cancer immune landscape and its immunotherapeutic implications. JCI Insight 2016, 1, e89829. Available online: https://insight.jci.org/articles/view/89829 (accessed on 15 October 2022). [CrossRef] [PubMed]
- Wood, O.; Woo, J.; Seumois, G.; Savelyeva, N.; McCann, K.J.; Singh, D.; Jones, T.; Peel, L.; Breen, M.S.; Ward, M.; et al. Gene expression analysis of TIL rich HPV-driven head and neck tumors reveals a distinct B-cell signature when compared to HPV independent tumors. Oncotarget 2016, 7, 56781–56797. [Google Scholar] [CrossRef]
- Wagner, S.; Wittekindt, C.; Reuschenbach, M.; Hennig, B.; Thevarajah, M.; Würdemann, N. CD56-positive lymphocyte infiltra-tion in relation to human papillomavirus association and prognostic significance in oropharyngeal squamous cell carcinoma. Int. J. Cancer. 2016, 138, 2263–2273. [Google Scholar] [CrossRef]
- Wieland, A.; Patel, M.R.; Cardenas, M.A.; Eberhardt, C.S.; Hudson, W.H.; Obeng, R.C.; Griffith, C.C.; Wang, X.; Chen, Z.G.; Kissick, H.T.; et al. Defining HPV-specific B cell responses in patients with head and neck cancer. Nature 2021, 597, 274–278. [Google Scholar] [CrossRef]
- Nulton, T.J.; Olex, A.L.; Dozmorov, M.; Morgan, I.M.; Windle, B. Analysis of The Cancer Genome Atlas sequencing data reveals novel properties of the human papillomavirus 16 genome in head and neck squamous cell carcinoma. Oncotarget 2017, 8, 17684–17699. [Google Scholar] [CrossRef] [PubMed]
- Matlung, S.E.; Wilhelmina van Kempen, P.M.; Bovenschen, N.; van Baarle, D.; Willems, S.M. Differences in T-cell infiltrates and sur-vival between HPV+ and HPV- oropharyngeal squamous cell carcinoma. Future Sci. 2016, 2, FSO88. [Google Scholar]
- Chakravarthy, A.; Henderson, S.; Thirdborough, S.M.; Ottensmeier, C.; Su, X.; Lechner, M.; Feber, A.; Thomas, G.J.; Fenton, T.R. Human Papillomavirus Drives Tumor Development Throughout the Head and Neck: Improved Prognosis Is Associated with an Immune Response Largely Restricted to the Oropharynx. J. Clin. Oncol. 2016, 34, 4132–4141. [Google Scholar] [CrossRef]
- Ward, M.J.; Thirdborough, S.M.; Mellows, T.; Riley, C.; Harris, S.; Suchak, K.; Webb, A.; Hampton, C.; Patel, N.N.; Randall, C.J.; et al. Tumour-infiltrating lymphocytes predict for outcome in HPV-positive oropharyngeal cancer. Br. J. Cancer 2014, 110, 489–500. [Google Scholar] [CrossRef]
- King, E.V.; Ottensmeier, C.H.; Thomas, G.J. The immune response in HPV+ oropharyngeal cancer. Oncoimmunology 2014, 3, e27254. [Google Scholar] [CrossRef]
- Näsman, A.; Andersson, E.; Marklund, L.; Tertipis, N.; Hammarstedt-Nordenvall, L.; Attner, P.; Nyberg, T.; Masucci, G.V.; Munck-Wikland, E.; Ramqvist, T.; et al. HLA Class I and II Expression in Oropharyngeal Squamous Cell Carcinoma in Relation to Tumor HPV Status and Clinical Outcome. PLoS ONE 2013, 8, e77025. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Yan, Z.; Lian, S.; Wei, L.; Zhou, C.; Feng, D.; Zhang, Y.; Yang, J.; Li, M.; Chen, Y. Prognostic value of novel immune-related genomic biomarkers identified in head and neck squamous cell carcinoma. J. Immunother. Cancer 2020, 8, e000444. [Google Scholar] [CrossRef]
- Krupar, R.; Robold, K.; Gaag, D.; Spanier, G.; Kreutz, M.; Renner, K.; Hellerbrand, C.; Hofstaedter, F.; Bosserhoff, A.K. Immunologic and metabolic characteristics of HPV-negative and HPV-positive head and neck squamous cell carcinomas are strikingly different. Virchows Arch. 2014, 465, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Eberhardt, C.S.; Kissick, H.T.; Patel, M.R.; Cardenas, M.A.; Prokhnevska, N.; Obeng, R.C.; Nasti, T.H.; Griffith, C.C.; Im, S.J.; Wang, X.; et al. Functional HPV-specific PD-1+ stem-like CD8 T cells in head and neck cancer. Nature 2021, 597, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Im, S.J.; Hashimoto, M.; Gerner, M.Y.; Lee, J.; Kissick, H.T.; Burger, M.C.; Shan, Q.; Hale, J.S.; Lee, J.; Nasti, T.H.; et al. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature 2016, 537, 417–421. [Google Scholar] [CrossRef]
- Subsets of Exhausted CD8+ T Cells Differentially Mediate Tumor Control and Respond to Checkpoint Blockade—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/30778252/ (accessed on 1 February 2023).
- Siddiqui, I.; Schaeuble, K.; Chennupati, V.; Marraco, S.A.F.; Calderon-Copete, S.; Ferreira, D.P.; Carmona, S.J.; Scarpellino, L.; Gfeller, D.; Pradervand, S.; et al. Intratumoral Tcf1+PD-1+CD8+ T Cells with Stem-like Properties Promote Tumor Control in Response to Vaccination and Checkpoint Blockade Immunotherapy. Immunity 2019, 50, 195–211.e10. [Google Scholar] [CrossRef]
- Zandberg, D.P.; Strome, S.E. The role of the PD-L1:PD-1 pathway in squamous cell carcinoma of the head and neck. Oral Oncol. 2014, 50, 627–632. [Google Scholar] [CrossRef]
- Badoual, C.; Hans, S.; Merillon, N.; Van Ryswick, C.; Ravel, P.; Benhamouda, N.; Levionnois, E.; Nizard, M.; Si-Mohamed, A.; Besnier, N.; et al. PD-1–Expressing Tumor-Infiltrating T Cells Are a Favorable Prognostic Biomarker in HPV-Associated Head and Neck Cancer. Cancer Res 2013, 73, 128–138. [Google Scholar] [CrossRef]
- Burtness, B.; Harrington, K.J.; Greil, R.; Soulières, D.; Tahara, M.; de Castro, G. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEY-NOTE-048): A randomised, open-label, phase 3 study. Lancet 2019, 394, 1915–1928. [Google Scholar] [CrossRef]
- Chen, L.; Han, X. Anti-PD-1/PD-L1 therapy of human cancer: Past, present, and future. J. Clin. Invest. 2015, 125, 3384–3391. [Google Scholar] [CrossRef]
- Castellano, L.R.C.; Cruz, S.B.S.C.; Hier, M.; Bonan, P.R.F.; Alaoui-Jamali, M.A.; da Silva, S.D. Implications and Emerging Therapeutic Avenues of Inflammatory Response in HPV+ Head and Neck Squamous Cell Carcinoma. Cancers 2022, 14, 5406. [Google Scholar] [CrossRef]
- Guidelines Detail. NCCN. Available online: https://www.nccn.org/guidelines/guidelines-detail (accessed on 3 February 2023).
- Pignon, J.-P.; le Maître, A.; Bourhis, J. Meta-Analyses of Chemotherapy in Head and Neck Cancer (MACH-NC): An Update. Int. J. Radiat. Oncol. 2007, 69, S112–S114. [Google Scholar] [CrossRef] [PubMed]
- Szturz, P.; Wouters, K.; Kiyota, N.; Tahara, M.; Prabhash, K.; Noronha, V. Weekly Low-Dose Versus Three-Weekly High-Dose Cisplatin for Concurrent Chemoradiation in Locoregionally Advanced Non-Nasopharyngeal Head and Neck Cancer: A Sys-tematic Review and Meta-Analysis of Aggregate Data. Oncologist 2017, 22, 1056–1066. [Google Scholar] [CrossRef]
- Geiger, J.L.; Lazim, A.F.; Walsh, F.J.; Foote, R.L.; Moore, E.J.; Okuno, S.H.; Olsen, K.D.; Kasperbauer, J.L.; Price, D.L.; Garces, Y.I.; et al. Adjuvant chemoradiation therapy with high-dose versus weekly cisplatin for resected, locally-advanced HPV/p16-positive and negative head and neck squamous cell carcinoma. Oral Oncol. 2014, 50, 311–318. [Google Scholar] [CrossRef]
- Behera, M.; Owonikoko, T.K.; Kim, S.; Chen, Z.; Higgins, K.; Ramalingam, S.S.; Shin, D.M.; Khuri, F.R.; Beitler, J.J.; Saba, N.F. Concurrent therapy with taxane versus non-taxane containing regimens in locally advanced squamous cell carcinomas of the head and neck (SCCHN): A systematic review. Oral Oncol. 2014, 50, 888–894. [Google Scholar] [CrossRef] [PubMed]
- Homma, A.; Shirato, H.; Furuta, Y.; Nishioka, T.; Oridate, N.; Tsuchiya, K. Randomized Phase II Trial of Concomitant Chemo-radiotherapy Using Weekly Carboplatin or Daily Low-Dose Cisplatin for Squamous Cell Carcinoma of the Head and Neck. Cancer J. 2004, 10, 326–332. [Google Scholar] [CrossRef]
- Bonner, J.A.; Harari, P.M.; Giralt, J.; Azarnia, N.; Shin, D.M.; Cohen, R.B. Radiotherapy plus Cetuximab for Squamous-Cell Car-cinoma of the Head and Neck. N. Engl. J. Med. 2006, 354, 567–578. [Google Scholar] [CrossRef]
- Vermorken, J.B.; Mesia, R.; Rivera, F.; Remenar, E.; Kawecki, A.; Rottey, S.; Erfan, J.; Zabolotnyy, D.; Kienzer, H.-R.; Cupissol, D.; et al. Platinum-Based Chemotherapy plus Cetuximab in Head and Neck Cancer. N. Engl. J. Med. 2008, 359, 1116–1127. [Google Scholar] [CrossRef]
- Szturz, P.; Seiwert, T.Y.; Vermorken, J.B. How Standard Is Second-Line Cetuximab in Recurrent or Metastatic Head and Neck Cancer in 2017? J. Clin. Oncol. 2017, 35, 2229–2231. [Google Scholar] [CrossRef]
- Moy, J.D.; Moskovitz, J.M.; Ferris, R.L. Biological mechanisms of immune escape and implications for immunotherapy in head and neck squamous cell carcinoma. Eur. J. Cancer 2017, 76, 152–166. [Google Scholar] [CrossRef] [PubMed]
- Moskovitz, J.; Moy, J.; Ferris, R.L. Immunotherapy for Head and Neck Squamous Cell Carcinoma. Curr. Oncol. Rep. 2018, 20, 22. [Google Scholar] [CrossRef] [PubMed]
- Chow, L.Q.M.; Haddad, R.; Gupta, S.; Mahipal, A.; Mehra, R.; Tahara, M. Antitumor Activity of Pembrolizumab in Bi-omarker-Unselected Patients with Recurrent and/or Metastatic Head and Neck Squamous Cell Carcinoma: Results from the Phase Ib KEYNOTE-012 Expansion Cohort. J. Clin. Oncol. 2016, 34, 3838–3845. [Google Scholar] [CrossRef] [PubMed]
- Bauml, J.; Seiwert, T.Y.; Pfister, D.G.; Worden, F.; Liu, S.V.; Gilbert, J.; Saba, N.F.; Weiss, J.; Wirth, L.; Sukari, A.; et al. Pembrolizumab for Platinum- and Cetuximab-Refractory Head and Neck Cancer: Results from a Single-Arm, Phase II Study. J. Clin. Oncol. 2017, 35, 1542–1549. [Google Scholar] [CrossRef] [PubMed]
- Cohen, E.E.W.; Soulières, D.; Le Tourneau, C.; Dinis, J.; Licitra, L.; Ahn, M.-J.; Soria, A.; Machiels, J.-P.; Mach, N.; Mehra, R.; et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): A randomised, open-label, phase 3 study. Lancet 2019, 393, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Ferris, R.L.; Blumenschein, G., Jr.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2016, 375, 1856–1867. [Google Scholar] [CrossRef]
- Ferris, R.L.; Blumenschein, G., Jr.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.J.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab vs investigator’s choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2-year long-term survival update of CheckMate 141 with analyses by tumor PD-L1 expression. Oral Oncol. 2018, 81, 45–51. [Google Scholar] [CrossRef]
- Haddad, R.I.; Harrington, K.; Tahara, M.; Ferris, R.L.; Gillison, M.; Fayette, J.; Daste, A.; Koralewski, P.; Zurawski, B.; Taberna, M.; et al. Nivolumab Plus Ipilimumab Versus EXTREME Regimen as First-Line Treatment for Recurrent/Metastatic Squamous Cell Carcinoma of the Head and Neck: The Final Results of CheckMate 651. J. Clin. Oncol. 2022, 22, 332. [Google Scholar] [CrossRef]
- Zandberg, D.P.; Algazi, A.P.; Jimeno, A.; Good, J.S.; Fayette, J.; Bouganim, N. Durvalumab for recurrent or metastatic head and neck squamous cell carcinoma: Results from a single-arm, phase II study in patients with ≥25% tumour cell PD-L1 expression who have progressed on platinum-based chemotherapy. Eur. J. Cancer 2019, 107, 142–152. [Google Scholar] [CrossRef]
- Ferris, R.; Haddad, R.; Even, C.; Tahara, M.; Dvorkin, M.; Ciuleanu, T.; Clement, P.; Mesia, R.; Kutukova, S.; Zholudeva, L.; et al. Durvalumab with or without tremelimumab in patients with recurrent or metastatic head and neck squamous cell carcinoma: EAGLE, a randomized, open-label phase III study. Ann. Oncol. 2020, 31, 942–950. [Google Scholar] [CrossRef]
- Wang, B.; Cao, R.; Li, P.; Fu, C. The effects and safety of PD-1/PD-L1 inhibitors on head and neck cancer: A systematic review and meta-analysis. Cancer Med. 2019, 8, 5969–5978. [Google Scholar] [CrossRef]
- Sieviläinen, M.; Saavalainen, J.; Adnan-Awad, S.; Salo, T.; Al-Samadi, A. IDO1 Inhibition Reduces Immune Cell Exclusion Through Inducing Cell Migration While PD-1 Blockage Increases IL-6 and -8 Secretion from T Cells in Head and Neck Cancer. Front. Immunol. 2022, 13, 844. Available online: https://www.frontiersin.org/articles/10.3389/fimmu.2022.812822 (accessed on 4 March 2023). [CrossRef]
- Ferris, R.L.; Saba, N.F.; Gitlitz, B.J.; Haddad, R.; Sukari, A.; Neupane, P.; Morris, J.C.; Misiukiewicz, K.; Bauman, J.E.; Fenton, M.; et al. Effect of Adding Motolimod to Standard Combination Chemotherapy and Cetuximab Treatment of Patients with Squamous Cell Carcinoma of the Head and Neck. JAMA Oncol. 2018, 4, 1583–1588. [Google Scholar] [CrossRef]
- Duhen, R.; Ballesteros-Merino, C.; Frye, A.K.; Tran, E.; Rajamanickam, V.; Chang, S.-C.; Koguchi, Y.; Bifulco, C.B.; Bernard, B.; Leidner, R.S.; et al. Neoadjuvant anti-OX40 (MEDI6469) therapy in patients with head and neck squamous cell carcinoma activates and expands antigen-specific tumor-infiltrating T cells. Nat. Commun. 2021, 12, 1047. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Fan, T.; Wu, L.; Yu, G.; Deng, W.; Chen, L. Selective blockade of B7-H3 enhances antitumour immune activity by re-ducing immature myeloid cells in head and neck squamous cell carcinoma. J. Cell Mol. Med. 2017, 21, 2199–2210. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Y. T cell receptor-engineered T cells for leukemia immunotherapy. Cancer Cell Int. 2019, 19, 2. [Google Scholar] [CrossRef]
- Jäger, E.; Maeurer, M.; Höhn, H.; Karbach, J.; Jäger, D.; Zidianakis, Z. Clonal expansion of Melan A-specific cytotoxic T lym-phocytes in a melanoma patient responding to continued immunization with melanoma-associated peptides. Int. J. Cancer. 2000, 86, 538–547. [Google Scholar] [CrossRef]
- Rosenberg, S. A Phase II Study of Lymphodepletion Followed by Autologous Tumor-Infiltrating Lymphocytes and High-Dose Aldesleukin for Human Papillomavirus-Associated Cancers. clinicaltrials.gov. Report No.: NCT01585428; 2018. Available online: https://clinicaltrials.gov/ct2/show/NCT01585428 (accessed on 6 September 2022).
- Jin, B.Y.; Campbell, T.E.; Draper, L.M.; Stevanović, S.; Weissbrich, B.; Yu, Z. Engineered T cells targeting E7 mediate regression of human papillomavirus cancers in a murine model. JCI Insight 2018, 3, 99488. [Google Scholar] [CrossRef] [PubMed]
- Nagarsheth, N.B.; Norberg, S.M.; Sinkoe, A.L.; Adhikary, S.; Meyer, T.J.; Lack, J.B.; Warner, A.C.; Schweitzer, C.; Doran, S.L.; Korrapati, S.; et al. TCR-engineered T cells targeting E7 for patients with metastatic HPV-associated epithelial cancers. Nat. Med. 2021, 27, 419–425. [Google Scholar] [CrossRef]
- Jia, Q. Two-Arm Open-Labeled Trial of HPV-E6-Specific TCR-T Cells with or without Anti-PD1 Auto-Secreted Element in the Treatment of HPV-Positive Head and Neck Carcinoma or Cervical Cancer. clinicaltrials.gov. Report No.: NCT03578406; 2019. Available online: https://clinicaltrials.gov/ct2/show/NCT03578406 (accessed on 6 September 2022).
- Doran, S.L.; Stevanović, S.; Adhikary, S.; Gartner, J.J.; Jia, L.; Kwong, M.L.M. T-Cell Receptor Gene Therapy for Human Papillo-mavirus-Associated Epithelial Cancers: A First-in-Human, Phase I/II Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2019, 37, 2759–2768. [Google Scholar] [CrossRef]
- Bendle, G.M.; Linnemann, C.; I Hooijkaas, A.; Bies, L.; De Witte, M.A.; Jorritsma, A.; Kaiser, A.D.M.; Pouw, N.; Debets, R.; Kieback, E.; et al. Lethal graft-versus-host disease in mouse models of T cell receptor gene therapy. Nat. Med. 2010, 16, 565–570. [Google Scholar] [CrossRef]
- Linette, G.P.; Stadtmauer, E.A.; Maus, M.V.; Rapoport, A.P.; Levine, B.L.; Emery, L.; Litzky, L.; Bagg, A.; Carreno, B.M.; Cimino, P.J.; et al. Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma. Blood 2013, 122, 863–871. [Google Scholar] [CrossRef]
- Parkhurst, M.R.; Yang, J.C.; Langan, R.C.; Dudley, M.E.; Nathan, D.-A.N.; Feldman, S.A.; Davis, J.L.; Morgan, R.A.; Merino, M.J.; Sherry, R.M.; et al. T Cells Targeting Carcinoembryonic Antigen Can Mediate Regression of Metastatic Colorectal Cancer but Induce Severe Transient Colitis. Mol. Ther. 2011, 19, 620–626. [Google Scholar] [CrossRef]
- Teachey, D.T.; Lacey, S.F.; Shaw, P.A.; Melenhorst, J.J.; Maude, S.L.; Frey, N.; Pequignot, E.; Gonzalez, V.E.; Chen, F.; Finklestein, J.; et al. Identification of Predictive Biomarkers for Cytokine Release Syndrome after Chimeric Antigen Receptor T-cell Therapy for Acute Lymphoblastic Leukemia. Cancer Discov. 2016, 6, 664–679. [Google Scholar] [CrossRef]
- Zeigler, S.; Woodham, A.; Li, M.; Zeyang, E.; Kolifrath, S.; Rashidian, M.; O’Connor, K.; Chaparro, R.; Seidel, R.; Mesyngier, M.; et al. 623 Immuno-STATs: Leveraging protein engineering to expand and track antigen-specific T cells in vivo. J. Immunother. Cancer 2020, 8, A659. Available online: https://jitc.bmj.com/content/8/Suppl_3/A374.2 (accessed on 1 February 2023).
- A Phase 1 Study in Patients with HPV16+ Recurrent/ Metastatic Head and Neck Squamous Cell Carcinoma—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03978689 (accessed on 27 December 2022).
- Gavrielatou, N.; Vathiotis, I.; Economopoulou, P.; Psyrri, A. The Role of B Cells in Head and Neck Cancer. Cancers 2021, 13, 5383. [Google Scholar] [CrossRef]
- Hladíková, K.; Koucký, V.; Bouček, J.; Laco, J.; Grega, M.; Hodek, M. Tumor-infiltrating B cells affect the progression of oro-pharyngeal squamous cell carcinoma via cell-to-cell interactions with CD8+ T cells. J. Immunother. Cancer 2019, 7, 261. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, B.; Ma, F.; Tong, F.; Yan, B.; Liu, T.; Xie, H.; Song, L.; Yu, S.; Wei, L. Characteristics of B lymphocyte infiltration in HPV + head and neck squamous cell carcinoma. Cancer Sci. 2021, 112, 1402–1416. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.S.; Shen, S.; Miyauchi, S.; Sanders, P.D.; Franiak-Pietryga, I.; Mell, L.; Gutkind, J.S.; Cohen, E.E.; Califano, J.A.; Sharabi, A.B. B Cells Improve Overall Survival in HPV-Associated Squamous Cell Carcinomas and Are Activated by Radiation and PD-1 Blockade. Clin. Cancer Res. 2020, 26, 3345–3359. [Google Scholar] [CrossRef] [PubMed]
- Yarchoan, M.; Johnson, B.A.; Lutz, E.R.; Laheru, D.A.; Jaffee, E.M. Targeting neoantigens to augment antitumour immunity. Nat. Rev. Cancer 2017, 17, 569. [Google Scholar] [CrossRef] [PubMed]
- Hollingsworth, R.E.; Jansen, K. Turning the corner on therapeutic cancer vaccines. NPJ Vaccines 2019, 4, 7. [Google Scholar] [CrossRef]
- Paolini, F.; Curzio, G.; Cordeiro, M.N.; Massa, S.; Mariani, L.; Pimpinelli, F.; de Freitas, A.C.; Franconi, R.; Venuti, A. HPV 16 E5 oncoprotein is expressed in early stage carcinogenesis and can be a target of immunotherapy. Hum. Vaccines Immunother. 2017, 13, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.H.; Jin, H.T.; Park, S.H.; Youn, J.I.; Sung, Y.-C. Optimal induction of HPV DNA vaccine-induced CD8+ T cell responses and therapeutic antitumor effect by antigen engineering and electroporation. Vaccine 2009, 27, 5906–5912. [Google Scholar] [CrossRef]
- Zandberg, D.P.; Rollins, S.; Goloubeva, O.G.; Morales, R.E.; Tan, M.T.; Taylor, R. A phase I dose escalation trial of MAGE-A3 and HPV-16 specific peptide immunomodulatory vaccines in patients with recurrent/metastatic (RM) squamous cell carcinoma of the head and neck (SCCHN). J. Clin. Oncol. 2014, 32 (Suppl. 15), e17014. [Google Scholar] [CrossRef]
- Chandra, J.; Woo, W.P.; Finlayson, N.; Liu, H.Y.; McGrath, M.; Ladwa, R.; Brauer, M.; Xu, Y.; Hanson, S.; Panizza, B.; et al. A phase 1, single centre, open label, escalating dose study to assess the safety, tolerability and immunogenicity of a therapeutic human papillomavirus (HPV) DNA vaccine (AMV002) for HPV-associated head and neck cancer (HNC). Cancer Immunol. Immunother. 2021, 70, 743–753. [Google Scholar] [CrossRef]
- Aggarwal, C.; Cohen, R.B.; Morrow, M.P.; Kraynyak, K.A.; Sylvester, A.J.; Knoblock, D.M. Immunotherapy Targeting HPV16/18 Generates Potent Immune Responses in HPV-Associated Head and Neck Cancer. Clin. Cancer Res. 2019, 25, 110–124. [Google Scholar] [CrossRef]
- Aggarwal, C.; Saba, N.F.; Algazi, A.P.; Sukari, A.; Seiwert, T.; Haigentz, M. 916MO Safety and efficacy of MEDI0457 plus dur-valumab in patients (pts) with human papillomavirus-associated recurrent/metastatic head and neck squamous cell carcinoma (HPV+ R/M HNSCC). Ann. Oncol. 2020, 31, S661–S662. [Google Scholar] [CrossRef]
- Transgene. A Phase Ib/II Trial Evaluating the Combination of TG4001 and Avelumab in Patients with HPV-16 Positive Re-current or Metastatic Malignancies [Internet]. clinicaltrials.gov; Report No.: NCT03260023; 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT03260023 (accessed on 26 October 2022).
- Van Poelgeest, M.I.E.; Welters, M.J.P.; van Esch, E.M.G.; Stynenbosch, L.F.M.; Kerpershoek, G.; van Persijn van Meerten, E.L. HPV16 synthetic long peptide (HPV16-SLP) vaccination therapy of patients with advanced or recurrent HPV16-induced gynecological carcinoma, a phase II trial. J. Transl. Med. 2013, 11, 88. [Google Scholar] [CrossRef] [PubMed]
- Kenter, G.G.; Welters, M.J.P.; Valentijn, A.R.P.M.; Lowik, M.J.G.; Berends-van der Meer, D.M.A.; Vloon, A.P.G. Phase I immunother-apeutic trial with long peptides spanning the E6 and E7 sequences of high-risk human papillomavirus 16 in end-stage cervical cancer patients shows low toxicity and robust immunogenicity. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2008, 14, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Massarelli, E.; William, W.; Johnson, F.; Kies, M.; Ferrarotto, R.; Guo, M. Combining Immune Checkpoint Blockade and Tu-mor-Specific Vaccine for Patients with Incurable Human Papillomavirus 16–Related Cancer: A Phase 2 Clinical Trial. JAMA Oncol. 2019, 5, 67–73. [Google Scholar] [CrossRef]
- M.D. Anderson Cancer Center. Phase II Trial of Nivolumab and HPV-16 Vaccination in Patients with HPV-16-Positive In-curable Solid Tumors. clinicaltrials.gov; Report No.: Study/NCT02426892; 2021. Available online: https://clinicaltrials.gov/ct2/show/study/NCT02426892 (accessed on 26 October 2022).
- M.D. Anderson Cancer Center. Phase II Trial of Utomilumab and ISA101b Vaccination in Patients with HPV-16-Positive In-curable Oropharyngeal Cancer. clinicaltrials.gov; Report No.: NCT03258008; 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT03258008 (accessed on 26 October 2022).
- Zandberg, D. Phase II Study Evaluating HPV-16 Vaccination (ISA101b) and Pembrolizumab Plus Cisplatin Chemoradiotherapy for “Intermediate Risk” HPV-16 Associated Head and Neck Squamous Cell Carcinoma (HNSCC). clinicaltrials.gov; Report No.: NCT04369937; 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT04369937 (accessed on 26 October 2022).
- Mayo Clinic. Stimulating Immune Response with Neoadjuvant Human Papilloma Virus (HPV)-16 Specific Vaccination in HPV-Oropharyngeal Squamous Cell Carcinoma (HPV-OPSCC). clinicaltrials.gov; Report No.: NCT05232851; 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT05232851 (accessed on 26 October 2022).
- Hookipa Biotech GmbH. A Phase I/II Study of TheraT® Vector(s) Expressing Human Papillomavirus 16 Positive (HPV 16+) Specific Antigens in Patients with HPV 16+ Confirmed Cancers [Internet]. clinicaltrials.gov; Report No.: NCT04180215; 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT04180215 (accessed on 27 October 2022).
- Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins. A Phase I Clinical Trial Assessing the Safety and Feasibility of Administration of pNGVL4a-CRT/E7(Detox) DNA Vaccine Using the Intramuscular TriGridTM Delivery System in Combina-tion With Cyclophosphamide in HPV-16 Associated Head and Neck Cancer Patients. clinicaltrials.gov; Report No.: NCT01493154; 2018. Available online: https://clinicaltrials.gov/ct2/show/NCT01493154 (accessed on 26 October 2022).
- ADXS 11-001 Vaccination Prior to Robotic Surgery, HPV-Positive Oropharyngeal Cancer—Study Results—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/results/NCT02002182 (accessed on 27 October 2022).
- Sehgal, K. A Phase Ib/II Trial to Test the Safety and Efficacy of Vaccination with HPV16-E711-19 Nanomer for the Treatment of Incurable HPV 16-Related Oropharyngeal, Cervical and Anal Cancer in HLA-A*02 Positive Patients. clinicaltri-als.gov; Report No.: NCT02865135; 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT02865135 (accessed on 26 October 2022).
- National Cancer Institute (NCI). Phase I/II Trial of HPV Vaccine PRGN-2009 Alone or in Combination with An-ti-PD-L1/TGF-Beta Trap (M7824) in Subjects with HPV Positive Cancers [Internet]. clinicaltrials.gov; Report No.: NCT04432597; 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT04432597 (accessed on 26 October 2022).
- Stanley, M. Immunobiology of HPV and HPV vaccines. Gynecol. Oncol. 2008, 109 (Suppl. 2), S15–S21. [Google Scholar] [CrossRef]
- Kamolratanakul, S.; Pitisuttithum, P. Human Papillomavirus Vaccine Efficacy and Effectiveness against Cancer. Vaccines 2021, 9, 1413. [Google Scholar] [CrossRef] [PubMed]
- Understanding and learning from the success of prophylactic human papillomavirus vaccines|Nature Reviews Microbiology. Available online: https://www.nature.com/articles/nrmicro2872 (accessed on 28 October 2022).
- Gillison, M.L.; Chaturvedi, A.K.; Lowy, D.R. HPV prophylactic vaccines and the potential prevention of noncervical cancers in both men and women. Cancer 2008, 113 (Suppl. 2), 3036–3046. [Google Scholar] [CrossRef] [PubMed]
- FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N. Engl. J. Med. 2007, 356, 1915–1927. [Google Scholar] [CrossRef]
- Paavonen, J.; Naud, P.; Salmerón, J.; Wheeler, C.M.; Chow, S.N.; Apter, D. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): Final analysis of a double-blind, randomised study in young women. Lancet 2009, 374, 301–314. [Google Scholar] [CrossRef]
- Research C for BE and GARDASIL 9. FDA; 2020. Available online: https://www.fda.gov/vaccines-blood-biologics/vaccines/gardasil-9 (accessed on 22 June 2022).
- Wilkin, T.J.; Chen, H.; Cespedes, M.S.; Leon-Cruz, J.T.; Godfrey, C.; Chiao, E.Y.; Bastow, B.; Webster-Cyriaque, J.; Feng, Q.; Dragavon, J.; et al. A Randomized, Placebo-Controlled Trial of the Quadrivalent Human Papillomavirus Vaccine in Human Immunodeficiency Virus-Infected Adults Aged 27 Years or Older: AIDS Clinical Trials Group Protocol A5298. Clin. Infect. Dis. 2018, 67, 1339–1346. [Google Scholar] [CrossRef]
- Herrero, R.; Quint, W.; Hildesheim, A.; Gonzalez, P.; Struijk, L.; Katki, H.A.; Porras, C.; Schiffman, M.; Rodriguez, A.C.; Solomon, D.; et al. Reduced Prevalence of Oral Human Papillomavirus (HPV) 4 Years after Bivalent HPV Vaccination in a Randomized Clinical Trial in Costa Rica. PLoS ONE 2013, 8, e68329. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, A.K.; Graubard, B.I.; Broutian, T.; Pickard, R.K.L.; Tong, Z.-Y.; Xiao, W.; Kahle, L.; Gillison, M.L. Effect of Prophylactic Human Papillomavirus (HPV) Vaccination on Oral HPV Infections Among Young Adults in the United States. J. Clin. Oncol. 2018, 36, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Schlecht, N.F.; Masika, M.; Diaz, A.; Nucci-Sack, A.; Salandy, A.; Pickering, S.; Strickler, H.D.; Shankar, V.; Burk, R.D. Risk of Oral Human Papillomavirus Infection Among Sexually Active Female Adolescents Receiving the Quadrivalent Vaccine. JAMA Netw. Open 2019, 2, e1914031. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, A.K.; Graubard, B.I.; Broutian, T.; Xiao, W.; Pickard, R.K.L.; Kahle, L. Prevalence of Oral HPV Infection in Unvac-cinated Men and Women in the United States, 2009–2016. JAMA 2019, 322, 977–979. [Google Scholar] [CrossRef]
- Mehanna, H.; Bryant, T.S.; Babrah, J.; Louie, K.; Bryant, J.L.; Spruce, R.J.; Batis, N.; Olaleye, O.; Jones, J.; Struijk, L.; et al. Human Papillomavirus (HPV) Vaccine Effectiveness and Potential Herd Immunity for Reducing Oncogenic Oropharyngeal HPV-16 Prevalence in the United Kingdom: A Cross-sectional Study. Clin. Infect. Dis. 2019, 69, 1296–1302. [Google Scholar] [CrossRef]
| Trial | Study Population | Regimen | Patient | Primary Endpoint | Results per HPV Status |
|---|---|---|---|---|---|
| KEYNOTE-048 (phase III) [51] | RM, untreated SCC | A: Pembrolizumab B: Pembrolizumab, platinum, 5-FU C: Cetuximab, platinum, 5-FU | A: 301 B: 281 C: 300 | OS (B vs. C) CPS > 20: 14.7 vs. 11.0 month CPS > 1: 13.6 vs. 10.4 month | No difference in OS between HPV+ and HPV− patients |
| CONDOR (phase II) [63] | RM, platinum-refractory with low PD-L1 | A: Durvalumab + Tremelimumab B: Durvalumab C: Tremelimumab | A: 133 B: 67 C: 67 | A vs. B vs. C ORR: 7.8%, 9.2%, 1.6% Grade ¾ AE: 15.8% vs. 12.3% vs. 16.9% | HPV+ vs. HPV− in three arms ORR: 5.4% vs. 16.7% vs. 0% |
| KEYNOTE-012 (phase IB) [65] | RM, untreated and treated SCC | Single Arm Pembrolizumab | 132 | ORR: 18% Any Grade AE: 62% Grade > 3: 9% | HPV+ vs. HPV− ORR: 32% vs. 14% 6-month PFS: 37% vs. 20% 6mo OS: 70% vs. 56% |
| KEYNOTE-055 (phase II) [66] | RM, platinum-refractory SCC | Single Arm Pembrolizumab | 171 | ORR: 16% Any Grade AE: 64% Grade > 3: 15% | HPV+ vs. HPV- ORR: 16% vs. 15% 6-month PFS: 25% vs. 21% 6 month OS: 72% vs. 55% |
| KEYNOTE-040 (phase III) [67] | RM, platinum-refractory SCC | A: Pembrolizumab B: Standard of Care | A: 247 B: 248 | OS A: 8.4 month B: 6.9 month | No difference in OS between HPV+ and HPV− patients |
| CHECKMATE-141 (phase III) [69] | RM, platinum-refractory SCC | A: Nivolumab B: Standard of Care | A: 240 B: 121 | OS (1/2 years) A: 36.0%/16.9% B: 16.6%/6.0% | OS benefit noted in all groups irrespective of PD-L1 expression or HPV status |
| CHECKMATE-651 (phase III) [70] | RM, untreated SCC | A: Nivolumab + Ipilimumab B: EXTREME | A: 472 B: 475 | OS (total/CPS > 20/CPS ≥ 1) A: 13.9/17.6/15.7 month B: 13.5/14.6/13.2 month | No difference in OS between HPV+ and HPV− patients |
| HAWK (phase II) [71] | RM, immunotherapy naïve with high PD-L1 | Single arm Durvalumab | 111 | ORR: 16.2% | HPV+ vs. HPV− ORR: 30% vs. 11.8% Median PFS: 3.6 vs. 1.8 month Median OS: 10.2 vs. 5.0 month |
| EAGLE (phase III) [72] | RM, platinum-refractory SCCC | A: Durvalumab B: Durvalumab + Tremelimumab C: Standard of Care | A: 240 B: 247 C: 249 | OS (A vs. C): 7.6 vs. 8.3 OS (B vs. C): 6.5 vs. 8.3 | NA |
| Trial | Target | Therapeutic Arms | Primary Endpoints/Results Related to HPV-Positive Opscc |
|---|---|---|---|
| NCT01585428 (phase II) [80] | HPV E6/E7 oncoproteins | Young TIL + Fludarabine + Cyclophosphamide + Aldesleukin | OTRR: 18% in (2/11 patients) with noncervical HPV-associated cancer. One patient with oropharyngeal cancer attained a PR of 5 months duration. |
| NCT02858310 (phase I/II) [82] | HPV E7 oncoprotein | E7 TCR cells + Fludarabine + Cyclophosphamide + Aldesleukin | OTRR: 50% in 12 patients with HPV-related cancers. Four patients with HPV-related oropharyngeal cancer, three had a PR, and one had SD. |
| NCT03578406 (phase I) [83] | HPV E6 oncoprotein | E6 TCR cells +/− anti-PD1 auto-secreted element | MTD |
| NCT02280811 (phase I/II) [84] | E6 TCR cells + Fludarabine + Cyclophosphamide + Aldesleukin | MTD, OTRR, DR. 1/12 patients with oropharyngeal cancer. Two patients with PR and four patients with SD, which included the patient with HPV-positive oropharyngeal cancer. | |
| NCT03978689 (phase I) [90] | HPV E7 oncoprotein | CUE-101 +/− Pembrolizumab | DLT, Serum PK parameters. In total, 14 evaluable patients in the monotherapy group, 1 with PR and 6 with SD. In total, 7 evaluable patients in the combination group, 2 with PR and 2 with SD. |
| Intervention/ Treatment | Primary Endpoints/Results Related to HPV-Positive Opscc | |
|---|---|---|
| NCT00257738 (phase I) [99] | Cohort 1: Peptide vaccine MAGE-A3 Cohort 2: Peptide vaccine GL-0817 | Patients with HPV16-positive HNC, no dose-limiting toxicity was observed. 67% (4/6 patients) in the MAGE-A3 arm and 80% (4/5 patients) in the GL-0817 arm developed a T cell and antibody response. |
| ACTRN12618000140257 (phase I) [100] | DNA vaccine AMV002 | Patients with previously treated HPV-positive OPSCC, 83% (10/12 patients) developed a vaccine-induced cell-mediated response. One patient developed a greater than four-fold increase in response post-vaccine administration. |
| NCT02163057 (phase I/II) [101] | DNA vaccine MED10457 | Patients with locally advanced HPV16-positive HNC, 85% (18/21) developed a HPV-16 antigen-specific response |
| NCT03162224 (phase I/II) [102] | DNA vaccine MED10457 + durvalumab | Patients with recurrent or metastatic HPV-positive HNC, OTRR of 22.2% (6/27 patients), 3 patients had a CR, and 3 patients had a PR. |
| NCT03260023 (phase I/II) [103] | Modified Ankara vector-based vaccine TG4001 +/− avelumab | 5/9 patients with HPV-positive OPSCC, 33% (3/9 patients) with PR. |
| NCT02426892 (phase II) [106] | Peptide vaccine ISA-101 + nivolumab | 22/24 patients with HPV-positive OPSCC, OTRR 33% (8/24 patients). Cure rate in patients with HPV-positive OPSCC 9% (2/22). |
| NCT03258008 (phase II) [108] | Peptide vaccine ISA-101 + utomilumab | OTRR, Patients with HPV16-positive OPSCC. |
| NCT04369937 (phase II) [109] | Peptide vaccine ISA-101 + pembrolizumab | PFS, Patients with HPV16-positive HNC. |
| NCT0523851 (phase I/II) [110] | Liposomal peptide vaccine PDS0101 +/− pembrolizumab | Proportion of successful response, OTRR, PFS, patients with locally advanced HPV-positive OPSCC. |
| NCT04180215 (phase I/II) [111] | Live attenuated arenavirus vaccine HB-201 +/− pembrolizumab | MTD, OTRR, patients with recurrent/metastatic HPV+ HNC. |
| NCT01493154 (phase I) [112] | DNA vaccine pNGVL4a-CRT/E7 | Patients with HPV16-positive HNC. Enrolled two patients but was terminated early due to serious adverse events. |
| NCT02002182 (phase I) [113] | Live attenuated Listeria monocytogenes vaccine ADXS11-001 | Patients with HPV-positive OPSCC, preliminary results showed 55.6% (5/9 patients) had grade 3 or 4 serious adverse events |
| NCT02865135 (phase I/II) [114] | Peptide vaccine DPX-E7 | Patients with HPV-positive cancers, of which OPSCC is included. |
| NCT04432597 (phase I/II) [115] | Gorilla adenovirus vaccine PRGN-2009 | T cell infiltration response, MTD, Patients with HPV-positive cancers, of which OPSCC is included |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saba, N.F.; Pamulapati, S.; Patel, B.; Mody, M.; Strojan, P.; Takes, R.; Mäkitie, A.A.; Cohen, O.; Pace-Asciak, P.; Vermorken, J.B.; et al. Novel Immunotherapeutic Approaches to Treating HPV-Related Head and Neck Cancer. Cancers 2023, 15, 1959. https://doi.org/10.3390/cancers15071959
Saba NF, Pamulapati S, Patel B, Mody M, Strojan P, Takes R, Mäkitie AA, Cohen O, Pace-Asciak P, Vermorken JB, et al. Novel Immunotherapeutic Approaches to Treating HPV-Related Head and Neck Cancer. Cancers. 2023; 15(7):1959. https://doi.org/10.3390/cancers15071959
Chicago/Turabian StyleSaba, Nabil F., Saagar Pamulapati, Bhamini Patel, Mayur Mody, Primož Strojan, Robert Takes, Antti A. Mäkitie, Oded Cohen, Pia Pace-Asciak, Jan B. Vermorken, and et al. 2023. "Novel Immunotherapeutic Approaches to Treating HPV-Related Head and Neck Cancer" Cancers 15, no. 7: 1959. https://doi.org/10.3390/cancers15071959
APA StyleSaba, N. F., Pamulapati, S., Patel, B., Mody, M., Strojan, P., Takes, R., Mäkitie, A. A., Cohen, O., Pace-Asciak, P., Vermorken, J. B., Bradford, C., Forastiere, A., Teng, Y., Wieland, A., & Ferlito, A. (2023). Novel Immunotherapeutic Approaches to Treating HPV-Related Head and Neck Cancer. Cancers, 15(7), 1959. https://doi.org/10.3390/cancers15071959











