Casting a Wider NET: Pancreatic Exocrine Insufficiency Induced by Somatostatin Analogues among Patients with Neuroendocrine Tumours?
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Consent
2.5. Variables
2.6. Quantitative Assessment of Pancreatic Exocrine Function
2.7. Qualitative Assessment of Pancreatic Exocrine Function
2.8. Assessment of Nutritional Status
2.9. Statistical Analysis
2.10. Ethical and Regulatory Considerations
3. Results
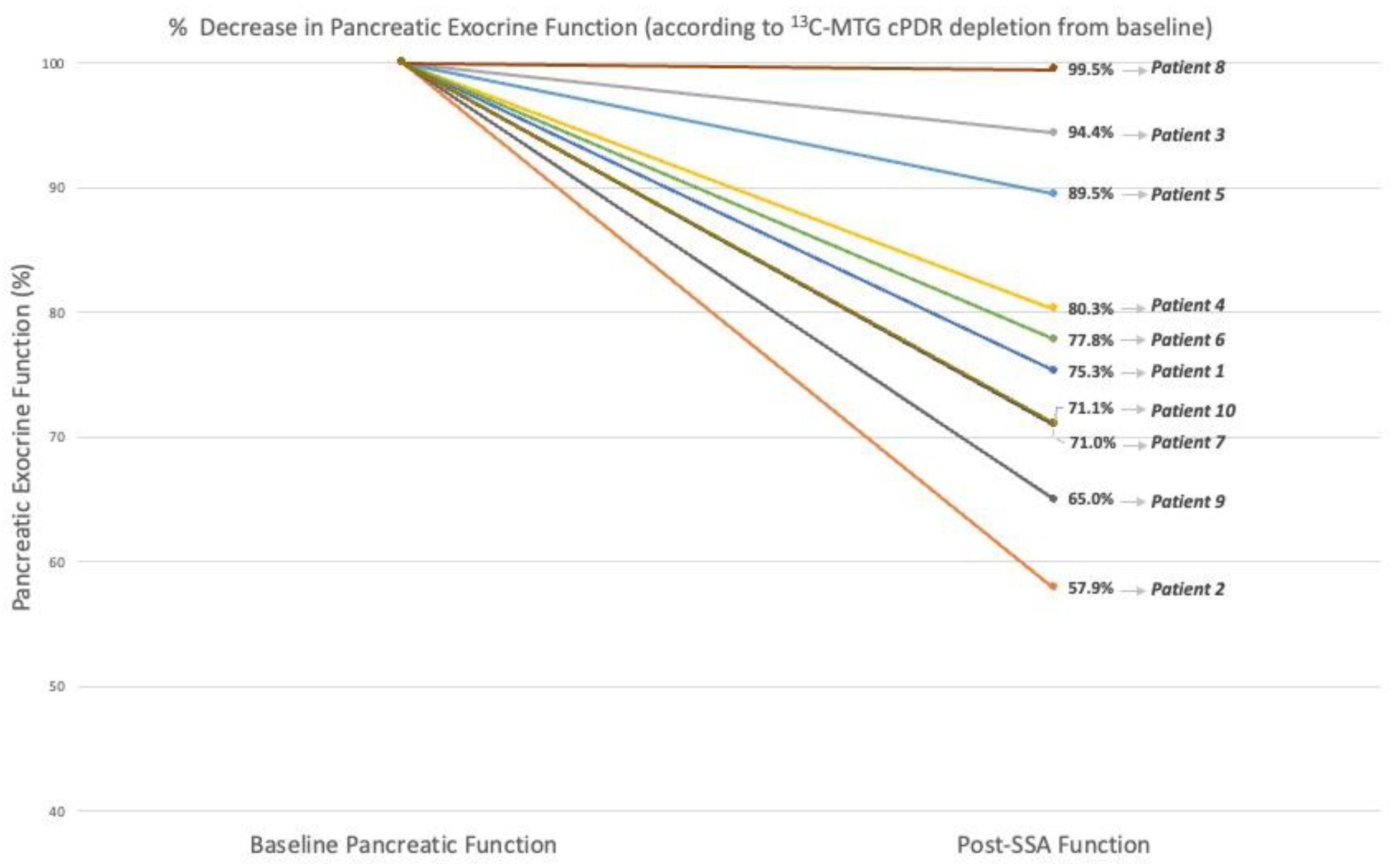
3.1. Quantitative Assessment of Pancreatic Exocrine Function
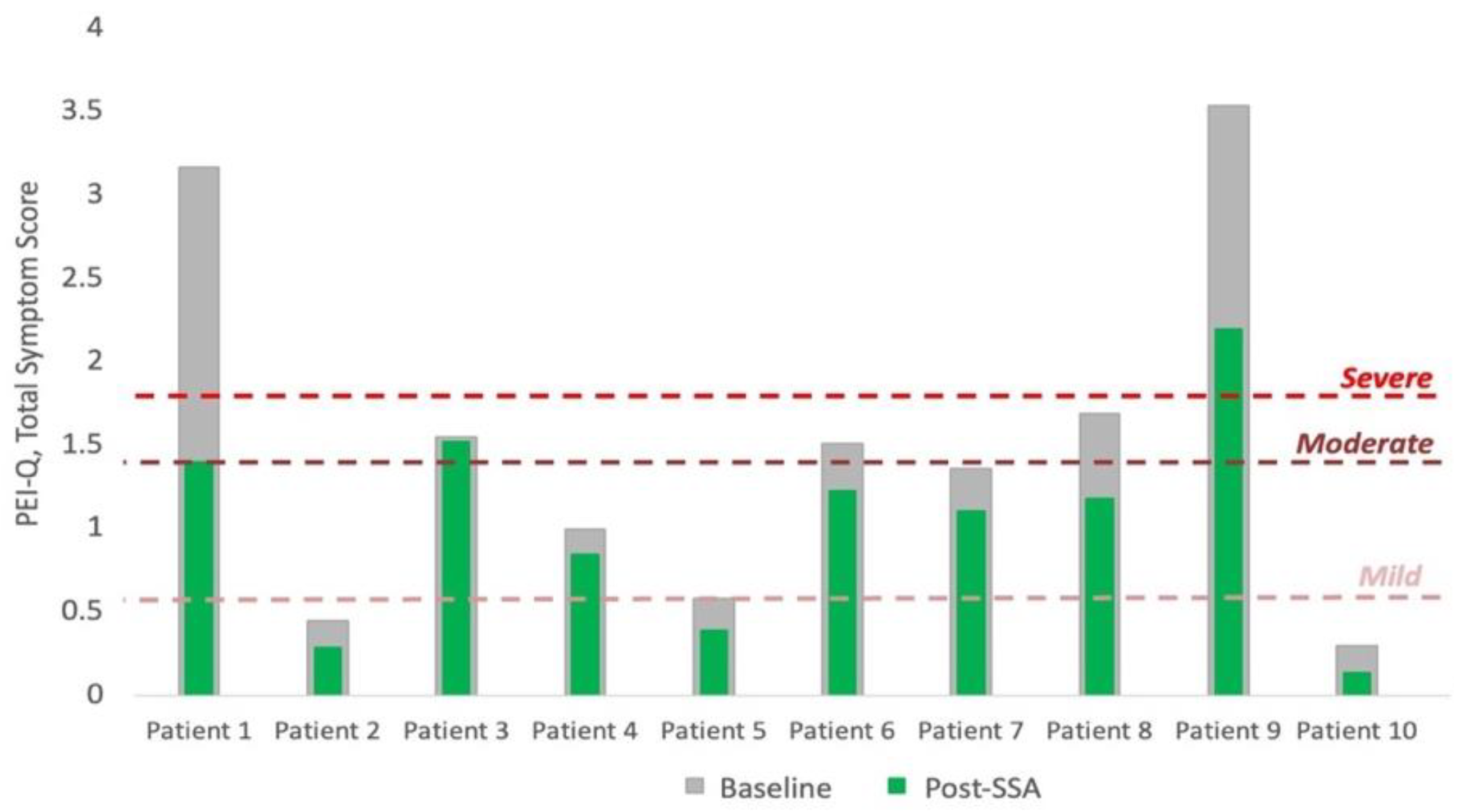
3.2. Qualitative Assessment of Pancreatic Exocrine Function
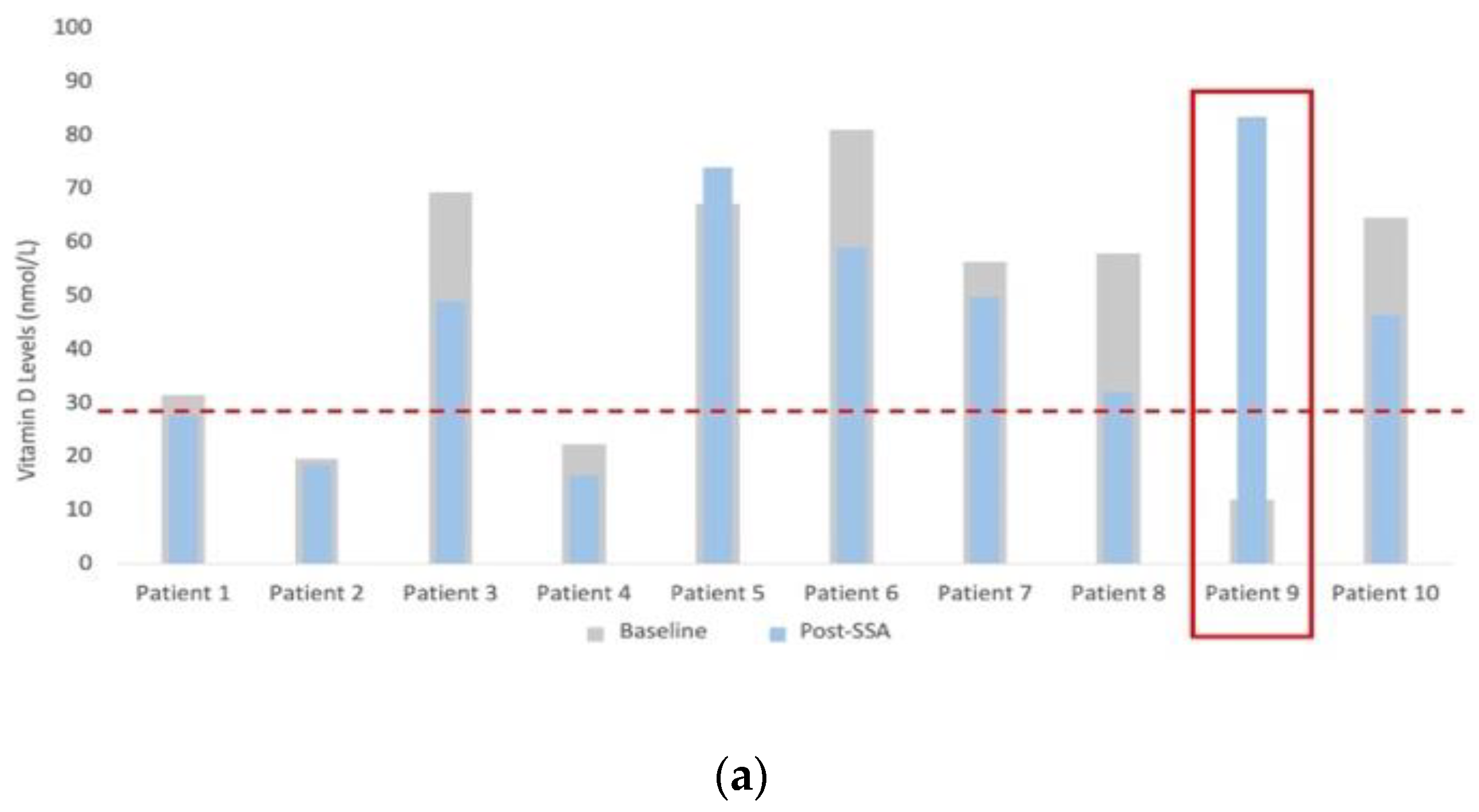
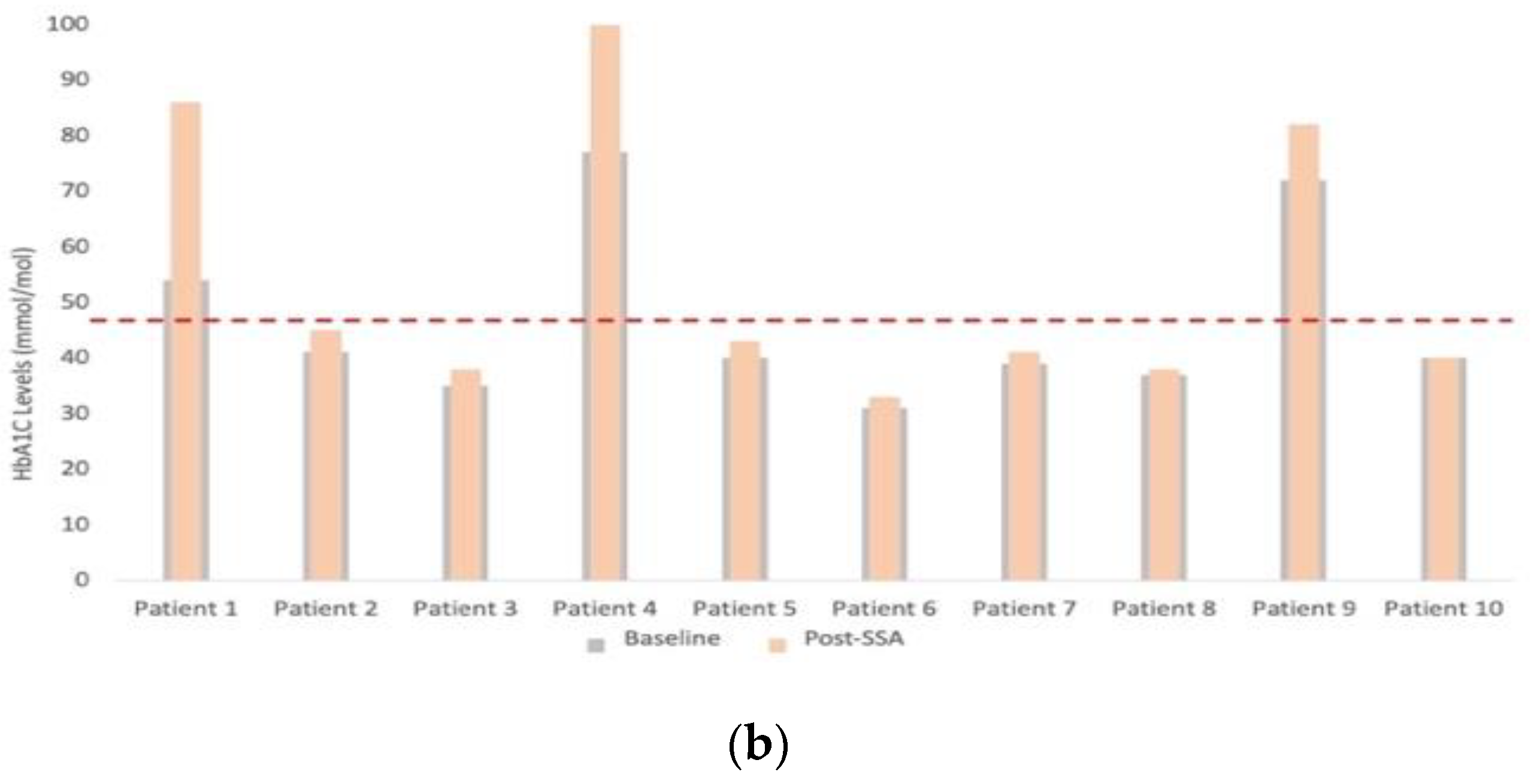
3.3. Assessment of Nutritional Status
3.4. PERT Use
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Capurso, G.; Traini, M.; Piciucchi, M.; Signoretti, M.; Arcidiacono, P.G. Exocrine pancreatic insufficiency: Prevalence, diagnosis, and management. Clin. Exp. Gastroenterol. 2019, 12, 129–139. [Google Scholar] [CrossRef]
- Lindkvist, B. Diagnosis and treatment of pancreatic exocrine insufficiency. World J. Gastroenterol. 2013, 19, 7258–7266. [Google Scholar] [PubMed]
- Nikfarjam, M.; Wilson, J.S.; Smith, R.C. Diagnosis and management of pancreatic exocrine insufficiency. Med. J. Aust. 2017, 207, 161–165. [Google Scholar] [PubMed]
- Johnson, C.D.; Williamson, N.; Janssen-van Solingen, G.; Arbuckle, R.; Johnson, C.; Simpson, S.; Staab, D.; Dominguez-Munoz, E.; Levy, P.; Connett, G.; et al. Psychometric evaluation of a patient-reported outcome measure in pancreatic exocrine insufficiency (PEI). Pancreatology 2019, 19, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Talley, N.J.; Holtmann, G.; Nguyen, Q.N.; Gibson, P.; Bampton, P.; Veysey, M.; Wong, J.; Philcox, S.; Koloski, N.; Bunby, L.; et al. Undiagnosed pancreatic exocrine insufficiency and chronic pancreatitis in functional GI disorder patients with diarrhea or abdominal pain. J. Gastroenterol. Hepatol. 2017, 32, 1813–1817. [Google Scholar] [PubMed]
- Pathanki, A.M.; Attard, J.A.; Bradley, E.; Powell-Brett, S.; Dasari, B.V.M.; Isaac, J.R.; Roberts, K.J.; A Chatzizacharias, N. Pancreatic exocrine insufficiency after pancreaticoduodenectomy: Current evidence and management. World J. Gastrointest. Pathophysiol. 2020, 11, 20–31. [Google Scholar]
- Vujasinovic, M.; Valente, R.; del Chiaro, M.; Permert, J.; Löhr, J.M. Pancreatic exocrine insufficiency in pancreatic cancer. Nutrients 2017, 9, 183. [Google Scholar]
- Huang, W.; de la Iglesia-García, D.; Baston-Rey, I.; Calviño-Suarez, C.; Lariño-Noia, J.; Iglesias-Garcia, J.; Shi, N.; Zhang, X.; Cai, W.; Deng, L.; et al. Exocrine Pancreatic Insufficiency Following Acute Pancreatitis: Systematic Review and Meta-Analysis. Dig. Dis. Sci. 2019, 64, 1985–2005. [Google Scholar] [CrossRef]
- Swart, G.; Baartman, E.; Wattimena, J.; Rietveld, T.; Overbeek, S.; van den Berg, J. Evaluation studies of the 13C-mixed triglyceride breath test in healthy controls and adult cystic fibrosis patients with exocrine pancreatic insufficiency. Digestion 1997, 58, 415–420. [Google Scholar]
- Singh, V.K.; Haupt, M.E.; Geller, D.E.; Hall, J.A.; Diez, P.M.Q. Less common etiologies of exocrine pancreatic insufficiency. World J. Gastroenterol. 2017, 23, 7059–7076. [Google Scholar]
- Pallagi, P.; Hegyi, P.; Rakonczay, Z. The physiology and pathophysiology of pancreatic ductal secretion the background for clinicians. Pancreas 2015, 44, 1211–1233. [Google Scholar] [PubMed]
- Saif, M.W.; Larson, H.; Kaley, K.; Shaib, W. Chronic octreotide therapy can induce pancreatic insufficiency: A common but under-recognized adverse effect. Expert Opin. Drug Saf. 2010, 9, 867–873. [Google Scholar] [CrossRef]
- Lamarca, A.; McCallum, L.; Nuttall, C.; Barriuso, J.; Backen, A.; Frizziero, M.; Leon, R.; Mansoor, W.; McNamara, M.G.; Hubner, R.A.; et al. Somatostatin analogue-induced pancreatic exocrine insufficiency in patients with neuroendocrine tumors: Results of a prospective observational study. Expert Rev. Gastroenterol. Hepatol. 2018, 12, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Saif, M.W.; Romano, A.; Smith, M.H.; Patel, R.; Relias, V. Chronic Use of Long-Acting Somatostatin Analogues (SSAs) and Exocrine Pancreatic Insufficiency (EPI) in Patients with Gastroenteropancreatic Neuroendocrine Tumors (GEP-NETs): An Under-recognized Adverse Effect. Cancer Med. J. 2020, 3, 75–84. [Google Scholar]
- Saif, W.M.; Smith, M.H.; Romano, A.; Patel, R.; Relias, V. Pancreatic exocrine insufficiency (PI) secondary to chronic use of long-acting (LA) somatostatin analogues (SA) in patients (pts) with gastroenteropancreatic neuroendocrine tumors (GEP-NETs). J. Clin. Oncol. 2018, 36, 454. Available online: http://ascopubs.org/doi/abs/10.1200/JCO.2018.36.4_suppl.454 (accessed on 2 December 2022).
- Rinzivillo, M.; de Felice, I.; Magi, L.; Annibale, B.; Panzuto, F. Occurrence of exocrine pancreatic insufficiency in patients with advanced neuroendocrine tumors treated with somatostatin analogs. Pancreatology 2020, 20, 875–879. [Google Scholar] [PubMed]
- Panzuto, F.; Magi, L.; Rinzivillo, M. Drug Safety Exocrine pancreatic insufficiency and somatostatin analogs in patients with neuroendocrine neoplasia. Expert Opin. Drug Saf. 2021, 20, 383–386. [Google Scholar] [CrossRef]
- Perbtani, Y.; Forsmark, C.E. Update on the diagnosis and management of exocrine pancreatic insufficiency. F1000Research 2019, 8, 1991. [Google Scholar]
- Carnie, L.E.; Lamarca, A.; McNamara, M.G.; Bibby, N.; O’Reilly, D.A.; Valle, J.W. The assessment of pancreatic exocrine function in patients with inoperable pancreatic cancer: In need of a new gold-standard. Pancreatology 2020, 20, 668–675. [Google Scholar]
- Vanga, R.R.; Tansel, A.; Sidiq, S.; El-Serag, H.B.; Othman, M.O. Diagnostic Performance of Measurement of Fecal Elastase-1 in Detection of Exocrine Pancreatic Insufficiency—Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2019, 16, 1220–1228. [Google Scholar]
- Enrique Domínguez-Muñoz, J.; Nieto, L.; Vilariño, M.; Lourido, M.V.; Iglesias-García, J. Development and Diagnostic Accuracy of a Breath Test for Pancreatic Exocrine Insufficiency in Chronic Pancreatitis. Pancreas 2016, 45, 241–247. [Google Scholar]
- Keller, J.; Hammer, H.F.; Afolabi, P.R.; Benninga, M.; Borrelli, O.; Dominguez-Munoz, E.; Dumitrascu, D.; Goetze, O.; Haas, S.L.; Hauser, B.; et al. European guideline on indications, performance and clinical impact of 13C-breath tests in adult and pediatric patients: An EAGEN, ESNM, and ESPGHAN consensus, supported by EPC. UEG J. 2021, 9, 598–625. [Google Scholar]
- Gharaibeh, A.; Koppikar, S.; Bonilla-Escobar, F.J. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) in the International Journal of Medical Students. Int. J. Med. Stud. 2014, 2, 36–37. [Google Scholar] [CrossRef]
- Kalivianakis, M.; Verkade, H.; Stellaard, F.; van der Werf, M.; Elzinga, H.; Vonk, R. The 13C-mixed triglyceride breath test in healthy adults: Determinants of the 13CO2 response. Eur. J. Clin. Investig. 1997, 27, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Löser, C.; Brauer, C.; Aygen, S.; Hennemann, O.; Fölsch, U. Comparative clinical evaluation of the 13C-mixed triglyceride breath test as an indirect pancreatic function test. Scand. J. Gastroenterol. 1998, 33, 327–334. [Google Scholar] [PubMed]
- Sudeep, K.; Chacko, A.; Thomas, N.; Selvakumar, R.; George, B.; Paul, T.; Seshadri, M.S. Predictors of osteodystrophy in patients with chronic nonalcoholic pancreatitis with or without diabetes. Endocr. Pract. 2011, 17, 897–905. [Google Scholar]
- Stoop, T.F.; Ateeb, Z.; Ghorbani, P.; Scholten, L.; Arnelo, U.; Besselink, M.G.; Del Chiaro, M. Impact of Endocrine and Exocrine Insufficiency on Quality of Life After Total Pancreatectomy. Ann. Surg. Oncol. 2020, 27, 587–596. [Google Scholar] [CrossRef]
- Layer, P.; Kashirskaya, N.; Gubergrits, N. Contribution of pancreatic enzyme replacement therapy to survival and quality of life in patients with pancreatic exocrine insufficiency. World J. Gastroenterol. 2019, 25, 2430–2441. [Google Scholar]
- Fiebrich, H.-B.; Van Den Berg, G.; Kema, I.P.; Links, T.P.; Kleibeuker, J.H.; Van Beek, A.P.; Walenkamp, A.M.E.; Sluiter, W.J.; De Vries, E.G.E. Alimentary Pharmacology and Therapeutics Deficiencies in fat-soluble vitamins in long-term users of somatostatin analogue. Aliment. Pharmacol. Ther. 2010, 32, 1398–1404. [Google Scholar]
- Lind, A.; Wängberg, B.; Ellegård, L. Vitamin D and vitamin B12 deficiencies are common in patients with midgut carcinoid (SI-NET). Eur. J. Clin. Nutr. 2016, 70, 990–994. [Google Scholar] [CrossRef]
- Robbins, H.L.; Symington, M.; Mosterman, B.; Goodby, J.; Davies, L.; Dimitriadis, G.K.; Kaltsas, G.; Randeva, H.S.; Weickert, M.O. Supplementation of Vitamin D Deficiency in Patients with Neuroendocrine Tumors Using Over- the-Counter Vitamin D3 Preparations. Nutr. Cancer 2018, 70, 748–754. [Google Scholar] [CrossRef]
- Massironi, S.; Zilli, A.; Bernasconi, S.; Fanetti, I.; Cavalcoli, F.; Ciafardini, C.; Felicetta, I.; Conte, D. A role for vitamin D in the Gastro-entero-pancreatic Neuroendocrine Neoplasms outcome: Report on a Series from a Single Institute. Neuroendocrinology 2017, 105, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Motylewska, E.; Gawronska, J.; Niedziela, A.; Melen, G.; Lawnicka, H.; Komorowski, J.; Swietoslawski, J.; Stepien, H. Somatostatin Analogs and Tumor Localization Do Not Influence Vitamin D Concentration in Patients with Neuroendocrine Tumors. Nutr. Cancer 2016, 68, 428–434. [Google Scholar] [PubMed]
- Laing, E.; Kiss, N.; Michael, M.; Krishnasamy, M. Nutritional Complications and the Management of Patients with Gastroenteropancreatic Neuroendocrine Tumors. Neuroendocrinology 2020, 110, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Gianotti, L.; Besselink, M.G.; Sandini, M.; Hackert, T.; Conlon, K.; Gerritsen, A.; Griffin, O.; Fingerhut, A.; Probst, P.; Abu Hilal, M.; et al. Nutritional support and therapy in pancreatic surgery: A position paper of the International Study Group on Pancreatic Surgery (ISGPS). Surgery 2018, 164, 1035–1048. [Google Scholar] [CrossRef] [PubMed]
- Keller, J.; Layer, P.; Brückel, S.; Jahr, C.; Rosien, U. 13C-mixed triglyceride breath test for evaluation of pancreatic exocrine function in diabetes mellitus. Pancreas 2014, 43, 842–848. [Google Scholar] [PubMed]
- Radlinger, B.; Ramoser, G.; Kaser, S. Exocrine Pancreatic Insufficiency in Type 1 and Type 2 Diabetes. Curr. Diabetes Rep. 2020, 20, 18. [Google Scholar]
- Patel, K.R.; Nahar, A.; Elhassan, Y.S.; Shetty, S.; Smith, S.; Vickrage, S.; Kemp-Blake, J.; Palani, R.; Geh, I.; Venkataraman, H.; et al. The effects of somatostatin analogues on glycaemia in the treatment of neuroendocrine tumours. J. Neuroendocrinol. 2022, 34, e13064. [Google Scholar]
- Thompson, O.; Hall, L.; Roberts, K.; Powell-Brett, S.; Bradley, E.; Pande, R.; Shah, T. Survival benefit of pancreatic enzyme replacement therapy in patients undergoing treatment of pancreatic neuroendocrine tumours. HPB 2022, 24, 1921–1929. [Google Scholar]
| Patient | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | Patient 9 | Patient 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Age [years] | 63 | 58 | 73 | 72 | 78 | 64 | 84 | 62 | 65 | 76 |
| Sex [M/F] | F | M | M | M | M | M | M | M | F | M |
| Weight [kg] | 83.5 | 100.9 | 72.6 | 107 | 68.8 | 86.1 | 79.2 | 86.4 | 81.1 | 70.6 |
| BMI [kg/m2] | 36.62 | 28.5 | 24 | 34.5 | 23.5 | 27.1 | 26.9 | 28.3 | 31.7 | 24.7 |
| SSA Type | L | L | L | O | O | L | L | L | L | L |
| Tumour | ||||||||||
| Location | Pancreas | Pancreas | Small Bowel | Small Bowel | Small Bowel | Lung | Small Bowel | Small Bowel | Small Bowel | Small Bowel |
| Grade | 1 | 1 | 2 | 1/2 | 1 | 2 | NR | 1 | 1 | 1 |
| Ki-67 [%] | <1% | <2% | 10–15% | <3% | 2% | 5% | NR | <1% | <1% | 3% |
| Functional Status * | NFu | NFu | Fu | Fu | Fu | NFu | Fu | NFu | NFu | Fu |
| Histology | WD | WD | WD | WD | WD | WD | WD | WD | WD | WD |
| Baseline | 2 Months (3 Injections) | Change (Median, IQR) | p-Value * | ||
|---|---|---|---|---|---|
| Quantitative Assessment of Function | 13C-MTG Breath Test (% cPDR) | 53.1 (44.6–58.9) | 38.7 (35.0–45.0) | −23.4% (−42.1–0.5) | 0.005 |
| FE-1 (ug/g) | 450 (158.8–500) | 500 (428–500) | 0% (−59.5–222.6) | 0.345 | |
| Qualitative Assessment of Function | PEI-Q (Total Symptom Score) | 1.4 (0.7–1.7) | 1.1 (0.5–1.4) | −31.5% (−56.8–−1.9) | 0.005 |
| Nutritional Markers | Vitamin D (nmol/L) | 57.1 (24.6–66.5) | 47.6 (28.9–56.7) | −26.5% (−44.7–10) | 0.038 |
| HbA1C (mmol/L) | 40 (37.5–50.8) | 42 (38.5–72.8) | 8.0% (0–59.3) | 0.008 | |
| Weight (kg) | 82.3 (74.3–86.3) | 82.6 (74.3–87.8) | −0.21% (−4.5–3.5) | 1 | |
| BMI (kg/m2) | 27.7 (25.3–30.9) | 27.2 (25.3–32.2) | 0.27% (−5.2–5.4) | 0.722 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hall, L.A.; Powell-Brett, S.; Thompson, O.; Smith, D.; Bradley, E.; Smith, S.; Vickrage, S.; Kemp-Blake, J.; Roberts, K.J.; Shah, T. Casting a Wider NET: Pancreatic Exocrine Insufficiency Induced by Somatostatin Analogues among Patients with Neuroendocrine Tumours? Cancers 2023, 15, 1933. https://doi.org/10.3390/cancers15071933
Hall LA, Powell-Brett S, Thompson O, Smith D, Bradley E, Smith S, Vickrage S, Kemp-Blake J, Roberts KJ, Shah T. Casting a Wider NET: Pancreatic Exocrine Insufficiency Induced by Somatostatin Analogues among Patients with Neuroendocrine Tumours? Cancers. 2023; 15(7):1933. https://doi.org/10.3390/cancers15071933
Chicago/Turabian StyleHall, Lewis A., Sarah Powell-Brett, Oscar Thompson, Daniel Smith, Elizabeth Bradley, Stacey Smith, Suzanne Vickrage, Joanne Kemp-Blake, Keith J. Roberts, and Tahir Shah. 2023. "Casting a Wider NET: Pancreatic Exocrine Insufficiency Induced by Somatostatin Analogues among Patients with Neuroendocrine Tumours?" Cancers 15, no. 7: 1933. https://doi.org/10.3390/cancers15071933
APA StyleHall, L. A., Powell-Brett, S., Thompson, O., Smith, D., Bradley, E., Smith, S., Vickrage, S., Kemp-Blake, J., Roberts, K. J., & Shah, T. (2023). Casting a Wider NET: Pancreatic Exocrine Insufficiency Induced by Somatostatin Analogues among Patients with Neuroendocrine Tumours? Cancers, 15(7), 1933. https://doi.org/10.3390/cancers15071933







