Sex Differences in Survival from Neuroendocrine Neoplasia in England 2012–2018: A Retrospective, Population-Based Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Data Source
2.2. NEN Classification and Analytic Process
2.3. Statistical Analytic Approach
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cives, M.; Pelle’, E.; Strosberg, J. Emerging Treatment Options for Gastroenteropancreatic Neuroendocrine Tumors. J. Clin. Med. 2020, 9, 3655. [Google Scholar] [CrossRef] [PubMed]
- Caplin, M.E.; Baudin, E.; Ferolla, P.; Filosso, P.; Garcia-Yuste, M.; Lim, E.; Oberg, K.; Pelosi, G.; Perren, A.; Rossi, R.E.; et al. Pulmonary neuroendocrine (carcinoid) tumors: European Neuroendocrine Tumor Society expert consensus and recommendations for best practice for typical and atypical pulmonary carcinoids. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2015, 26, 1604–1620. [Google Scholar] [CrossRef] [PubMed]
- Clement, D.; Ramage, J.; Srirajaskanthan, R. Update on Pathophysiology, Treatment, and Complications of Carcinoid Syndrome. J. Oncol. 2020, 2020, 8341426. [Google Scholar] [CrossRef]
- Das, S.; Dasari, A. Epidemiology, Incidence, and Prevalence of Neuroendocrine Neoplasms: Are There Global Differences? Curr. Oncol. Rep. 2021, 23, 43. [Google Scholar] [CrossRef]
- Hassan, M.M.; Phan, A.; Li, D.; Dagohoy, C.G.; Leary, C.; Yao, J.C. Risk factors associated with neuroendocrine tumors: A U.S.-based case-control study. Int. J. Cancer 2008, 123, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Haugvik, S.-P.; Hedenström, P.; Korsæth, E.; Valente, R.; Hayes, A.; Siuka, D.; Maisonneuve, P.; Gladhaug, I.P.; Lindkvist, B.; Capurso, G. Diabetes, Smoking, Alcohol Use, and Family History of Cancer as Risk Factors for Pancreatic Neuroendocrine Tumors: A Systematic Review and Meta-Analysis. Neuroendocrinology 2015, 101, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Feola, T.; Puliani, G.; Sesti, F.; Modica, R.; Centello, R.; Minotta, R.; Cannavale, G.; Di Meglio, S.; Di Vito, V.; Lauretta, R.; et al. Risk factors for gastroenteropancreatic neuroendocrine neoplasms (GEP-NENs): A three-centric case–control study. J. Endocrinol. Investig. 2022, 45, 849–857. [Google Scholar] [CrossRef] [PubMed]
- White, B.E.; Rous, B.; Chandrakumaran, K.; Wong, K.; Bouvier, C.; Van Hemelrijck, M.; George, G.; Russell, B.; Srirajaskanthan, R.; Ramage, J.K. Incidence and survival of neuroendo-crine neoplasia in England 1995–2018: A retrospective, population-based study. Lancet Reg. Health-Eur. 2022, 23, 100510. [Google Scholar] [CrossRef]
- Man, D.; Wu, J.; Shen, Z.; Zhu, X. Prognosis of patients with neuroendocrine tumor: A SEER database analysis. Cancer Manag. Res. 2018, 10, 5629–5638. [Google Scholar] [CrossRef]
- Abdel-Rahman, O.; Fazio, N. Sex-Based Differences in Prognosis of Patients With Gastroenteropancreatic-Neuroendocrine Neoplasms: A Population-Based Study. Pancreas 2021, 50, 727–731. [Google Scholar] [CrossRef] [PubMed]
- Hallet, J.; Law, C.H.L.; Cukier, M.; Saskin, R.; Liu, N.; Singh, S. Exploring the rising incidence of neuroendocrine tumors: A population-based analysis of epidemiology, metastatic presentation, and outcomes. Cancer 2015, 121, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Boyar Cetinkaya, R.; Aagnes, B.; Myklebust, T.Å.; Thiis-Evensen, E. Survival in neuroendocrine neoplasms; A report from a large Norwegian population-based study. Int. J. Cancer 2018, 142, 1139–1147. [Google Scholar] [CrossRef] [PubMed]
- Genus, T.S.E.; Bouvier, C.; Wong, K.F.; Srirajaskanthan, R.; Rous, B.A.; Talbot, D.C.; Valle, J.W.; Khan, M.; Pearce, N.; Elshafie, M.; et al. Impact of neuroendocrine morphology on cancer outcomes and stage at diagnosis: A UK nationwide cohort study 2013–2015. Br. J. Cancer 2019, 121, 966–972. [Google Scholar] [CrossRef]
- Wyld, D.; Wan, M.H.; Moore, J.; Dunn, N.; Youl, P. Epidemiological trends of neuroendocrine tumours over three decades in Queensland, Australia. Cancer Epidemiol. 2019, 63, 101598. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.S.; Chen, L.-T.; Shan, Y.-S.; Chu, P.-Y.; Tsai, C.-R.; Tsai, H.-J. An updated analysis of the epidemiologic trends of neuroendocrine tumors in Taiwan. Sci. Rep. 2021, 11, 7881. [Google Scholar] [CrossRef] [PubMed]
- Mauvais-Jarvis, F.; Merz, N.B.; Barnes, P.J.; Brinton, R.D.; Carrero, J.J.; DeMeo, D.L.; De Vries, G.J.; Epperson, C.N.; Govindan, R.; Klein, S.L.; et al. Sex and gender: Modifiers of health, disease, and medicine. Lancet 2020, 396, 565–582. [Google Scholar] [CrossRef]
- Claessens, F.; Tilley, W. Androgen signalling and steroid receptor crosstalk in endocrine cancers. Endocr.-Relat. Cancer 2014, 21, E3–E5. [Google Scholar] [CrossRef] [PubMed]
- Clocchiatti, A.; Cora, E.; Zhang, Y.; Dotto, G.P. Sexual dimorphism in cancer. Nat. Rev. Cancer 2016, 16, 330–339. [Google Scholar] [CrossRef]
- McCartney, G.; Mahmood, L.; Leyland, A.; Batty, G.D.; Hunt, K. Contribution of smoking-related and alcohol-related deaths to the gender gap in mortality: Evidence from 30 European countries. Tob. Control. 2011, 20, 166–168. [Google Scholar] [CrossRef]
- Wagner, A.; Oertelt-Prigione, S.; Adjei, A.; Buclin, T.; Cristina, V.; Csajka, C.; Coukos, G.; Dafni, U.; Dotto, G.-P.; Ducreux, M.; et al. Gender medicine and oncology: Report and consensus of an ESMO workshop. Ann. Oncol. 2019, 30, 1914–1924. [Google Scholar] [CrossRef]
- Li, Y.; Xu, A.; Jia, S.; Huang, J. Recent advances in the molecular mechanism of sex disparity in hepatocellular carcinoma. Oncol. Lett. 2019, 17, 4222–4228. [Google Scholar] [CrossRef]
- Estrella, J.S.; Ma, L.T.; Milton, D.R.; Yao, J.C.; Wang, H.; Rashid, A.; Broaddus, R.R. Expression of estrogen-induced genes and estrogen receptor β in pancreatic neuroendocrine tumors: Implications for targeted therapy. Pancreas 2014, 43, 996–1002. [Google Scholar] [CrossRef]
- Blažević, A.; Zandee, W.; Franssen, G.J.H.; Hofland, J.; van Velthuysen, M.-L.; Hofland, L.J.; Feelders, R.A.; De Herder, W.W. Mesenteric fibrosis and palliative surgery in small intestinal neuroendocrine tumours. Endocr.-Relat. Cancer 2018, 25, 245–254. [Google Scholar] [CrossRef]
- Blažević, A.; Brabander, T.; Zandee, W.; Hofland, J.; Franssen, G.; van Velthuysen, M.-L.; Feelders, R.; De Herder, W. Evolution of the Mesenteric Mass in Small Intestinal Neuroendocrine Tumours. Cancers 2021, 13, 443. [Google Scholar] [CrossRef] [PubMed]
- Blažević, A.; Iyer, A.M.; van Velthuysen, M.-L.F.; Hofland, J.; Oudijk, L.; de Herder, W.W.; Hofland, L.J.; Feelders, R.A. Sexual Dimorphism in Small-intestinal Neuroendocrine Tumors: Lower Prevalence of Mesenteric Disease in Premenopausal Women. J. Clin. Endocrinol. Metab. 2022, 107, e1969–e1975. [Google Scholar] [CrossRef] [PubMed]
- Arnason, T.; Sapp, H.L.; Barnes, P.J.; Drewniak, M.; Abdolell, M.; Rayson, D. Immunohistochemical expression and prognostic value of ER, PR and HER2/neu in pancreatic and small intestinal neuroendocrine tumors. Neuroendocrinology 2011, 93, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Estrella, J.S.; Broaddus, R.R.; Mathews, A.; Milton, D.R.; Yao, J.C.; Wang, H.; Rashid, A. Progesterone Receptor and PTEN Expression Predict Survival in Patients With Low- and Intermediate-Grade Pancreatic Neuroendocrine Tumors. Arch. Pathol. Lab. Med. 2014, 138, 1027–1036. [Google Scholar] [CrossRef]
- Daskalakis, K.; Kaltsas, G.; Öberg, K.; Tsolakis, A.V. Lung Carcinoids: Long-Term Surgical Results and the Lack of Prognostic Value of Somatostatin Receptors and Other Novel Immunohistochemical Markers. Neuroendocrinology 2018, 107, 355–365. [Google Scholar] [CrossRef]
- Barros, M.; Felismino, T.; de Jesus, V.; Mello, C.; Silva, V.; Camandaroba, M.; Rodrigues, N.; Donadio, M.; Nobrega, E.; Chinen, L.; et al. HORMONET: Study of tamoxifen in well differentiated neuroendocrine tumours and hormone receptor positive expression. Ann. Oncol. 2019, 30, v573. [Google Scholar] [CrossRef]
- Henson, K.E.; Elliss-Brookes, L.; Coupland, V.H.; Payne, E.; Vernon, S.; Rous, B.; Rashbass, J. Data Resource Profile: National Cancer Registration Dataset in England. Int. J. Epidemiol. 2020, 49, 16–16h. [Google Scholar] [CrossRef]
- NCIN. Collecting and Using Data. Available online: http://www.ncin.org.uk/collecting_and_using_data/ (accessed on 24 January 2022).
- Rindi, G.; Klöppel, G.; Alhman, H.; Caplin, M.; Couvelard, A.; de Herder, W.W.; Erikssson, B.; Falchetti, A.; Falconi, M.; Komminoth, P.; et al. TNM staging of foregut (neuro)endocrine tumors: A consensus proposal including a grading system. Virchows Arch. 2006, 449, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Rindi, G.; Klöppel, G.; Couvelard, A.; Komminoth, P.; Körner, M.; Lopes, J.M.; McNicol, A.-M.; Nilsson, O.; Perren, A.; Scarpa, A.; et al. TNM staging of midgut and hindgut (neuro) endocrine tumors: A consensus proposal including a grading system. Virchows Arch. 2007, 451, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Brierley, J.D.; Gospodarowicz, M.K.; Wittekind, C. (Eds.) TNM Classification of Malignant Tumours, 8th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Jack, A.; Percy, C.L.; Sobin, L.; Whelan, S. International Classification of Diseases for Oncology (ICD-O), 3rd ed.; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Nagtegaal, I.D.; Odze, R.D.; Klimstra, D.; Paradis, V.; Rugge, M.; Schirmacher, P.; Washington, K.M.; Carneiro, F.; Cree, I.A.; the WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020, 76, 182–188. [Google Scholar] [CrossRef] [PubMed]
- NCIN. National Cancer Intelligence Network: Cancer Survival in England by Stage. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/347275/Cancer_survival_in_England_by_stage_report_.pdf (accessed on 24 January 2022).
- Berrino, F. The EUROCARE Study: Strengths, limitations and perspectives of population-based, comparative survival studies. Ann. Oncol. 2003, 14, v9–v13. [Google Scholar] [CrossRef] [PubMed]
- Hayati Rezvan, P.; Lee, K.J.; Simpson, J.A. The rise of multiple imputation: A review of the reporting and implementation of the method in medical research Data collection, quality, and reporting. BMC Med. Res. Methodol. 2015, 15, 30. [Google Scholar] [CrossRef]
- Dasari, A.; Shen, C.; Halperin, D.M.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar] [CrossRef]
- Rindi, G.; Klimstra, D.S.; Abedi-Ardekani, B.; Asa, S.L.; Bosman, F.T.; Brambilla, E.; Busam, K.J.; De Krijger, R.R.; Dietel, M.; El-Naggar, A.K.; et al. A common classification framework for neuroendocrine neoplasms: An International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Mod. Pathol. 2018, 31, 1770–1786. [Google Scholar] [CrossRef] [PubMed]
- Han, K.; Jung, I. Restricted Mean Survival Time for Survival Analysis: A Quick Guide for Clinical Researchers. Korean J. Radiol. 2022, 23, 495–499. [Google Scholar] [CrossRef]
- Discacciati, A.; Bellavia, A.; Lee, J.J.; Mazumdar, M.; Valeri, L. Med4way: A Stata command to investigate mediating and interactive mechanisms using the four-way effect decomposition. Int. J. Epidemiol. 2018, 48, 15–20. [Google Scholar] [CrossRef]
- Padmanabhan, S.; Carty, L.; Cameron, E.; Ghosh, R.E.; Williams, R.; Strongman, H. Approach to record linkage of primary care data from Clinical Practice Research Datalink to other health-related patient data: Overview and implications. Eur. J. Epidemiol. 2019, 34, 91–99. [Google Scholar] [CrossRef]
- Luong, T.V.; Watkins, J.; Chakrabarty, B.; Wang, L.M. Standards and Datasets for Reporting Cancers Dataset for Histopathological Reporting of Neuroendocrine Neoplasms of the Gastroenteropancreatic Tract DRAFT. 2019. Available online: https://www.acpgbi.org.uk/_userfiles/import/2019/05/Dataset-for-histopathological-reporting-of-neuroendocrine-neoplasms-of-the-gastroenteropancreatic-tract-For-Consultation-002.pdf (accessed on 4 November 2022).
- Niu, Y.; Guo, C.; Wen, S.; Tian, J.; Luo, J.; Wang, K.; Tian, H.; Yeh, S.; Chang, C. ADT with antiandrogens in prostate cancer induces adverse effect of in-creasing resistance, neuroendocrine differentiation and tumor metastasis. Cancer Lett. 2018, 439, 47–55. [Google Scholar] [CrossRef] [PubMed]
| Total | 14,834 | ||
|---|---|---|---|
| Age (Median, IQR) | 65 (53–73) | ||
| n | % | ||
| Age group | 0–30 | 909 | 6.1% |
| 31–54 | 3141 | 21.2% | |
| 55–64 | 3117 | 21.0% | |
| 65–74 | 4450 | 30.0% | |
| 75+ | 3217 | 21.7% | |
| Ethnicity | Asian | 425 | 2.9% |
| Black | 338 | 2.3% | |
| Mixed race | 64 | 0.4% | |
| Other | 192 | 1.3% | |
| White | 13,197 | 89.0% | |
| Not stated | 618 | 4.2% | |
| Site | Appendix | 2146 | 14.5% |
| Caecum | 528 | 3.6% | |
| Colon | 509 | 3.4% | |
| Lung | 4661 | 31.4% | |
| Pancreas | 2183 | 14.7% | |
| Rectum | 948 | 6.4% | |
| Small intestine | 3201 | 21.6% | |
| Stomach | 658 | 4.4% | |
| Stage | I | 5040 | 34.0% |
| II | 2004 | 13.5% | |
| III | 2669 | 18.0% | |
| IV | 5121 | 34.5% | |
| Morphology | NET | 11,080 | 74.7% |
| NEC | 3754 | 25.3% | |
| Deprivation quintile | 1—least deprived | 3048 | 20.5% |
| 2 | 3194 | 21.5% | |
| 3 | 3148 | 21.2% | |
| 4 | 2722 | 18.3% | |
| 5—most deprived | 2722 | 18.3% | |
| Age Group | Ethnicity | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total 14,834 | Median Age (IQR) | 0–30 | 31–54 | 55–64 | 65–74 | 75+ | Asian | Black | Mixed Race | Other | White | Not Stated | |
| Male | 7196 | 65.5 (54–73) | 355 | 1447 | 1562 | 2257 | 1575 | 218 | 145 | 30 | 101 | 6389 | 313 |
| 48.5% | 39.1% | 46.1% | 50.1% | 50.7% | 49.0% | 51.3% | 42.9% | 46.9% | 52.6% | 48.4% | 50.6% | ||
| Female | 7638 | 65 (51–73) | 554 | 1694 | 1555 | 2193 | 1642 | 207 | 193 | 34 | 91 | 6808 | 305 |
| 51.5% | 60.9% | 53.9% | 49.9% | 49.3% | 51.0% | 48.7% | 57.1% | 53.1% | 47.4% | 51.6% | 49.4% | ||
| Site | Stage | ||||||||||||
| Appendix | Caecum | Colon | Lung | Pancreas | Rectum | Small Intestine | Stomach | I | II | III | IV | ||
| Male | 831 | 228 | 298 | 1854 | 1230 | 544 | 1804 | 407 | 1996 | 929 | 1425 | 2846 | |
| 38.7% | 43.2% | 58.5% | 39.8% | 56.3% | 57.4% | 56.4% | 61.9% | 39.6% | 46.4% | 53.4% | 55.6% | ||
| Female | 1315 | 300 | 211 | 2807 | 953 | 404 | 1397 | 251 | 3044 | 1075 | 1244 | 2275 | |
| 61.3% | 56.8% | 41.5% | 60.2% | 43.7% | 42.6% | 43.6% | 38.1% | 60.4% | 53.6% | 46.6% | 44.4% | ||
| Deprivation Quintile | Morphology | ||||||||||||
| 1—least deprived | 2 | 3 | 4 | 5—most deprived | NET | NEC | |||||||
| Male | 1524 | 1547 | 1488 | 1354 | 1283 | 5093 | 2103 | ||||||
| 50.0% | 48.4% | 47.3% | 49.7% | 47.1% | 46.0% | 56.0% | |||||||
| Female | 1524 | 1647 | 1660 | 1368 | 1439 | 5987 | 1651 | ||||||
| 50.0% | 51.6% | 52.7% | 50.3% | 52.9% | 54.0% | 44.0% | |||||||
| Multivariable Survival Analysis | Analysis of Sex Difference in Survival | ||||
|---|---|---|---|---|---|
| 5-year RMST | Age-Adjusted Female Survival Advantage | ||||
| Variable | HR (95%CI) | Sex | Months (95% CI) | Months (95% CI), p-Value | |
| Site | Appendix | Reference | M | 4.64 (4.56–4.72) | 0.95 (−0.18 to 2.10), p = 0.098 |
| p = 0.041 | F | 4.76 (4.71–4.82) | |||
| Caecum | 1.01 (0.9–1.11) | M | 3.11 (2.83–3.39) | 2.77 (−1.61 to 7.15), p = 0.215 | |
| F | 3.30 (3.07–3.54) | ||||
| Colon | 1.14 (1.04–1.25) | M | 2.22 (1.98–2.47) | −2.44(−6.90 to 2.04), p = 0.287 | |
| F | 1.99 (1.71–2.28) | ||||
| Lung | 1.22 (1.13–1.31) | M | 2.96 (2.86–3.06) | 9.85 (8.40 to 11.30), p < 0.001 | |
| F | 3.76 (3.69–3.83) | ||||
| Pancreas | 1.18 (1.09–1.28) | M | 3.16 (3.05–3.28) | 3.62 (1.73 to 5.90), p < 0.001 | |
| F | 3.52 (3.39–3.65) | ||||
| Rectum | 1.27 (1.15–1.41) | M | 3.24 (3.05–3.42) | 5.68 (2.38 to 8.96), p < 0.001 | |
| F | 3.75 (3.55–3.94) | ||||
| Small intestine | 0.94 (0.87–1.02) | M | 4.01 (3.93–4.09) | 1.31 (−0.16 to 2.75), p = 0.081 | |
| F | 4.09 (4.00–4.18) | ||||
| Stomach | 1.26 (1.14–1.33) | M | 2.18 (1.97–2.38) | 10.26 (6.6 to 14.45), p < 0.001 | |
| F | 3.22 (2.95–3.49) | ||||
| Stage | I | Reference | M | 4.66 (4.62–4.71) | 1.2 (0.48 to 1.92), p < 0.001 |
| p < 0.001 | F | 4.74 (4.71–4.78) | |||
| II | 1.38 (1.29–1.46) | M | 4.60 (4.53–4.67) | 3.24 (1.76 to 4.72), p < 0.001 | |
| F | 4.25 (0.05–4.35) | ||||
| III | 1.58 (1.49–1.68) | M | 3.86 (3.77–3.95) | 2.23 (0.58 to 3.89), p = 0.008 | |
| F | 4.07 (3.97–4.16) | ||||
| IV | 2.11 (2.01–2.23) | M | 2.02 (1.94–2.10) | 3.19 (1.84 to 4.54), p < 0.001 | |
| F | 2.28 (2.19–2.37) | ||||
| Morphology | NET | Reference | M | 4.20 (4.15– 4.24) | 2.44 (1.74 to 3.13), p < 0.001 |
| p < 0.001 | F | 4.43 (4.39–4.46) | |||
| NEC | 1.29 (1.25–1.33) | M | 1.52 (1.45–1.60) | 4.92 (3.46 to 6.37), p < 0.001 | |
| F | 1.94 (1.85–2.04) | ||||
| Deprivation | 1—least deprived | Reference | M | 3.53 (3.43–3.64) | 5.14 (3.49 to 6.78), p < 0.001 |
| p < 0.001 | F | 4.02 (3.93–4.11) | |||
| 2 | 1.11 (1.02–1.21) | M | 3.36 (3.26–3.46) | 5.68 (4.00 to 7.36), p < 0.001 | |
| F | 3.90 (3.81–3.99) | ||||
| 3 | 1.09 (1.02–1.19) | M | 3.46 (3.35–3.56) | 5.16 (3.48 to 6.84), p < 0.001 | |
| F | 3.91 (3.82–4.00) | ||||
| 4 | 1.21 (1.11–1.33) | M | 3.34 (3.23–3.46) | 4.95 (3.32 to 9.98), p < 0.001 | |
| F | 3.87 (3.77–3.97) | ||||
| 5—most deprived | 1.32 (1.22–1.45) | M | 3.32 (3.20–3.43) | 4.45 (2.58 to 6.31), p < 0.001 | |
| F | 3.68 (3.58–3.78) | ||||
| Four-Way Decomposition | |||||||||
|---|---|---|---|---|---|---|---|---|---|
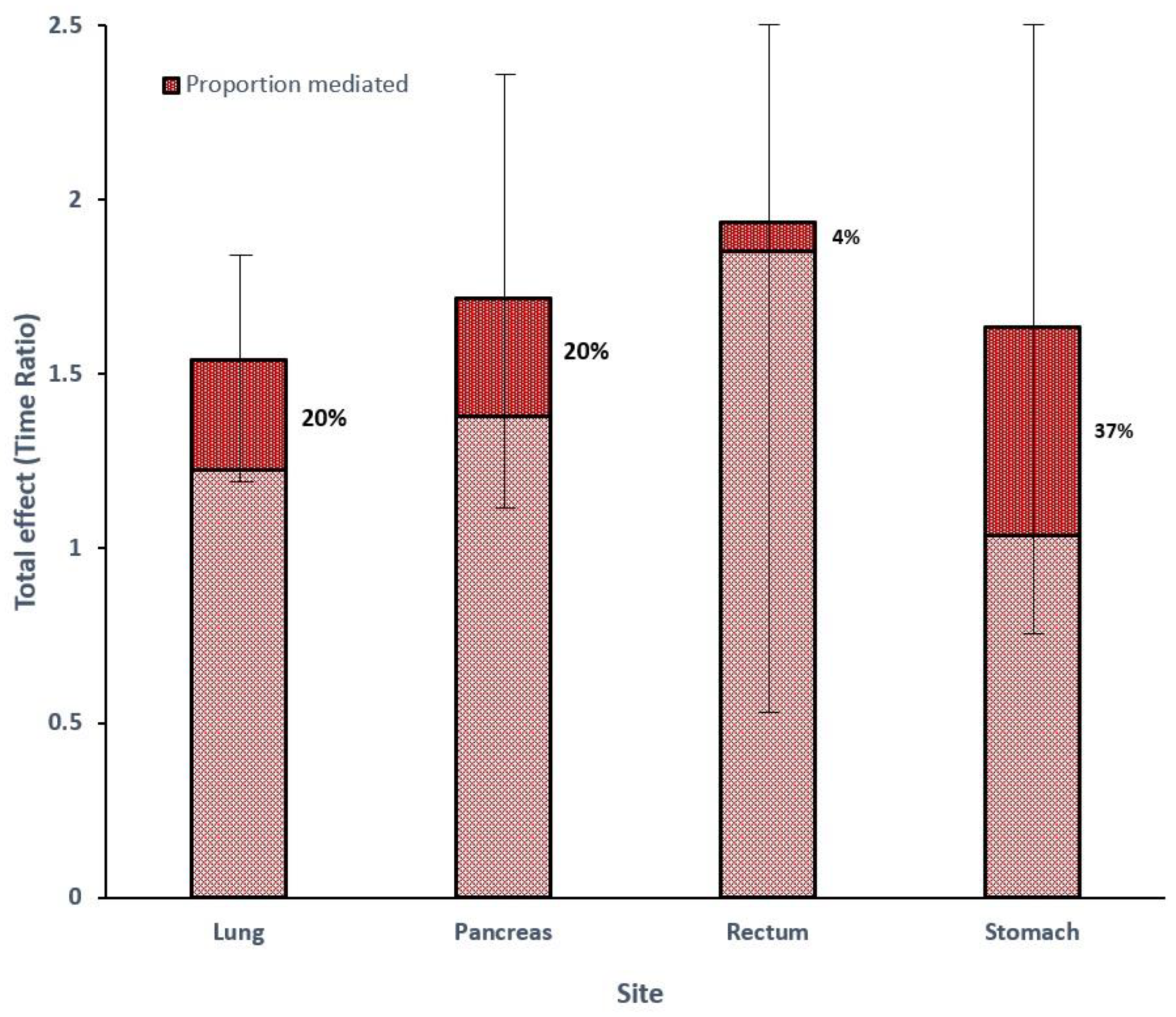
| Site | n | %F | Model for Outcome (TR) (95% CI) | Model for Mediator (OR) (95% CI) | Total Effect (TR) (95% CI) | CDE% | INT% | IE% | MED% |
| Lung | 4661 | 60 | 1.43 (1.16–1.76) | 0.61 (0.53–0.71) | 1.54 (1.22–1.86) | 97% | −12% | 14% | 20% |
| Pancreas | 2183 | 44 | 1.70 (1.16–2.50) | 0.81 (0.67–0.97) | 1.72 (1.13–2.30) | 170% | −80% | 10% | 20% |
| Rectum | 948 | 43 | 1.91 (0.93–3.91) | 0.78 (0.52–1.19) | 1.94 (0.55–3.32) | 107% | −9% | 2% | 4% |
| Stomach | 658 | 38 | 1.50 (0.88–2.56) | 0.48 (0.32–0.73) | 1.64 (0.82–2.45) | 103% | −24% | 20% | 37% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
White, B.E.; Russell, B.; Remmers, S.; Rous, B.; Chandrakumaran, K.; Wong, K.F.; Van Hemelrijck, M.; Srirajaskanthan, R.; Ramage, J.K. Sex Differences in Survival from Neuroendocrine Neoplasia in England 2012–2018: A Retrospective, Population-Based Study. Cancers 2023, 15, 1863. https://doi.org/10.3390/cancers15061863
White BE, Russell B, Remmers S, Rous B, Chandrakumaran K, Wong KF, Van Hemelrijck M, Srirajaskanthan R, Ramage JK. Sex Differences in Survival from Neuroendocrine Neoplasia in England 2012–2018: A Retrospective, Population-Based Study. Cancers. 2023; 15(6):1863. https://doi.org/10.3390/cancers15061863
Chicago/Turabian StyleWhite, Benjamin E., Beth Russell, Sebastiaan Remmers, Brian Rous, Kandiah Chandrakumaran, Kwok F. Wong, Mieke Van Hemelrijck, Rajaventhan Srirajaskanthan, and John K. Ramage. 2023. "Sex Differences in Survival from Neuroendocrine Neoplasia in England 2012–2018: A Retrospective, Population-Based Study" Cancers 15, no. 6: 1863. https://doi.org/10.3390/cancers15061863
APA StyleWhite, B. E., Russell, B., Remmers, S., Rous, B., Chandrakumaran, K., Wong, K. F., Van Hemelrijck, M., Srirajaskanthan, R., & Ramage, J. K. (2023). Sex Differences in Survival from Neuroendocrine Neoplasia in England 2012–2018: A Retrospective, Population-Based Study. Cancers, 15(6), 1863. https://doi.org/10.3390/cancers15061863






