Selection of Chemotherapy in Advanced Poorly Differentiated Extra-Pulmonary Neuroendocrine Carcinoma
Abstract
:Simple Summary
Abstract
1. Introduction
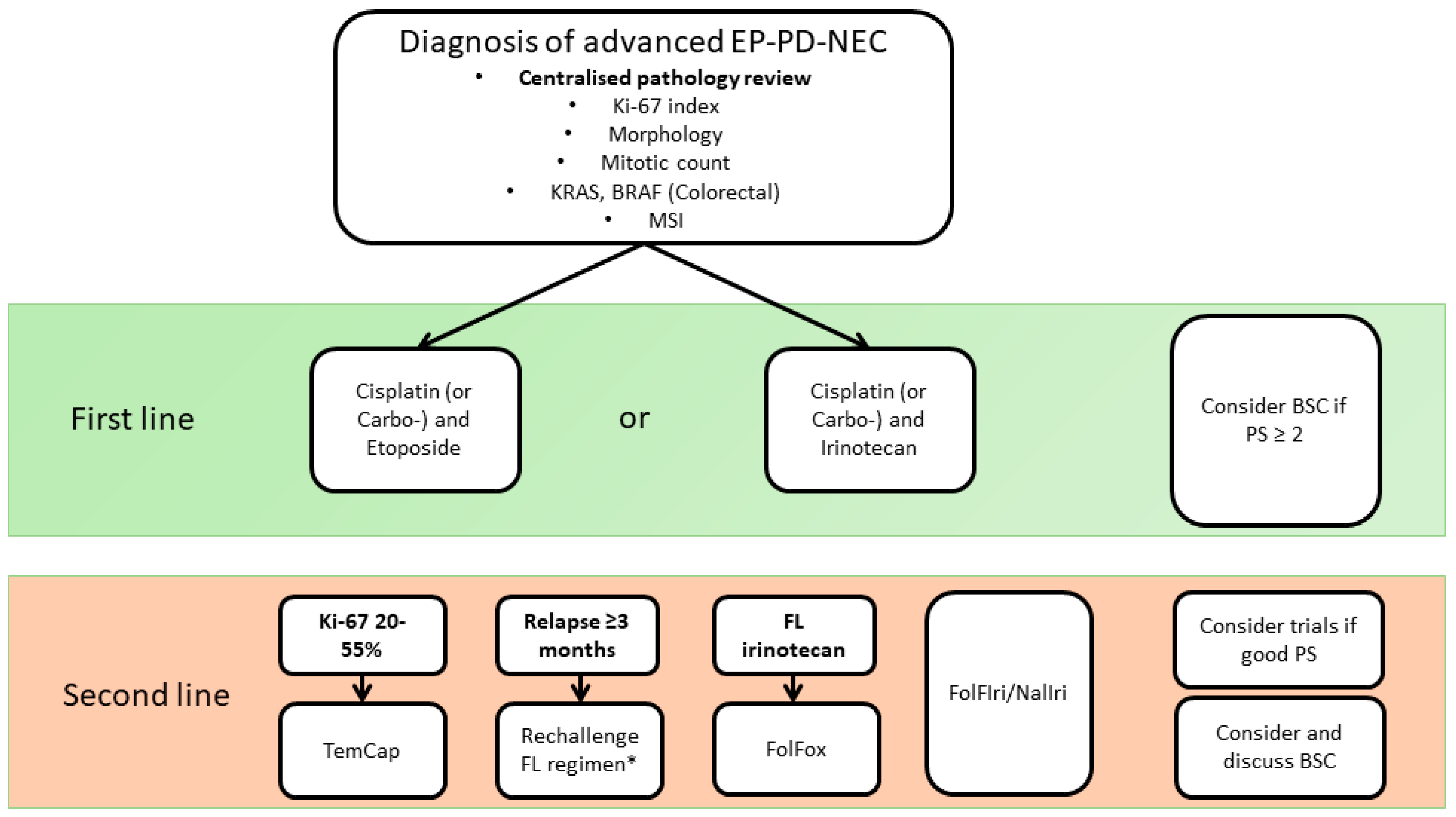
2. First-Line Systemic Therapy Options for Patients with Advanced EP-PD-NEC
3. Second-Line Treatment for EP-PD-NEC in the Advanced Setting
3.1. Re-Challenge in the Second-Line Setting
3.2. Single-Agent Chemotherapy in the Second-Line Setting
3.3. Combination Chemotherapy Regimen in the Second-Line Setting
4. Discussion
Improving on and Moving beyond Current Chemotherapy Regimens in Patients with EP-PD-NEC
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nagtegaal, I.D.; Odze, R.D.; Klimstra, D.; Paradis, V.; Rugge, M.; Schirmacher, P.; Washington, K.M.; Carneiro, F.; Cree, I.A.; the WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020, 76, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Dasari, A.; Mehta, K.; Byers, L.A.; Sorbye, H.; Yao, J.C. Comparative study of lung and extrapulmonary poorly differentiated neuroendocrine carcinomas: A SEER database analysis of 162,983 cases. Cancer 2018, 124, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Morizane, C.; Machida, N.; Honma, Y.; Okusaka, T.; Boku, N.; Kato, K.; Nomura, S.; Hiraoka, N.; Sekine, S.; Taniguchi, H.; et al. Effectiveness of Etoposide and Cisplatin vs Irinotecan and Cisplatin Therapy for Patients with Advanced Neuroendocrine Carcinoma of the Digestive System: The TOPIC-NEC Phase 3 Randomized Clinical Trial. JAMA Oncol. 2022, 8, 1447–1455. [Google Scholar] [CrossRef] [PubMed]
- Frizziero, M.; Kilgour, E.; Simpson, K.L.; Rothwell, D.G.; Moore, D.A.; Frese, K.K.; Galvin, M.; Lamarca, A.; Hubner, R.A.; Valle, J.W.; et al. Expanding Therapeutic Opportunities for Extrapulmonary Neuroendocrine Carcinoma. Clin. Cancer Res. 2022, 28, 1999–2019. [Google Scholar] [CrossRef] [PubMed]
- Van Riet, J.; van de Werken, H.J.G.; Cuppen, E.; Eskens, F.A.L.M.; Tesselaar, M.; van Veenendaal, L.M.; Klümpen, H.-J.; Dercksen, M.W.; Valk, G.D.; Lolkema, M.P.; et al. The genomic landscape of 85 advanced neuroendocrine neoplasms reveals subtype-heterogeneity and potential therapeutic targets. Nat. Commun. 2021, 12, 4612. [Google Scholar] [CrossRef] [PubMed]
- Hijioka, S.; Hosoda, W.; Matsuo, K.; Ueno, M.; Furukawa, M.; Yoshitomi, H.; Kobayashi, N.; Ikeda, M.; Ito, T.; Nakamori, S.; et al. Rb Loss and KRAS Mutation Are Predictors of the Response to Platinum-Based Chemotherapy in Pancreatic Neuroendocrine Neoplasm with Grade 3: A Japanese Multicenter Pancreatic NEN-G3 Study. Clin. Cancer Res. 2017, 23, 4625–4632. [Google Scholar] [CrossRef] [PubMed]
- Yachida, S.; Vakiani, E.; White, C.M.B.; Zhong, Y.; Saunders, T.H.; Morgan, R.; de Wilde, R.F.; Maitra, A.M.; Hicks, J.B.; DeMarzo, A.M.; et al. Small cell and large cell neuroendocrine carcinomas of the pancreas are genetically similar and distinct from well-differentiated pancreatic neuroendocrine tumors. Am. J. Surg. Pathol. 2012, 36, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Frizziero, M.; Durand, A.; Taboada, R.G.; Zaninotto, E.; Luchini, C.; Chakrabarty, B.; Hervieu, V.; Claro, L.C.; Zhou, C.; Cingarlini, S.; et al. Is the Morphological Subtype of Extra-Pulmonary Neuroendocrine Carcinoma Clinically Relevant? Cancers 2021, 13, 4152. [Google Scholar] [CrossRef] [PubMed]
- Vélayoudom-Céphise, F.L.; Duvillard, P.; Foucan, L.; Hadoux, J.; Chougnet, C.N.; Leboulleux, S.; Malka, D.; Guigay, J.; Goere, D.; Debaere, T.; et al. Are G3 ENETS neuroendocrine neoplasms heterogeneous? Endocr. Relat. Cancer 2013, 20, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Sorbye, H.; Welin, S.; Langer, S.W.; Vestermark, L.W.; Holt, N.; Osterlund, P.; Dueland, S.; Hofsli, E.; Guren, M.G.; Ohrling, K.; et al. Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal neuroendocrine carcinoma (WHO G3): The NORDIC NEC study. Ann. Oncol. 2013, 24, 152–160. [Google Scholar] [CrossRef]
- McNamara, M.G.; Frizziero, M.; Jacobs, T.; Lamarca, A.; Hubner, R.A.; Valle, J.W.; Amir, E. Second-line treatment in patients with advanced extra-pulmonary poorly differentiated neuroendocrine carcinoma: A systematic review and meta-analysis. Ther. Adv. Med. Oncol. 2020, 12, 1758835920915299. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Hijioka, S.; Hosoda, W.; Ueno, M.; Kobayashi, N.; Ikeda, M.; Ito, T.; Kodama, Y.; Morizane, C.; Notohara, K.; et al. Pancreatic neuroendocrine carcinoma G3 may be heterogeneous and could be classified into two distinct groups. Pancreatology 2020, 20, 1421–1427. [Google Scholar] [CrossRef] [PubMed]
- McNamara, M.G.; Swain, J.; Craig, Z.; Sharma, R.; Faluyi, O.O.; Wadsley, J.; Morgan, C.; Wall, L.R.; Chau, I.; Reed, N.; et al. NET-02: A multicenter, randomized, phase II trial of liposomal irinotecan (nal-IRI) and 5-fluorouracil (5-FU)/folinic acid or docetaxel as second-line therapy in patients (pts) with progressive poorly differentiated extra-pulmonary neuroendocrine carcinoma (PD-EP-NEC). J. Clin. Oncol. 2022, 40, 4005. [Google Scholar]
- Walter, T.; Lievre, A.; Coriat, R.; Malka, D.; Elhajbi, F.; Di Fiore, F.; Hentic, O.; Smith, D.; Hautefeuille, V.; Roquin, G.; et al. Bevacizumab plus FOLFIRI after failure of platinum–etoposide first-line chemotherapy in patients with advanced neuroendocrine carcinoma (PRODIGE 41-BEVANEC): A randomised, multicentre, non-comparative, open-label, phase 2 trial. Lancet Oncol. 2023, 24, 297–306. [Google Scholar] [CrossRef]
- Kitagawa, Y.; Osumi, H.; Shinozaki, E.; Ota, Y.; Nakayama, I.; Suzuki, T.; Wakatsuki, T.; Ichimura, T.; Ogura, M.; Ooki, A.; et al. Safety and efficacy of amrubicin monotherapy in patients with platinum-refractory metastatic neuroendocrine carcinoma of the gastrointestinal tract: A single cancer center retrospective study. Cancer Manag. Res. 2019, 11, 5757–5764. [Google Scholar] [CrossRef]
- La Rosa, S.; Marando, A.; Furlan, D.; Sahnane, N.; Capella, C. Colorectal Poorly Differentiated Neuroendocrine Carcinomas and Mixed Adenoneuroendocrine Carcinomas: Insights into the Diagnostic Immunophenotype, Assessment of Methylation Profile, and Search for Prognostic Markers. Am. J. Surg. Pathol. 2012, 36, 601. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Di Maio, M.; Chiodini, P.; Rudd, R.M.; Okamoto, H.; Skarlos, D.V.; Früh, M.; Qian, W.; Tamura, T.; Samantas, E.; et al. Carboplatin- or cisplatin-based chemotherapy in first-line treatment of small-cell lung cancer: The COCIS meta-analysis of individual patient data. J. Clin. Oncol. 2012, 30, 1692–1698. [Google Scholar] [CrossRef] [PubMed]
- Sorbye, H.; Grande, E.; Pavel, M.; Tesselaar, M.; Fazio, N.; Reed, N.S.; Knigge, U.; Christ, E.; Ambrosini, V.; Couvelard, A.; et al. European Neuroendocrine Tumor Society (ENETS) 2023 guidance paper for digestive neuroendocrine carcinoma. J. Neuroendocrinol. 2023, 35, e13249. [Google Scholar] [CrossRef] [PubMed]
- Moertel, C.G.; Kvols, L.K.; O’Connell, M.J.; Rubin, J. Treatment of neuroendocrine carcinomas with combined etoposide and cisplatin. Evidence of major therapeutic activity in the anaplastic variants of these neoplasms. Cancer 1991, 68, 227–232. [Google Scholar] [CrossRef]
- Mitry, E.; Baudin, E.; Ducreux, M.; Sabourin, J.C.; Rufié, P.; Aparicio, T.; Lasser, P.; Elias, D.; Duvillard, P.; Schlumberger, M.; et al. Treatment of poorly differentiated neuroendocrine tumours with etoposide and cisplatin. Br. J. Cancer 1999, 81, 1351–1355. [Google Scholar] [CrossRef]
- Frizziero, M.; Spada, F.; Lamarca, A.; Kordatou, Z.; Barriuso, J.; Nuttall, C.; McNamara, M.G.; Hubner, R.A.; Mansoor, W.; Manoharan, P.; et al. Carboplatin in Combination with Oral or Intravenous Etoposide for Extra-Pulmonary, Poorly-Differentiated Neuroendocrine Carcinomas. Neuroendocrinology 2019, 109, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Machida, N.; Morizane, C.; Kasuga, A.; Takahashi, H.; Sudo, K.; Nishina, T.; Tobimatsu, K.; Ishido, K.; Furuse, J.; et al. Multicenter retrospective analysis of systemic chemotherapy for advanced neuroendocrine carcinoma of the digestive system. Cancer Sci. 2014, 105, 1176–1181. [Google Scholar] [CrossRef] [PubMed]
- Pavel, M.; Öberg, K.; Falconi, M.; Krenning, E.P.; Sundin, A.; Perren, A.; Berruti, A. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 844–860. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.H.; Goldner, W.S.; Benson, A.B.; Bergsland, E.; Blaszkowsky, L.S.; Brock, P.; Chan, J.; Das, S.; Dickson, P.V.; Fanta, P.; et al. Neuroendocrine and Adrenal Tumors, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 839–868. [Google Scholar] [CrossRef]
- Lamarca, A.; Frizziero, M.; Barriuso, J.; McNamara, M.G.; Hubner, R.A.; Valle, J.W. Urgent need for consensus: International survey of clinical practice exploring use of platinum-etoposide chemotherapy for advanced extra-pulmonary high grade neuroendocrine carcinoma (EP-G3-NEC). Clin. Transl. Oncol. 2019, 21, 950–953. [Google Scholar] [CrossRef]
- Abdel-Rahman, O.; Koski, S.L. Cisplatin-Based versus Carboplatin-Based Chemotherapy for Extrapulmonary Neuroendocrine Carcinomas: A Real-World Study. Neuroendocrinology 2021, 112, 777–783. [Google Scholar] [CrossRef]
- Johnson, D.H.; Ruckdeschel, J.C.; Keller, J.H.; Lyman, G.H.; Kallas, G.J.; Macdonald, J.; Deconti, R.C.; Lee, J.; Ringenberg, Q.S.; Patterson, W.P.; et al. A randomized trial to compare intravenous and oral etoposide in combination with cisplatin for the treatment of small cell lung cancer. Cancer 1991, 67, 245–249. [Google Scholar] [CrossRef]
- Ali, A.S.; Grönberg, M.; Langer, S.W.; Ladekarl, M.; Hjortland, G.O.; Vestermark, L.W.; Österlund, P.; Welin, S.; Grønbæk, H.; Knigge, U.; et al. Intravenous versus oral etoposide: Efficacy and correlation to clinical outcome in patients with high-grade metastatic gastroenteropancreatic neuroendocrine neoplasms (WHO G3). Med. Oncol. 2018, 35, 47. [Google Scholar] [CrossRef]
- Liu, G.; Franssen, E.; Fitch, M.I.; Warner, E. Patient preferences for oral versus intravenous palliative chemotherapy. J. Clin. Oncol. 1997, 15, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Veslemes, M.; Polyzos, A.; Latsi, P.; Dimitroulis, J.; Stamatiadis, D.; Dardoufas, C.; Rasidakis, A.; Katsilambros, N.; Jordanoglou, J. Optimal duration of chemotherapy in small cell lung cancer: A randomized study of 4 versus 6 cycles of cisplatin-etoposide. J. Chemother. 1998, 10, 136–140. [Google Scholar] [CrossRef]
- Sallam, M.; Wong, H.; Escriu, C. Treatment beyond four cycles of first line Platinum and Etoposide chemotherapy in real-life patients with stage IV Small Cell Lung Cancer: A retrospective study of the Merseyside and Cheshire Cancer network. BMC Pulm. Med. 2019, 19, 195. [Google Scholar] [CrossRef] [PubMed]
- Eads, J.R.; Catalano, P.J.; Fisher, G.A.; Rubin, D.; Iagaru, A.; Klimstra, D.S.; Konda, B.; Kwong, M.S.; Chan, J.A.; De Jesus-Acosta, A.; et al. Randomized phase II study of platinum and etoposide (EP) versus temozolomide and capecitabine (CAPTEM) in patients (pts) with advanced G3 non-small cell gastroenteropancreatic neuroendocrine neoplasms (GEPNENs): ECOG-ACRIN EA2142. J. Clin. Oncol. 2022, 40, 4020. [Google Scholar] [CrossRef]
- Butt, B.; Stokmo, H.; Ladekarl, M.; Tabaksblat, E.M.; Sorbye, H.; Revheim, M.; Hjortland, G. 1108P Folfirinox in the treatment of advanced gastroenteropancreatic neuroendocrine carsinomas. Ann. Oncol. 2021, 32, S915. [Google Scholar] [CrossRef]
- Hadoux, J.; Afchain, P.; Walter, T.; Tougeron, D.; Hautefeuille, V.; Monterymard, C.; Lorgis, V.; Thuillier, F.; Baudin, E.; Scoazec, J.Y.; et al. FOLFIRINEC: A randomized phase II trial of mFOLFIRINOX vs platinum-etoposide for metastatic neuroendocrine carcinoma of gastroenteropancreatic or unknown origin. Dig. Liver Dis. 2021, 53, 824–829. [Google Scholar] [CrossRef] [PubMed]
- Hainsworth, J.D.; Spigel, D.R.; Litchy, S.; Greco, F.A. Phase II trial of paclitaxel, carboplatin, and etoposide in advanced poorly differentiated neuroendocrine carcinoma: A Minnie Pearl Cancer Research Network Study. J. Clin. Oncol. 2006, 24, 3548–3554. [Google Scholar] [CrossRef] [PubMed]
- Baize, N.; Monnet, I.; Greillier, L.; Geier, M.; Lena, H.; Janicot, H.; Vergnenegre, A.; Crequit, J.; Lamy, R.; Auliac, J.-B.; et al. Carboplatin plus etoposide versus topotecan as second-line treatment for patients with sensitive relapsed small-cell lung cancer: An open-label, multicentre, randomised, phase 3 trial. Lancet Oncol. 2020, 21, 1224–1233. [Google Scholar] [CrossRef]
- Hadoux, J.; Walter, T.; Kanaan, C.; Hescot, S.; Hautefeuille, V.; Perrier, M.; Tauveron, I.; Laboureau, S.; Cao, C.D.; Petorin, C.; et al. Second-line treatment and prognostic factors in neuroendocrine carcinoma: The RBNEC study. Endocr. Relat. Cancer 2022, 29, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Apostolidis, L.; Bergmann, F.; Jäger, D.; Winkler, E.C. Efficacy of topotecan in pretreated metastatic poorly differentiated extrapulmonary neuroendocrine carcinoma. Cancer Med. 2016, 5, 2261–2267. [Google Scholar] [CrossRef] [PubMed]
- Olsen, I.H.; Knigge, U.; Federspiel, B.; Hansen, C.P.; Skov, A.; Kjær, A.; Langer, S.W. Topotecan monotherapy in heavily pretreated patients with progressive advanced stage neuroendocrine carcinomas. J. Cancer 2014, 5, 628–632. [Google Scholar] [CrossRef]
- O’Brien, M.E.; Ciuleanu, T.-E.; Tsekov, H.; Shparyk, Y.; Čučeviá, B.; Juhasz, G.; Thatcher, N.; Ross, G.A.; Dane, G.C.; Crofts, T. Phase III trial comparing supportive care alone with supportive care with oral topotecan in patients with relapsed small-cell lung cancer. J. Clin. Oncol. 2006, 24, 5441–5447. [Google Scholar] [CrossRef]
- Araki, T.; Takashima, A.; Hamaguchi, T.; Honma, Y.; Iwasa, S.; Okita, N.; Kato, K.; Yamada, Y.; Hashimoto, H.; Taniguchi, H.; et al. Amrubicin in patients with platinum-refractory metastatic neuroendocrine carcinoma and mixed adenoneuroendocrine carcinoma of the gastrointestinal tract. Anticancer Drugs 2016, 27, 794–799. [Google Scholar] [CrossRef] [PubMed]
- Ebata, T.; Shimoi, T.; Ishiwata, T.; Iwasawa, S.; Bun, S.; Yunokawa, M.; Yonemori, K.; Takiguchi, Y.; Tamura, K. Amrubicin Monotherapy for Patients with Platinum-Pretreated Non-Gastrointestinal Non-Pancreatic Extrapulmonary Neuroendocrine Carcinoma. Oncology 2017, 93, 177–182. [Google Scholar] [CrossRef]
- Chen, M.H.; Chou, W.C.; Hsiao, C.F.; Jiang, S.S.; Tsai, H.J.; Liu, Y.C.; Hsu, C.; Shan, Y.S.; Hung, Y.P.; Hsich, C.H.; et al. An Open-Label, Single-Arm, Two-Stage, Multicenter, Phase II Study to Evaluate the Efficacy of TLC388 and Genomic Analysis for Poorly Differentiated Neuroendocrine Carcinomas. Oncologist 2020, 25, e782–e788. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Wang, H.; Yang, L.-X. Enhancement of radiation-induced DNA damage and inhibition of its repair by a novel camptothecin analog. Anticancer Res. 2010, 30, 937–944. [Google Scholar] [PubMed]
- Kobayashi, N.; Takeda, Y.; Okubo, N.; Suzuki, A.; Tokuhisa, M.; Hiroshima, Y.; Ichikawa, Y. Phase II study of temozolomide monotherapy in patients with extrapulmonary neuroendocrine carcinoma. Cancer Sci. 2021, 112, 1936–1942. [Google Scholar] [CrossRef] [PubMed]
- Longo-Muñoz, F.; Castellano, D.; Alexandre, J.; Chawla, S.P.; Fernández, C.; Kahatt, C.; Alfaro, V.; Siguero, M.; Zeaiter, A.; Moreno, V.; et al. Lurbinectedin in patients with pretreated neuroendocrine tumours: Results from a phase II basket study. Eur. J. Cancer 2022, 172, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Hentic, O.; Hammel, P.; Couvelard, A.; Rebours, V.; Zappa, M.; Palazzo, M.; Maire, F.; Goujon, G.; Gillet, A.; Lévy, P.; et al. FOLFIRI regimen: An effective second-line chemotherapy after failure of etoposide-platinum combination in patients with neuroendocrine carcinomas grade 3. Endocr. Relat. Cancer 2012, 19, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Hadoux, J.; Malka, D.; Planchard, D.; Scoazec, J.Y.; Caramella, C.; Guigay, J.; Boige, V.; Leboulleux, S.; Burtin, P.; Berdelou, A.; et al. Post-first-line FOLFOX chemotherapy for grade 3 neuroendocrine carcinoma. Endocr. Relat. Cancer 2015, 22, 289–298. [Google Scholar] [CrossRef]
- Thomas, K.; Voros, B.A.; Meadows-Taylor, M.; Smeltzer, M.P.; Griffin, R.; Boudreaux, J.P.; Thiagarajan, R.; Woltering, E.A.; Ramirez, R.A. Outcomes of Capecitabine and Temozolomide (CAPTEM) in Advanced Neuroendocrine Neoplasms (NENs). Cancers 2020, 12, 206. [Google Scholar] [CrossRef]
- Chan, D.L.; Bergsland, E.K.; Chan, J.A.; Gadgil, R.; Halfdanarson, T.R.; Hornbacker, K.; Kelly, V.; Kunz, P.L.; McGarrah, P.W.; Raj, N.P.; et al. Temozolomide in Grade 3 Gastroenteropancreatic Neuroendocrine Neoplasms: A Multicenter Retrospective Review. Oncologist 2021, 26, 950–955. [Google Scholar] [CrossRef]
- Elvebakken, H.; Perren, A.; Scoazec, J.Y.; Tang, L.H.; Federspiel, B.; Klimstra, D.S.; Vestermark, L.W.; Ali, A.S.; Zlobec, I.; Myklebust, T.Å.; et al. A Consensus-Developed Morphological Re-Evaluation of 196 High-Grade Gastroenteropancreatic Neuroendocrine Neoplasms and Its Clinical Correlations. Neuroendocrinology 2021, 111, 883–894. [Google Scholar] [CrossRef] [PubMed]
- De Mestier, L.; Couvelard, A.; Blazevic, A.; Hentic, O.; de Herder, W.W.; Rebours, V.; Paradis, V.; Ruszniewski, P.; Hofland, L.J.; Cros, J. Critical appraisal of MGMT in digestive NET treated with alkylating agents. Endocr. Relat. Cancer 2020, 27, R391–R405. [Google Scholar] [CrossRef]
- Welin, S.; Sorbye, H.; Sebjornsen, S.; Knappskog, S.; Busch, C.; Öberg, K. Clinical effect of temozolomide-based chemotherapy in poorly differentiated endocrine carcinoma after progression on first-line chemotherapy. Cancer 2011, 117, 4617–4622. [Google Scholar] [CrossRef] [PubMed]
- Lamarca, A.; Walter, T.; Pavel, M.; Borbath, I.; Freis, P.; Nuñez, B.; Childs, A.; McNamara, M.G.; Hubner, R.A.; Garcia-Carbonero, R.; et al. Design and Validation of the GI-NEC Score to Prognosticate Overall Survival in Patients with High-Grade Gastrointestinal Neuroendocrine Carcinomas. J. Natl. Cancer Inst. 2017, 109, djw277. [Google Scholar] [CrossRef] [PubMed]
- Horn, L.; Mansfield, A.S.; Szczęsna, A.; Havel, L.; Krzakowski, M.; Hochmair, M.J.; Huemer, F.; Losonczy, G.; Johnson, M.L.; Nishio, M.; et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2220–2229. [Google Scholar] [CrossRef] [PubMed]
- Paz-Ares, L.; Dvorkin, M.; Chen, Y.; Reinmuth, N.; Hotta, K.; Trukhin, D.; Statsenko, G.; Hochmair, M.J.; Özgüroğlu, M.; Ji, J.H.; et al. Durvalumab plus platinum–etoposide versus platinum–etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): A randomised, controlled, open-label, phase 3 trial. Lancet 2019, 394, 1929–1939. [Google Scholar] [CrossRef]
- Raj, N.; Chan, J.A.; Wang, S.J.; Aggarwal, R.R.; Calabrese, S.; DeMore, A.; Fong, L.; Grabowsky, J.; Hope, T.A.; Kolli, K.P.; et al. Pembrolizumab alone and pembrolizumab plus chemotherapy in previously treated, extrapulmonary poorly differentiated neuroendocrine carcinomas. Br. J. Cancer 2023, 129, 291–300. [Google Scholar] [CrossRef]
- Vijayvergia, N.; Dasari, A.; Deng, M.; Litwin, S.; Al-Toubah, T.; Alpaugh, R.K.; Dotan, E.; Hall, M.J.; Ross, N.M.; Runyen, M.M.; et al. Pembrolizumab monotherapy in patients with previously treated metastatic high-grade neuroendocrine neoplasms: Joint analysis of two prospective, non-randomised trials. Br. J. Cancer 2020, 122, 1309–1314. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.C.; Strosberg, J.; Fazio, N.; Pavel, M.E.; Bergsland, E.; Ruszniewski, P.; Halperin, D.M.; Li, D.; Tafuto, S.; Raj, N.; et al. Spartalizumab in metastatic, well/poorly differentiated neuroendocrine neoplasms. Endocr. Relat. Cancer 2021, 28, 161–172. [Google Scholar] [CrossRef]
- Patel, S.P.; Mayerson, E.; Chae, Y.K.; Strosberg, J.; Wang, J.; Konda, B.; Hayward, J.; McLeod, C.M.; Chen, H.X.; Sharon, E.; et al. A phase II basket trial of Dual Anti-CTLA-4 and Anti-PD-1 Blockade in Rare Tumors (DART) SWOG S1609: High-grade neuroendocrine neoplasm cohort. Cancer 2021, 127, 3194–3201. [Google Scholar] [CrossRef]
- Patel, S.P.; Othus, M.; Chae, Y.K.; Giles, F.J.; Hansel, D.E.; Singh, P.P.; Fontaine, A.; Shah, M.H.; Kasi, A.; Baghdadi, T.A.; et al. A Phase II Basket Trial of Dual Anti-CTLA-4 and Anti-PD-1 Blockade in Rare Tumors (DART SWOG 1609) in Patients with Nonpancreatic Neuroendocrine Tumors. Clin. Cancer Res. 2020, 26, 2290–2296. [Google Scholar] [CrossRef]
- Burkart, J.; Owen, D.; Shah, M.H.; Abdel-Misih, S.R.; Roychowdhury, S.; Wesolowski, R.; Haraldsdottir, S.; Reeser, J.W.; Samorodnitsky, E.; Smith, A.; et al. Targeting BRAF Mutations in High-Grade Neuroendocrine Carcinoma of the Colon. J. Natl. Compr. Canc. Netw. 2018, 16, 1035–1040. [Google Scholar] [CrossRef] [PubMed]
- Capdevila, J.; Arqués, O.; Hernández Mora, J.R.; Matito, J.; Caratù, G.; Mancuso, F.M.; Landolfi, S.; Barriuso, J.; Jimenez-Fonseca, P.; Lopez Lopez, C.; et al. Epigenetic EGFR Gene Repression Confers Sensitivity to Therapeutic BRAFV600E Blockade in Colon Neuroendocrine Carcinomas. Clin. Cancer Res. 2020, 26, 902–909. [Google Scholar] [CrossRef]
- Dizdar, L.; Werner, T.A.; Drusenheimer, J.C.; Möhlendick, B.; Raba, K.; Boeck, I.; Anlauf, M.; Schott, M.; Göring, W.; Esposito, I.; et al. BRAFV600E mutation: A promising target in colorectal neuroendocrine carcinoma. Int. J. Cancer 2019, 144, 1379–1390. [Google Scholar] [CrossRef] [PubMed]
- Klempner, S.J.; Gershenhorn, B.; Tran, P.; Lee, T.K.; Erlander, M.G.; Gowen, K.; Schrock, A.B.; Morosini, D.; Ross, J.S.; Miller, V.A.; et al. BRAFV600E Mutations in High-Grade Colorectal Neuroendocrine Tumors May Predict Responsiveness to BRAF-MEK Combination Therapy. Cancer Discov. 2016, 6, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, K.; Toshimitsu, K.; Matano, M.; Fujita, M.; Fujii, M.; Togasaki, K.; Ebisudani, T.; Shimokawa, M.; Takano, A.; Takahashi, S.; et al. An Organoid Biobank of Neuroendocrine Neoplasms Enables Genotype-Phenotype Mapping. Cell 2020, 183, 1420–1435.e21. [Google Scholar] [CrossRef]
| Clinicaltrials.gov Trial Identifier | Trial Regimen | Line of Treatment | Phase | Recruitment Target | Primary Endpoint |
|---|---|---|---|---|---|
| NCT02595424 | TEMCAP vs. EP | First | II | 59 | PFS |
| NCT05058651 | Atezolizumab + EP vs. EP | First | II/III | 189 | OS |
| NCT04325425 | mFolFIrinOx vs. EP | First | II | 218 | PFS |
| NCT04042714 | TAS-102 | Second | II | 14 | ORR |
| NCT03387592 | FolFIri vs. TEMCAP | Second | II | 112 | DCR/AE incidence |
| Trial ID | Trial Regimen | Line of Treatment | Phase | Recruitment Numbers | Main Primary Sites | Ki-67 ≥ 55% | ORR | PFS (Months) | OS |
|---|---|---|---|---|---|---|---|---|---|
| TOPIC-NEC | EP vs. IP | 1 | III | 170 | Upper GI | 82% vs. 81% | 54% vs. 52% | 5.6 vs. 5.1 | 12.5 vs. 10.9 months |
| Bevanec | FOLFIRI + Bev vs. FOLFIRI | 2 | II | 150 | Colorectal | 86% vs. 76% | 25% vs. 18% | 3.7 vs. 3.5 | 6 months, 53% vs. 61% |
| NET-02 | Nal-IRI/5FU vs. Docetaxel | 2 | II | 59 | Upper GI | 90% vs. 90% | 11% vs. 10% | 3 vs. 2 | 6 months, 29.6% vs. 13.8% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weaver, J.M.J.; Hubner, R.A.; Valle, J.W.; McNamara, M.G. Selection of Chemotherapy in Advanced Poorly Differentiated Extra-Pulmonary Neuroendocrine Carcinoma. Cancers 2023, 15, 4951. https://doi.org/10.3390/cancers15204951
Weaver JMJ, Hubner RA, Valle JW, McNamara MG. Selection of Chemotherapy in Advanced Poorly Differentiated Extra-Pulmonary Neuroendocrine Carcinoma. Cancers. 2023; 15(20):4951. https://doi.org/10.3390/cancers15204951
Chicago/Turabian StyleWeaver, Jamie M. J., Richard A. Hubner, Juan W. Valle, and Mairead G. McNamara. 2023. "Selection of Chemotherapy in Advanced Poorly Differentiated Extra-Pulmonary Neuroendocrine Carcinoma" Cancers 15, no. 20: 4951. https://doi.org/10.3390/cancers15204951
APA StyleWeaver, J. M. J., Hubner, R. A., Valle, J. W., & McNamara, M. G. (2023). Selection of Chemotherapy in Advanced Poorly Differentiated Extra-Pulmonary Neuroendocrine Carcinoma. Cancers, 15(20), 4951. https://doi.org/10.3390/cancers15204951






