Current Research and Development in Hyperthermic Intraperitoneal Chemotherapy (HIPEC)—A Cross-Sectional Analysis of Clinical Trials Registered on ClinicalTrials.gov
Abstract
Simple Summary
Abstract
1. Introduction
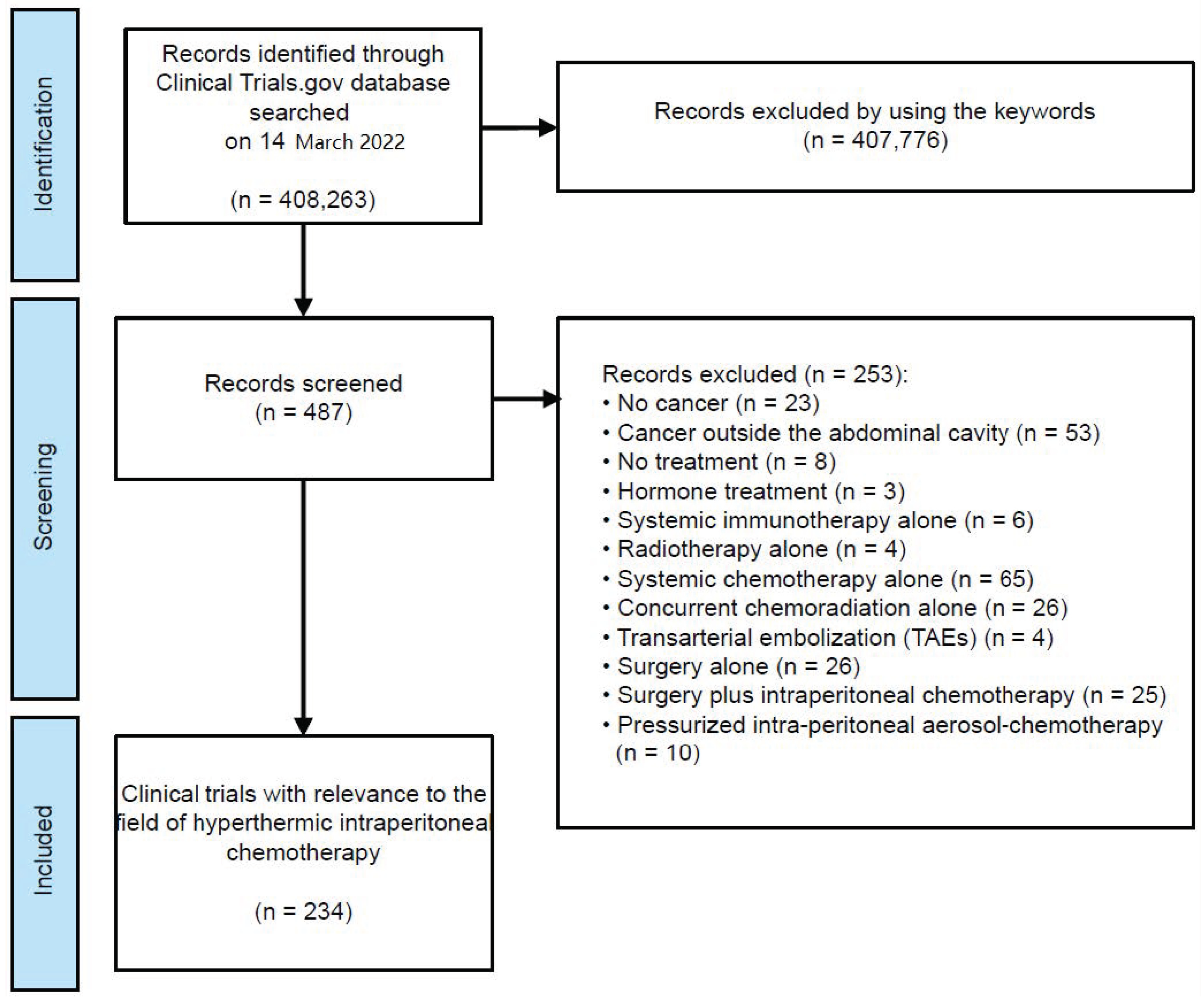
2. Materials and Methods
2.1. Selection of Clinical Trials
2.2. Data from ClinicalTrials.gov
2.3. Search for Publications of Trial Results
2.4. Outcome Parameters
2.5. Ethical Statement
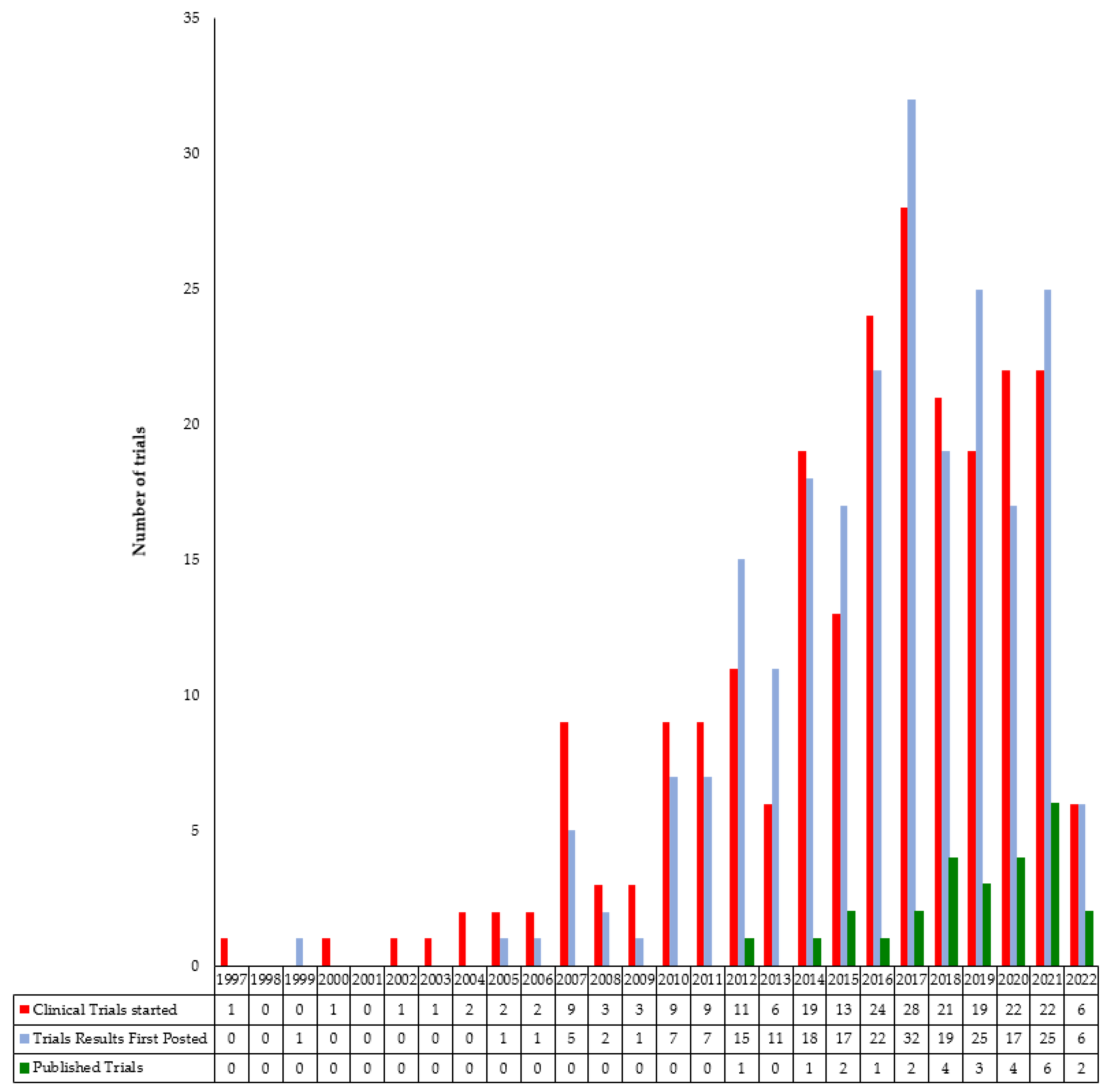
3. Results
4. Discussion
4.1. Main Findings
4.2. Results in Context
4.3. Strengths/Limitations
4.4. Implications for Future Research
4.5. Implications for the Practice
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Franko, J.; Shi, Q.; Meyers, J.P.; Maughan, T.S.; Adams, R.A.; Seymour, M.T.; Saltz, L.; Punt, C.J.; Koopman, M.; Tournigand, C.; et al. Prognosis of patients with peritoneal metastatic colorectal cancer given systemic therapy: An analysis of individual patient data from prospective randomised trials from the analysis and research in cancers of the digestive system (ARCAD) database. Lancet Oncol. 2016, 17, 1709–1719. [Google Scholar] [CrossRef]
- Lambert, L.A. Looking up: Recent advances in understanding and treating peritoneal carcinomatosis. CA Cancer J. Clin. 2015, 65, 284–298. [Google Scholar] [CrossRef]
- Glehen, O.; Kwiatkowski, F.; Sugarbaker, P.H.; Elias, D.; Levine, E.A.; De Simone, M.; Barone, R.; Yonemura, Y.; Cavaliere, F.; Quenet, F.; et al. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: A multi-institutional study. J. Clin. Oncol. 2004, 22, 3284–3292. [Google Scholar] [CrossRef]
- Jayne, D.G.; Fook, S.; Loi, C.; Seow-Choen, F. Peritoneal carcinomatosis from colorectal cancer. Br. J. Surg. 2002, 89, 1545–1550. [Google Scholar] [CrossRef]
- Sadeghi, B.; Arvieux, C.; Glehen, O.; Beaujard, A.C.; Rivoire, M.; Baulieux, J.; Fontaumard, E.; Brachet, A.; Caillot, J.L.; Faure, J.L.; et al. Peritoneal carcinomatosis from non-gynecologic malignancies: Results of the EVOCAPE 1 multicentric prospective study. Cancer 2000, 88, 358–363. [Google Scholar] [CrossRef]
- Sugarbaker, P.H. New standard of care for appendiceal epithelial neoplasms and pseudomyxoma peritonei syndrome? Lancet Oncol. 2006, 7, 69–76. [Google Scholar] [CrossRef]
- Yan, T.D.; Welch, L.; Black, D.; Sugarbaker, P.H. A systematic review on the efficacy of cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for diffuse malignancy peritoneal mesothelioma. Ann. Oncol. 2007, 18, 827–834. [Google Scholar] [CrossRef]
- Friedrich, M.; Zinn, W.; Kolnsberg, L.; Kraft, C.; Kuhn, W. Hyperthermic Intraperitoneal Chemotherapy (HIPEC) for Ovarian Cancer: Evaluation of Side Effects in a Single Institution Cohort. Anticancer Res. 2020, 40, 1481–1486. [Google Scholar] [CrossRef]
- Spratt, J.S.; Adcock, R.A.; Muskovin, M.; Sherrill, W.; McKeown, J. Clinical delivery system for intraperitoneal hyperthermic chemotherapy. Cancer Res. 1980, 40, 256–260. [Google Scholar]
- Girshally, R.; Demtroder, C.; Albayrak, N.; Zieren, J.; Tempfer, C.; Reymond, M.A. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) as a neoadjuvant therapy before cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. World J. Surg. Oncol. 2016, 14, 253. [Google Scholar] [CrossRef]
- Sugarbaker, P.H.; Ryan, D.P. Cytoreductive surgery plus hyperthermic perioperative chemotherapy to treat peritoneal metastases from colorectal cancer: Standard of care or an experimental approach? Lancet Oncol. 2012, 13, e362–e369. [Google Scholar] [CrossRef]
- van Driel, W.J.; Koole, S.N.; Sonke, G.S. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. N. Engl. J. Med. 2018, 378, 1363–1364. [Google Scholar] [CrossRef]
- Verwaal, V.J.; van Ruth, S.; de Bree, E.; van Sloothen, G.W.; van Tinteren, H.; Boot, H.; Zoetmulder, F.A. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J. Clin. Oncol. 2003, 21, 3737–3743. [Google Scholar] [CrossRef]
- Chua, T.C.; Yan, T.D.; Saxena, A.; Morris, D.L. Should the treatment of peritoneal carcinomatosis by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy still be regarded as a highly morbid procedure?: A systematic review of morbidity and mortality. Ann. Surg. 2009, 249, 900–907. [Google Scholar] [CrossRef]
- Quere, P.; Facy, O.; Manfredi, S.; Jooste, V.; Faivre, J.; Lepage, C.; Bouvier, A.M. Epidemiology, Management, and Survival of Peritoneal Carcinomatosis from Colorectal Cancer: A Population-Based Study. Dis. Colon. Rectum. 2015, 58, 743–752. [Google Scholar] [CrossRef]
- DeAngelis, C.D.; Drazen, J.M.; Frizelle, F.A.; Haug, C.; Hoey, J.; Horton, R.; Kotzin, S.; Laine, C.; Marusic, A.; Overbeke, A.J.; et al. Clinical trial registration: A statement from the International Committee of Medical Journal Editors. JAMA 2004, 292, 1363–1364. [Google Scholar] [CrossRef]
- Califf, R.M.; Zarin, D.A.; Kramer, J.M.; Sherman, R.E.; Aberle, L.H.; Tasneem, A. Characteristics of clinical trials registered in ClinicalTrials.gov, 2007-2010. JAMA 2012, 307, 1838–1847. [Google Scholar] [CrossRef]
- Cihoric, N.; Tsikkinis, A.; van Rhoon, G.; Crezee, H.; Aebersold, D.M.; Bodis, S.; Beck, M.; Nadobny, J.; Budach, V.; Wust, P.; et al. Hyperthermia-related clinical trials on cancer treatment within the ClinicalTrials.gov registry. Int. J. Hyperthermia 2015, 31, 609–614. [Google Scholar] [CrossRef]
- Hartung, D.M.; Zarin, D.A.; Guise, J.M.; McDonagh, M.; Paynter, R.; Helfand, M. Reporting discrepancies between the ClinicalTrials.gov results database and peer-reviewed publications. Ann. Intern. Med. 2014, 160, 477–483. [Google Scholar] [CrossRef]
- AstraZeneca Clinical Trials. Available online: www.astrazenecaclinicaltrials.com (accessed on 25 October 2022).
- GlaxoSmithKline Clinical Study Register. Available online: www.gsk-clinicalstudyregister.com (accessed on 25 October 2022).
- ISI Web of Knowledge, Journal Citation Reports, Thomson Reuters. Available online: https://jcr.clarivate.com/ (accessed on 14 October 2022).
- ISI Web of Science, Thomson Reuters. Available online: http://www.isiknowledge.com (accessed on 25 October 2022).
- van Driel, W.J.; Koole, S.N.; Sikorska, K.; Schagen van Leeuwen, J.H.; Schreuder, H.W.R.; Hermans, R.H.M.; de Hingh, I.; van der Velden, J.; Arts, H.J.; Massuger, L.; et al. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. N. Engl. J. Med. 2018, 378, 230–240. [Google Scholar] [CrossRef]
- Lim, M.C.; Chang, S.J.; Park, B.; Yoo, H.J.; Yoo, C.W.; Nam, B.H.; Park, S.Y.; Collaborators, H.f.O.C. Survival After Hyperthermic Intraperitoneal Chemotherapy and Primary or Interval Cytoreductive Surgery in Ovarian Cancer: A Randomized Clinical Trial. JAMA Surg. 2022, 157, 374–383. [Google Scholar] [CrossRef]
- Quenet, F.; Elias, D.; Roca, L.; Goere, D.; Ghouti, L.; Pocard, M.; Facy, O.; Arvieux, C.; Lorimier, G.; Pezet, D.; et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 256–266. [Google Scholar] [CrossRef]
- Goere, D.; Glehen, O.; Quenet, F.; Guilloit, J.M.; Bereder, J.M.; Lorimier, G.; Thibaudeau, E.; Ghouti, L.; Pinto, A.; Tuech, J.J.; et al. Second-look surgery plus hyperthermic intraperitoneal chemotherapy versus surveillance in patients at high risk of developing colorectal peritoneal metastases (PROPHYLOCHIP-PRODIGE 15): A randomised, phase 3 study. Lancet Oncol. 2020, 21, 1147–1154. [Google Scholar] [CrossRef]
- Alistar, A.; Morris, B.B.; Desnoyer, R.; Klepin, H.D.; Hosseinzadeh, K.; Clark, C.; Cameron, A.; Leyendecker, J.; D’Agostino, R., Jr.; Topaloglu, U.; et al. Safety and tolerability of the first-in-class agent CPI-613 in combination with modified FOLFIRINOX in patients with metastatic pancreatic cancer: A single-centre, open-label, dose-escalation, phase 1 trial. Lancet Oncol. 2017, 18, 770–778. [Google Scholar] [CrossRef]
- Klaver, C.E.L.; Wisselink, D.D.; Punt, C.J.A.; Snaebjornsson, P.; Crezee, J.; Aalbers, A.G.J.; Brandt, A.; Bremers, A.J.A.; Burger, J.W.A.; Fabry, H.F.J.; et al. Adjuvant hyperthermic intraperitoneal chemotherapy in patients with locally advanced colon cancer (COLOPEC): A multicentre, open-label, randomised trial. Lancet Gastroenterol. Hepatol. 2019, 4, 761–770. [Google Scholar] [CrossRef]
- Stewart, J.H.t.; Shen, P.; Russell, G.; Fenstermaker, J.; McWilliams, L.; Coldrun, F.M.; Levine, K.E.; Jones, B.T.; Levine, E.A. A phase I trial of oxaliplatin for intraperitoneal hyperthermic chemoperfusion for the treatment of peritoneal surface dissemination from colorectal and appendiceal cancers. Ann. Surg. Oncol. 2008, 15, 2137–2145. [Google Scholar] [CrossRef]
- Badgwell, B.; Ikoma, N.; Murphy, M.B.; Wang, X.; Estrella, J.; Roy-Chowdhuri, S.; Das, P.; Minsky, B.D.; Lano, E.; Song, S.; et al. A Phase II Trial of Cytoreduction, Gastrectomy, and Hyperthermic Intraperitoneal Perfusion with Chemotherapy for Patients with Gastric Cancer and Carcinomatosis or Positive Cytology. Ann. Surg. Oncol. 2021, 28, 258–264. [Google Scholar] [CrossRef]
- Franko, J.; Brahmbhatt, R.; Tee, M.; Raman, S.; Ferrel, B.; Gorvet, M.; Andres, M. Cellular Immunoprofile of Peritoneal Environment During a HIPEC Procedure. Ann. Surg. Oncol. 2020, 27, 5005–5013. [Google Scholar] [CrossRef]
- Baratti, D.; Kusamura, S.; Iusco, D.; Gimondi, S.; Pietrantonio, F.; Milione, M.; Guaglio, M.; Bonomi, S.; Grassi, A.; Virzi, S.; et al. Hyperthermic Intraperitoneal Chemotherapy (HIPEC) at the Time of Primary Curative Surgery in Patients with Colorectal Cancer at High Risk for Metachronous Peritoneal Metastases. Ann. Surg. Oncol. 2017, 24, 167–175. [Google Scholar] [CrossRef]
- Yurttas, C.; Horvath, P.; Fischer, I.; Meisner, C.; Nadalin, S.; Konigsrainer, I.; Konigsrainer, A.; Beckert, S.; Loffler, M.W. A Prospective, Phase I/II, Open-Label Pilot Trial to Assess the Safety of Hyperthermic Intraperitoneal Chemotherapy After Oncological Resection of Pancreatic Adenocarcinoma. Ann. Surg. Oncol. 2021, 28, 9086–9095. [Google Scholar] [CrossRef]
- Chua, T.C.; Morris, D.L.; Saxena, A.; Esquivel, J.; Liauw, W.; Doerfer, J.; Germer, C.T.; Kerscher, A.G.; Pelz, J.O. Influence of modern systemic therapies as adjunct to cytoreduction and perioperative intraperitoneal chemotherapy for patients with colorectal peritoneal carcinomatosis: A multicenter study. Ann. Surg. Oncol. 2011, 18, 1560–1567. [Google Scholar] [CrossRef] [PubMed]
- Goere, D.; Malka, D.; Tzanis, D.; Gava, V.; Boige, V.; Eveno, C.; Maggiori, L.; Dumont, F.; Ducreux, M.; Elias, D. Is there a possibility of a cure in patients with colorectal peritoneal carcinomatosis amenable to complete cytoreductive surgery and intraperitoneal chemotherapy? Ann. Surg. 2013, 257, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Klaver, C.E.; Groenen, H.; Morton, D.G.; Laurberg, S.; Bemelman, W.A.; Tanis, P.J.; Research Committee of the European Society of Coloproctology. Recommendations and consensus on the treatment of peritoneal metastases of colorectal origin: A systematic review of national and international guidelines. Color. Dis. 2017, 19, 224–236. [Google Scholar] [CrossRef]
- Li, Y.; Yu, Y.; Liu, Y. Report on the 9(th) International Congress on Peritoneal Surface Malignancies. Cancer Biol. Med. 2014, 11, 281–284. [Google Scholar]
- Quenet, F.; Goere, D.; Mehta, S.S.; Roca, L.; Dumont, F.; Hessissen, M.; Saint-Aubert, B.; Elias, D. Results of two bi-institutional prospective studies using intraperitoneal oxaliplatin with or without irinotecan during HIPEC after cytoreductive surgery for colorectal carcinomatosis. Ann. Surg. 2011, 254, 294–301. [Google Scholar] [CrossRef]
- General Definition of Institution of Higher ed- Ucation, 20 USC ch 28, x1001 (2012). Available online: https://www.law.cornell.edu/uscode/text/ (accessed on 14 March 2023).
- Tse, T.; Fain, K.M.; Zarin, D.A. How to avoid common problems when using ClinicalTrials.gov in research: 10 issues to consider. BMJ 2018, 361, k1452. [Google Scholar] [CrossRef]
- Zarin, D.A.; Tse, T.; Williams, R.J.; Carr, S. Trial Reporting in ClinicalTrials.gov—The Final Rule. N. Engl. J. Med. 2016, 375, 1998–2004. [Google Scholar] [CrossRef]
- Anderson, M.L.; Chiswell, K.; Peterson, E.D.; Tasneem, A.; Topping, J.; Califf, R.M. Compliance with results reporting at ClinicalTrials.gov. N. Engl. J. Med. 2015, 372, 1031–1039. [Google Scholar] [CrossRef]
- DeVito, N.J.; Bacon, S.; Goldacre, B. Compliance with legal requirement to report clinical trial results on ClinicalTrials.gov: A cohort study. Lancet 2020, 395, 361–369. [Google Scholar] [CrossRef]
- Pereira, F.; Serrano, A.; Manzanedo, I.; Pérez-Viejo, E.; González-Moreno, S.; González-Bayón, L.; Arjona-Sánchez, A.; Torres, J.; Ramos, I.; Barrios, M.E.; et al. GECOP-MMC: Phase IV randomized clinical trial to evaluate the efficacy of hyperthermic intraperitoneal chemotherapy (HIPEC) with mytomicin-C after complete surgical cytoreduction in patients with colon cancer peritoneal metastases. BMC Cancer 2022, 22, 536. [Google Scholar] [CrossRef]
- Zarin, D.A.; Tse, T.; Williams, R.J.; Rajakannan, T. Update on Trial Registration 11 Years after the ICMJE Policy Was Established. N. Engl. J. Med. 2017, 376, 383–391. [Google Scholar] [CrossRef]
- Chan, A.W.; Altman, D.G. Epidemiology and reporting of randomised trials published in PubMed journals. Lancet 2005, 365, 1159–1162. [Google Scholar] [CrossRef] [PubMed]
- Meinert, C.L.; Tonascia, S.; Higgins, K. Content of reports on clinical trials: A critical review. Control Clin. Trials 1984, 5, 328–347. [Google Scholar] [CrossRef] [PubMed]
- Turaga, K.; Levine, E.; Barone, R.; Sticca, R.; Petrelli, N.; Lambert, L.; Nash, G.; Morse, M.; Adbel-Misih, R.; Alexander, H.R.; et al. Consensus guidelines from The American Society of Peritoneal Surface Malignancies on standardizing the delivery of hyperthermic intraperitoneal chemotherapy (HIPEC) in colorectal cancer patients in the United States. Ann. Surg. Oncol. 2014, 21, 1501–1505. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | All Trials | Journal Publications | Not Published in Journals | |
|---|---|---|---|---|
| n = 234 (100%) | n = 26 (11%) | n = 208 (89%) | ||
| Results Reported Elsewhere | No Results Available | |||
| n = 15 (6%) | n = 193 (83%) | |||
| Primary purpose of trial | ||||
| Treatment | 158 | 18 | 12 | 128 |
| Prevention | 13 | 1 | 0 | 12 |
| Diagnostic | 5 | 0 | 0 | 5 |
| Supportive care | 5 | 0 | 0 | 5 |
| Other a | 9 | 1 | 2 | 6 |
| Missing | 44 | 6 | 1 | 37 |
| Intervention type b | ||||
| Drug | 130 | 16 | 10 | 104 |
| Procedural | 114 | 13 | 9 | 92 |
| Biological | 3 | 0 | 0 | 3 |
| Behavioral | 5 | 1 | 0 | 4 |
| Device | 9 | 0 | 0 | 9 |
| Dietary supplement | 1 | 0 | 0 | 1 |
| Diagnostic Test | 6 | 0 | 0 | 6 |
| Radiation | 3 | 0 | 0 | 3 |
| Combination Product | 3 | 0 | 1 | 2 |
| Other | 33 | 4 | 5 | 24 |
| Missing | 12 | 0 | 0 | 12 |
| Funding b | ||||
| Academic | 195 | 17 | 11 | 167 |
| Industry | 5 | 0 | 0 | 5 |
| Cancer foundation | 48 | 11 | 6 | 31 |
| Missing | 1 | 0 | 0 | 1 |
| Region b | ||||
| Africa | 2 | 0 | 0 | 2 |
| Australia | 1 | 0 | 0 | 1 |
| Europe | 79 | 14 | 6 | 59 |
| North America | 76 | 7 | 9 | 60 |
| Central and South America | 2 | 1 | 0 | 1 |
| Asia and Pacific | 53 | 4 | 0 | 49 |
| Middle East | 1 | 0 | 0 | 1 |
| Missing | 22 | 0 | 0 | 22 |
| Number of institutions/Collaboration | ||||
| 1 | 170 | 18 | 13 | 139 |
| 2 | 9 | 0 | 1 | 8 |
| 3–10 | 20 | 3 | 1 | 16 |
| >10 | 15 | 1 | 0 | 14 |
| Missing | 20 | 4 | 0 | 16 |
| Anticipated enrollment, No. of patients | ||||
| 1–9 | 4 | 0 | 0 | 4 |
| 10–49 | 88 | 12 | 6 | 70 |
| 50–99 | 57 | 7 | 2 | 48 |
| 100–499 | 75 | 7 | 6 | 62 |
| 500–999 | 7 | 0 | 0 | 7 |
| >1000 | 2 | 0 | 1 | 1 |
| Missing | 1 | 0 | 0 | 1 |
| Sex | ||||
| Female only | 52 | 4 | 4 | 44 |
| Male only | 0 | 0 | 0 | 0 |
| Both | 182 | 22 | 11 | 149 |
| Age of study population | ||||
| Children only | 1 | 0 | 0 | 1 |
| Children and adults | 18 | 0 | 2 | 16 |
| Adults only | 215 | 26 | 13 | 176 |
| Study type | ||||
| Interventional | 190 | 20 | 14 | 156 |
| Observational | 44 | 6 | 1 | 37 |
| Allocation status c | ||||
| Randomized | 90 | 9 | 7 | 74 |
| Nonrandomized | 20 | 3 | 1 | 16 |
| Missing | 80 | 8 | 6 | 66 |
| Interventional group | ||||
| Single group | 87 | 10 | 6 | 71 |
| Parallel | 101 | 10 | 8 | 83 |
| Sequential | 1 | 0 | 0 | 1 |
| Missing | 1 | 0 | 0 | 1 |
| Blinding c | ||||
| None (open label) | 157 | 18 | 12 | 127 |
| Single blind | 14 | 2 | 1 | 11 |
| Double blind | 9 | 0 | 0 | 9 |
| Triple blind | 6 | 0 | 1 | 5 |
| Quadruple blind | 3 | 0 | 0 | 3 |
| Missing | 1 | 0 | 0 | 1 |
| Trial phase | ||||
| Phase I | 25 | 2 | 0 | 23 |
| Phase I/II | 10 | 2 | 0 | 8 |
| Phase II | 69 | 8 | 6 | 55 |
| Phase II/III | 5 | 1 | 2 | 2 |
| Phase III | 44 | 5 | 3 | 36 |
| Phase IV | 1 | 0 | 0 | 1 |
| Missing | 80 | 8 | 4 | 68 |
| Overall status | ||||
| Not yet recruiting | 21 | 0 | 0 | 21 |
| Recruiting | 75 | 0 | 3 | 72 |
| Completed | 63 | 18 | 6 | 39 |
| Suspended | 1 | 0 | 0 | 1 |
| Terminated | 15 | 2 | 5 | 8 |
| Withdrawn | 8 | 0 | 0 | 8 |
| Active, not recruiting | 14 | 3 | 0 | 11 |
| Enrolling by invitation | 1 | 0 | 0 | 1 |
| Unknown status | 36 | 3 | 1 | 32 |
| Length of study conduct | ||||
| <1 y | 14 | 3 | 0 | 11 |
| 1–2 y | 29 | 4 | 0 | 25 |
| 2–5 y | 93 | 7 | 6 | 80 |
| 5–10 y | 79 | 11 | 8 | 60 |
| >10 y | 16 | 1 | 1 | 14 |
| Missing | 3 | 0 | 0 | 3 |
| Primary Outcome d | ||||
| Efficacy | 123 | 14 | 9 | 100 |
| Safety | 79 | 12 | 4 | 63 |
| Feasibility | 21 | 1 | 4 | 16 |
| Pharmacodynamics/pharmacokinetics | 29 | 4 | 0 | 25 |
| Quality of life of patients | 9 | 1 | 0 | 8 |
| Other e | 37 | 3 | 3 | 31 |
| Colorectal a (n = 67) | Ovarian a (n = 61) | Gastric a (n = 54) | Primary Peritoneal a (n = 35) | From Each Origin a,b (n = 33) | Appendiceal a (n = 21) | Fallopian Tube a (n = 20) | Non-Carcinoma Tumors a,c (n = 8) | Pancreatic a (n = 7) | Uterine a (n = 5) | Cervical a (n = 3) | Bile Duct Cancer a,d (n = 2) | Small Intestine a (n = 1) | Bladder a (n = 1) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Used drugs, n (%) e | ||||||||||||||
| Cisplatin | 4 (6) | 20 (33) | 16 (30) | 11 (31) | 5 (15) | 1 (5) | 9 (45) | 3 (38) | 2 (29) | 1 (20) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Mitomycin C | 18 (27) | 7 (11) | 15 (28) | 7 (20) | 2 (6) | 7 (33) | 4 (20) | 2 (25) | 2 (29) | 3 (60) | 3 (100) | 1 (50) | 1 (100) | 1 (100) |
| Irinotecan | 3 (4) | 0 (0) | 3 (6) | 1 (3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Doxorubicin | 1 (1) | 5 (8) | 3 (6) | 1 (3) | 0 (0) | 1 (0) | 3 (15) | 3 (38) | 1 (14) | 2 (40) | 2 (67) | 1 (50) | 1 (100) | 0 (0) |
| Paclitaxel | 1 (1) | 8 (13) | 18 (33) | 2 (6) | 1 (3) | 0 (0) | 3 (15) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Oxaliplatin | 13 (19) | 2 (3) | 6 (11) | 4 (11) | 0 (0) | 3 (14) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 5-Fluorouracil | 3 (4) | 0 (0) | 3 (6) | 1 (3) | 1 (3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Docetaxel | 0 (0) | 3 (5) | 3 (6) | 1 (3) | 0 (0) | 0 (0) | 1 (5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Lobaplatin | 2 (3) | 1 (2) | 2 (4) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Carboplatin | 1 (1) | 12 (20) | 1 (2) | 6 (17) | 1 (3) | 0 (0) | 8 (40) | 0 (0) | 0 (0) | 1 (20) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Anti-PD-1 antibody | 0 (0) | 0 (0) | 1 (2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Thalidomide | 2 (3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Leucovorin | 1 (1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Melphalan | 1 (1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| MOC31PE | 1 (1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Capecitabine | 1 (1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Raltitrexed | 2 (3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Pasireotide | 1 (1) | 1 (2) | 0 (0) | 1 (3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Gemcitabine | 0 (0) | 1 (2) | 1 (2) | 1 (3) | 0 (0) | 0 (0) | 1 (5) | 1 (13) | 3 (42) | 1 (20) | 0 (0) | 1 (50) | 0 (0) | 0 (0) |
| Cantrixil | 0 (0) | 1 (2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Missing | 2 (3) | 1 (2) | 0 (0) | 1 (3) | 24 (75) | 11 (52) | 0 (0) | 1 (13) | 1 (14) | 1 (20) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Allocation status | ||||||||||||||
| Randomized | 27 (40) | 22 (36) | 21 (39) | 10 (29) | 12 (36) | 6 (29) | 5 (25) | 0 (0) | 2 (29) | 0 (0) | 0 (0) | 1 (50) | 0 (0) | 0 (0) |
| Nonrandomized | 5 (8) | 9 (15) | 1 (2) | 3 (8) | 2 (6) | 3 (14) | 2 (10) | 2 (25) | 3 (42) | 2 (40) | 2 (67) | 1 (50) | 1 (100) | 0 (0) |
| Missing | 35 (52) | 30 (49) | 32 (59) | 22 (63) | 19 (58) | 12 (57) | 13 (65) | 6 (75) | 2 (29) | 3 (60) | 1 (33) | 0 (0) | 0 (0) | 1 (100) |
| Trial phase, n (%) | ||||||||||||||
| Phase I | 7 (10) | 11 (18) | 4 (7) | 7 (20) | 1 (3) | 2 (10) | 5 (25) | 3 (37.5) | 1 (14) | 1 (20) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Phase I/II | 4 (6) | 2 (3) | 3 (6) | 3 (8) | 0 (0) | 0 (0) | 1 (5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Phase II | 17 (25) | 19 (31) | 21 (39) | 12 (35) | 5 (15) | 8 (38) | 8 (40) | 3 (37.5) | 4 (58) | 2 (40) | 2 (67) | 2 (100) | 1 (100) | 0 (0) |
| Phase II/III | 2 (3) | 0 (0) | 1 (2) | 0 (0) | 0 (0) | 1 (5) | 0 (0) | 0 (0) | 1 (14) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Phase III | 11 (17) | 12 (20) | 13 (24) | 3 (8) | 3 (9) | 0 (0) | 3 (15) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Phase IV | 1 (1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Missing | 25 (38) | 17 (28) | 12 (22) | 10 (29) | 24 (73) | 10 (47) | 3 (15) | 2 (25) | 1 (14) | 2 (40) | 1 (33) | 0 (0) | 0 (0) | 1 (100) |
| ClinicalTrials.Gov Trial ID | Phase | Treatment Allocation | Number of Participants | Primary Purpose of Trial | Condition or Disease | Chemotherapy Drugs | Trial Status |
|---|---|---|---|---|---|---|---|
| NCT00052962 | Phase III | Randomized | 30 | Treatment | Peritoneal carcinomatosis from each origin | Cisplatin | Completed |
| NCT04981639 | Phase III | Randomized | 72 | Supportive care | Peritoneal carcinomatosis from each origin | N/A | Recruiting |
| NCT03359811 | Phase III | Randomized | 75 | Treatment | Peritoneal carcinomatosis from each origin | N/A | Completed |
| NCT03180177 | Phase III | Randomized | 263 | Treatment | Primary peritoneal carcinoma AND Ovarian AND Tube carcinoma | Paclitaxel AND/OR cisplatin | Not yet recruiting |
| NCT03373058 | Phase III | Randomized | 310 | Treatment | Primary peritoneal carcinoma AND Ovarian AND Tube carcinoma | Docetaxel AND/OR Cisplatin | Recruiting |
| NCT02328716 | Phase III | Randomized | 32 | Other | Primary peritoneal carcinoma AND Ovarian AND Tube carcinoma | Cisplatin | Unknown status |
| NCT01628380 | Phase III | Randomized | 94 | Treatment | Ovarian cancer | Cisplatin AND Paclitaxel | Unknown status |
| NCT02681432 | Phase III | Randomized | 60 | Treatment | Ovarian cancer | Paclitaxel | Unknown status |
| NCT03220932 | Phase III | Randomized | 132 | Treatment | Ovarian cancer | Cisplatin | Not yet recruiting |
| NCT03371693 | Phase III | Randomized | 112 | Treatment | Ovarian cancer | Lobaplatin | Active, not recruiting |
| NCT03717610 | Phase III | N/A | 10 | Treatment | Ovarian cancer | Mitomycin C | Recruiting |
| NCT03842982 | Phase III | Randomized | 362 | Treatment | Ovarian cancer | N/A | Recruiting |
| NCT04473339 | Phase III | Randomized | 280 | Treatment | Ovarian cancer | N/A | Recruiting |
| NCT04111978 | Phase III | Randomized | 540 | Treatment | Ovarian cancer | N/A | Recruiting |
| NCT01376752 | Phase III | Randomized | 415 | Treatment | Recurrent ovarian cancer | Cisplatin | Active, not recruiting |
| NCT02158988 | Phase III | Randomized | 105 | Treatment | Gastric cancer | Mitomycin C AND/OR Cisplatin | Completed |
| NCT02240524 | Phase III | Randomized | 582 | Treatment | Gastric cancer | Paclitaxel | Unknown status |
| NCT02356276 | Phase III | Randomized | 584 | Treatment | Gastric cancer | Paclitaxel | Unknown status |
| NCT02381847 | Phase III | Randomized | 60 | Treatment | Gastric cancer | Cisplatin | Unknown status |
| NCT02960061 | Phase III | Randomized | 640 | Treatment | Gastric cancer | Paclitaxel | Unknown status |
| NCT03179579 | Phase III | Randomized | 88 | Treatment | Gastric cancer | Paclitaxel AND/OR Cisplatin | Not yet recruiting |
| NCT03917173 | Phase III | Randomized | 240 | Treatment | Gastric cancer | Mitomycin C AND Cisplatin | Recruiting |
| NCT04447352 | Phase III | Randomized | 200 | Treatment | Gastric cancer | Cisplatin | Recruiting |
| NCT04597294 | Phase III | Randomized | 600 | Prevention | Gastric cancer | Irinotecan | Recruiting |
| NCT03772028 | Phase III | Randomized | 538 | Treatment | Gastric cancer | Mitomycin C | Recruiting |
| NCT01882933 | Phase III | Randomized | 367 | Treatment | Gastric cancer | Oxaliplatin | Active, not recruiting |
| NCT03023436 | Phase III | N/A | 220 | Treatment | Gastric cancer | Cisplatin | Recruiting |
| NCT03348150 | Phase III | Randomized | 182 | Treatment | Gastric cancer | Oxaliplatin AND Docetaxel | Recruiting |
| NCT02614534 | Phase III | Randomized | 200 | Treatment | Colorectal cancer | Mitomycin C | Active, not recruiting |
| NCT02179489 | Phase III | Randomized | 300 | Treatment | Colorectal cancer | Mitomycin C | Recruiting |
| NCT02965248 | Phase III | Randomized | 147 | Treatment | Colorectal cancer | Oxaliplatin | Recruiting |
| NCT02974556 | Phase III | Randomized | 140 | Prevention | Colorectal cancer | Oxaliplatin | Not yet recruiting |
| NCT03028155 | Phase III | Randomized | 60 | Treatment | Colorectal cancer | Oxaliplatin | Unknown status |
| NCT03221608 | Phase III | Randomized | 300 | Prevention | Colorectal cancer | Lobaplatin | Not yet recruiting |
| NCT03413254 | Phase III | Randomized | 389 | Diagnostic | Colorectal cancer | N/A | Recruiting |
| NCT03914820 | Phase III | Randomized | 330 | Treatment | Colorectal cancer | Mitomycin C | Recruiting |
| NCT04370925 | Phase III | Randomized | 688 | Treatment | Colorectal cancer | Mitomycin C | Recruiting |
| NCT04861558 | Phase III | Randomized | 356 | Treatment | Colorectal cancer | 5 Fluorouracil AND/OR Irinotecan AND/OR Oxaliplatin | Recruiting |
| NCT03733184 | Phase III | N/A | 200 | N/A | Colorectal cancer | Mitomycin C | Unknown status |
| NCT05250648 | Phase IV | Randomized | 216 | Treatment | Colorectal cancer | Mytomicin C | Not yet recruiting |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ukegjini, K.; Guidi, M.; Lehmann, K.; Süveg, K.; Putora, P.M.; Cihoric, N.; Steffen, T. Current Research and Development in Hyperthermic Intraperitoneal Chemotherapy (HIPEC)—A Cross-Sectional Analysis of Clinical Trials Registered on ClinicalTrials.gov. Cancers 2023, 15, 1926. https://doi.org/10.3390/cancers15071926
Ukegjini K, Guidi M, Lehmann K, Süveg K, Putora PM, Cihoric N, Steffen T. Current Research and Development in Hyperthermic Intraperitoneal Chemotherapy (HIPEC)—A Cross-Sectional Analysis of Clinical Trials Registered on ClinicalTrials.gov. Cancers. 2023; 15(7):1926. https://doi.org/10.3390/cancers15071926
Chicago/Turabian StyleUkegjini, Kristjan, Marisa Guidi, Kuno Lehmann, Krisztian Süveg, Paul Martin Putora, Nikola Cihoric, and Thomas Steffen. 2023. "Current Research and Development in Hyperthermic Intraperitoneal Chemotherapy (HIPEC)—A Cross-Sectional Analysis of Clinical Trials Registered on ClinicalTrials.gov" Cancers 15, no. 7: 1926. https://doi.org/10.3390/cancers15071926
APA StyleUkegjini, K., Guidi, M., Lehmann, K., Süveg, K., Putora, P. M., Cihoric, N., & Steffen, T. (2023). Current Research and Development in Hyperthermic Intraperitoneal Chemotherapy (HIPEC)—A Cross-Sectional Analysis of Clinical Trials Registered on ClinicalTrials.gov. Cancers, 15(7), 1926. https://doi.org/10.3390/cancers15071926






