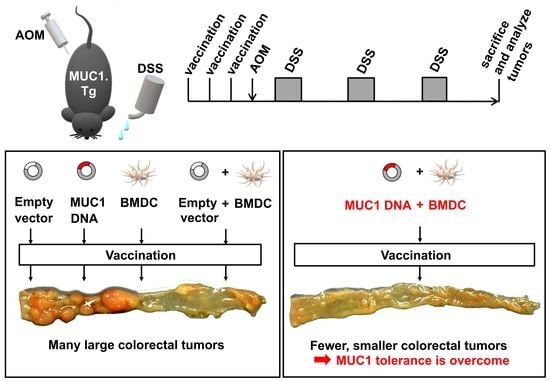
Prevention of Inflammation-Driven Colon Carcinogenesis in Human MUC1 Transgenic Mice by Vaccination with MUC1 DNA and Dendritic Cells
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Antibodies
2.3. Construction of MUC1 cDNA
2.4. Preparation of BMDCs
2.5. Vaccination
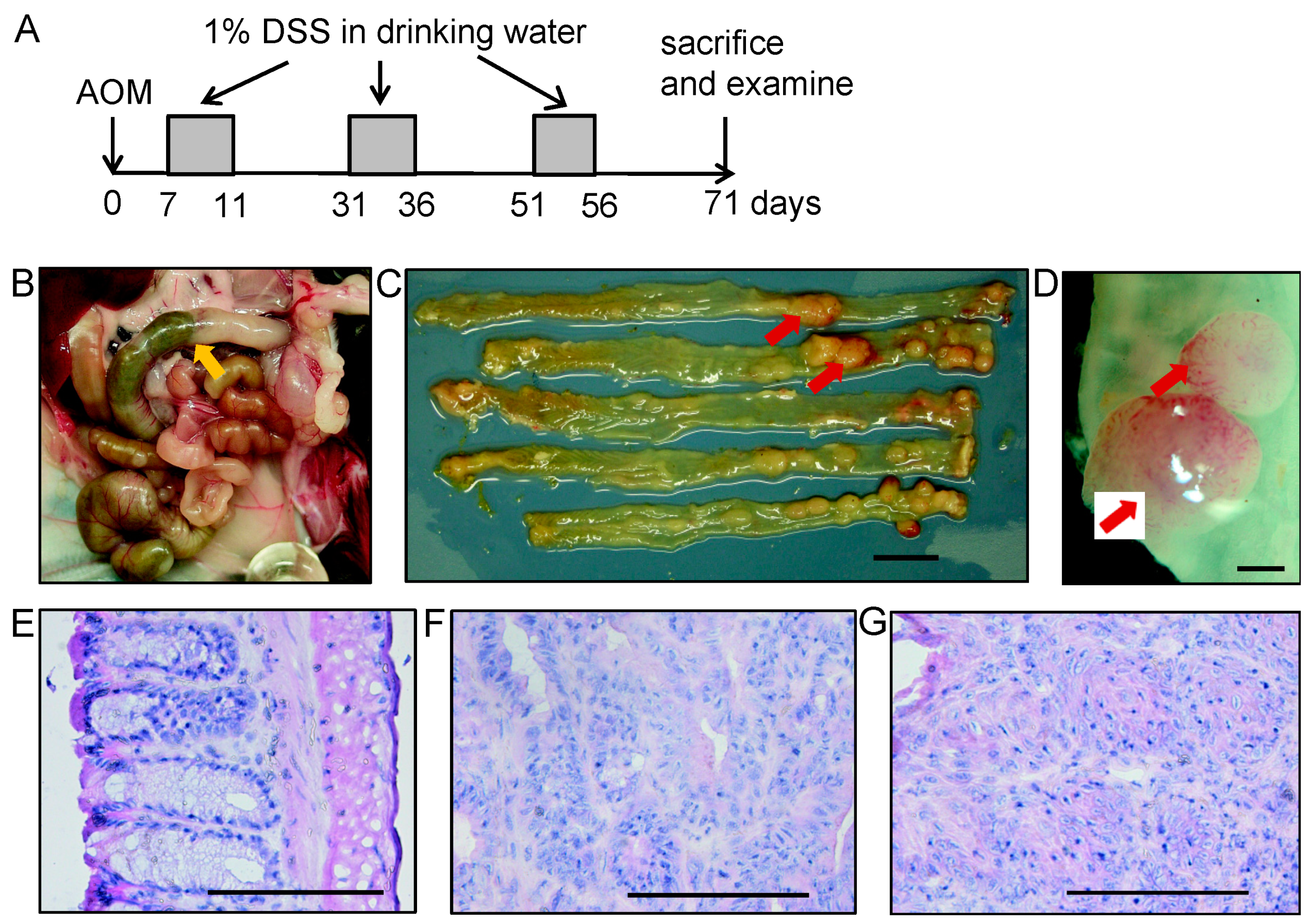
2.6. Experimental Colitis-Associated Colorectal Carcinogenesis
2.7. Gross and Histopathological Examination
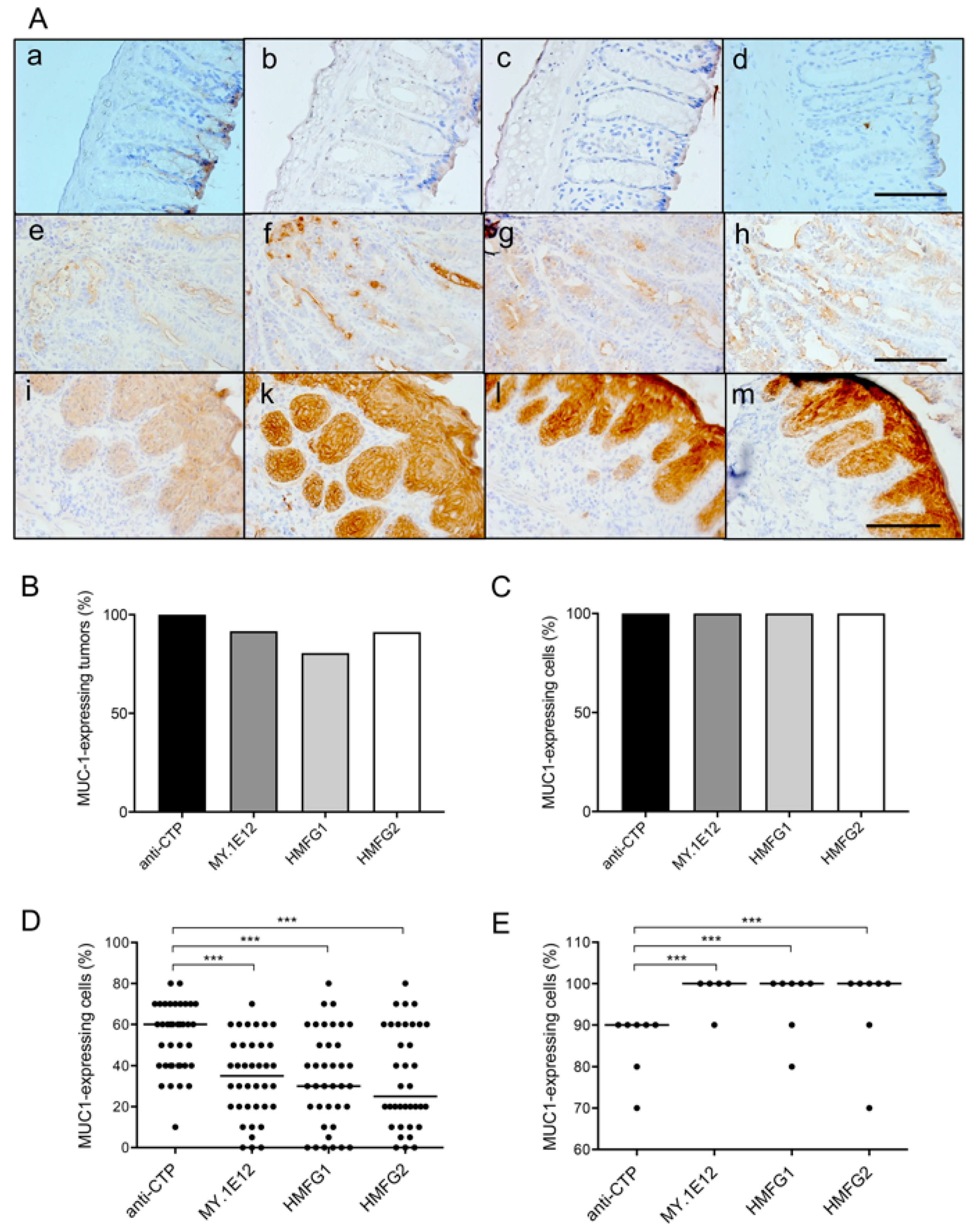
2.8. Immunohistochemical Analysis
2.9. Effect of MUC1 DNA Vaccination on Azoxymethane-Dextran Sulfate Sodium (AOM-DSS)-Induced Colorectal Inflammation
2.10. Statistical Analysis
3. Results
3.1. MUC1 Expression in Tumors Developed by Colitis-Associated Colorectal Carcinogenesis in MUC1.Tg Mice
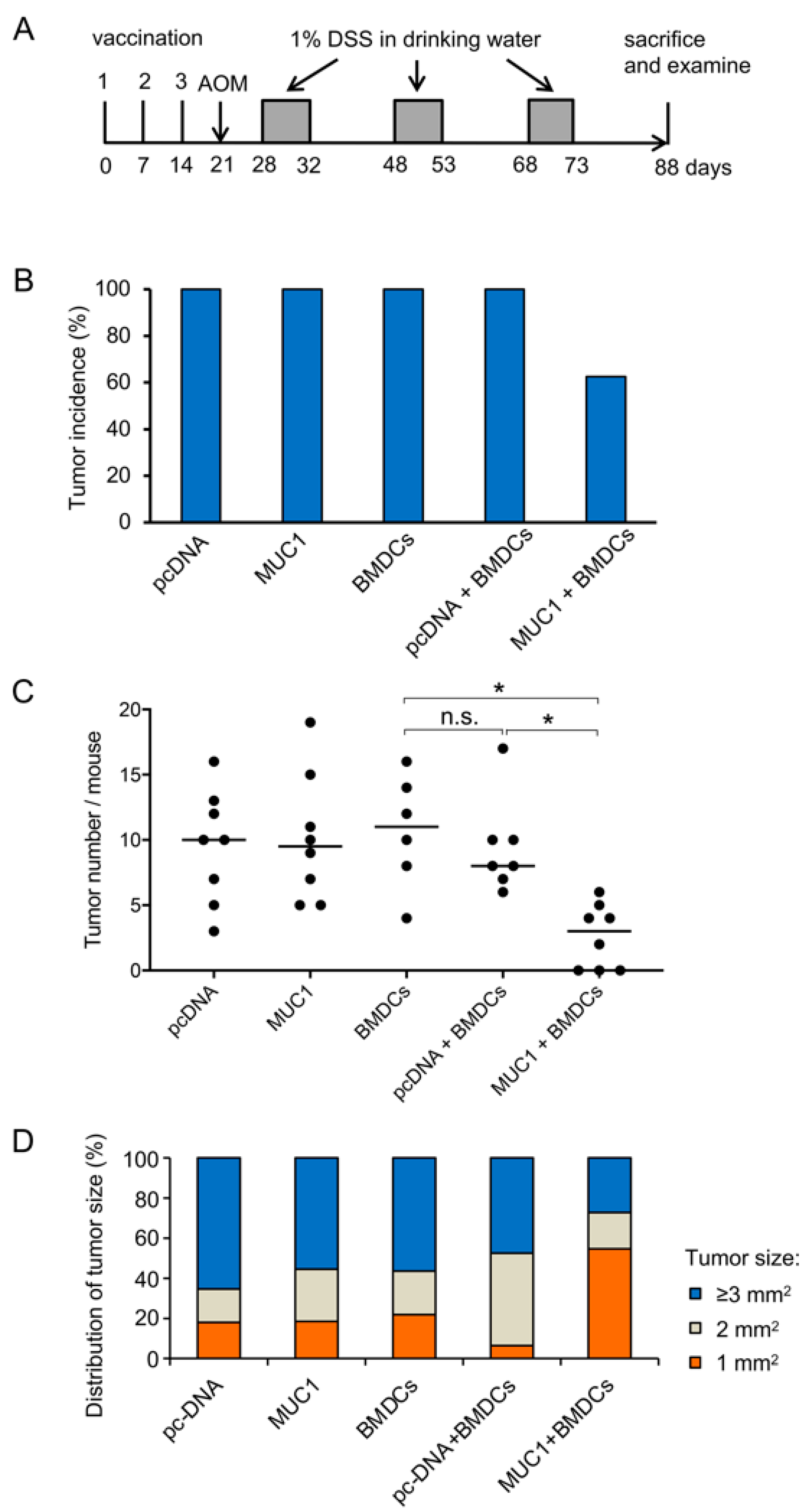
3.2. Preventive Efficacy of MUC1 DNA Vaccine on Experimental Colitis-Associated Colorectal Carcinogenesis
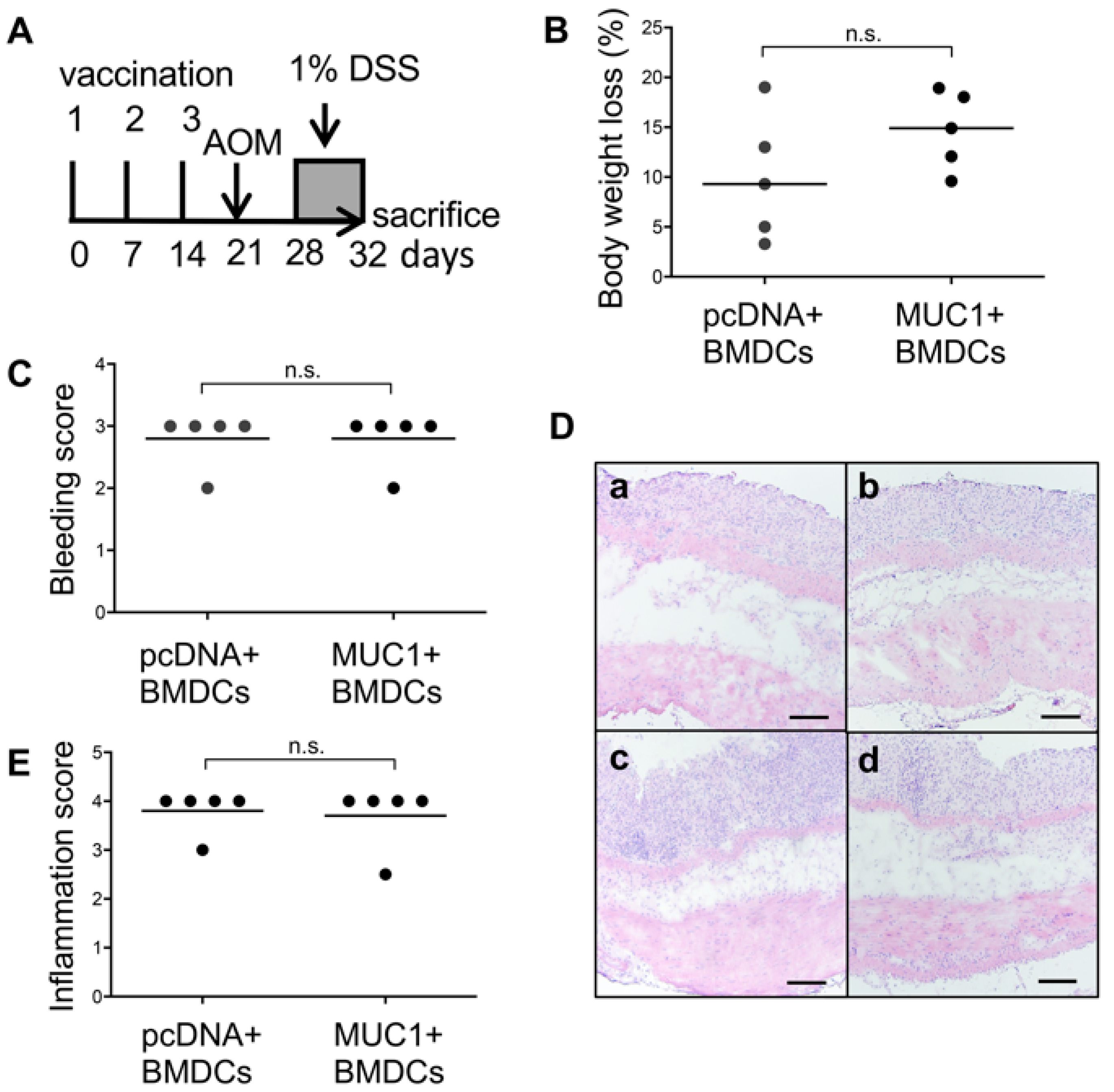
3.3. Effect of MUC1 DNA Vaccination on AOM-DSS Induced Colorectal Inflammation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Terzic, J.; Grivennikov, S.; Karin, E.; Karin, M. Inflammation and colon cancer. Gastroenterology 2010, 138, 2101–2114.e2105. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Jeang, J.; Yang, A.; Wu, T.C.; Hung, C.F. DNA vaccine for cancer immunotherapy. Hum. Vaccines Immunother. 2014, 10, 3153–3164. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, J.J.; Ulmer, J.B.; Shiver, J.W.; Liu, M.A. DNA vaccines. Annu. Rev. Immunol. 1997, 15, 617–648. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Klinman, D.M.; Seder, R.A. DNA vaccines: Immunology, application, and optimization. Annu. Rev. Immunol. 2000, 18, 927–974. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Chang, M.C.; Chiang, Y.C.; Lin, H.W.; Sun, N.Y.; Chen, C.A.; Sun, W.Z.; Cheng, W.F. Immuno-modulators enhance antigen-specific immunity and anti-tumor effects of mesothelin-specific chimeric DNA vaccine through promoting DC maturation. Cancer Lett. 2018, 425, 152–163. [Google Scholar] [CrossRef]
- Iwasaki, A.; Torres, C.A.; Ohashi, P.S.; Robinson, H.L.; Barber, B.H. The dominant role of bone marrow-derived cells in CTL induction following plasmid DNA immunization at different sites. J. Immunol. 1997, 159, 11–14. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, D.; Eling, D.J.; Robbins, J.; Kipps, T.J. DNA vaccines encoding full-length or truncated Neu induce protective immunity against Neu-expressing mammary tumors. Cancer Res. 1998, 58, 1965–1971. [Google Scholar]
- Chen, C.H.; Wang, T.L.; Hung, C.F.; Yang, Y.; Young, R.A.; Pardoll, D.M.; Wu, T.C. Enhancement of DNA vaccine potency by linkage of antigen gene to an HSP70 gene. Cancer Res. 2000, 60, 1035–1042. [Google Scholar]
- Hanisch, F.G.; Muller, S. MUC1: The polymorphic appearance of a human mucin. Glycobiology 2000, 10, 439–449. [Google Scholar] [CrossRef]
- Yang, Y.; Shao, Z.; Gao, J. Antitumor Effect of a DNA Vaccine Harboring Prostate Cancer-Specific Antigen with IL-12 as an Intramolecular Adjuvant. J. Mol. Microbiol. Biotechnol. 2017, 27, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Baghban Rahimi, S.; Mohebbi, A.; Vakilzadeh, G.; Biglari, P.; Razeghi Jahromi, S.; Mohebi, S.R.; Shirian, S.; Gorji, A.; Ghaemi, A. Enhancement of therapeutic DNA vaccine potency by melatonin through inhibiting VEGF expression and induction of antitumor immunity mediated by CD8+ T cells. Arch. Virol. 2018, 163, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Perez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Patel, A.; Tursi, N.J.; Zhu, X.; Muthumani, K.; Kulp, D.W.; Weiner, D.B. Harnessing Recent Advances in Synthetic DNA and Electroporation Technologies for Rapid Vaccine Development Against COVID-19 and Other Emerging Infectious Diseases. Front. Med. Technol. 2020, 2, 571030. [Google Scholar] [CrossRef]
- Miao, L.; Zhang, Y.; Huang, L. mRNA vaccine for cancer immunotherapy. Mol. Cancer 2021, 20, 41. [Google Scholar] [CrossRef]
- Nakamori, S.; Ota, D.M.; Cleary, K.R.; Shirotani, K.; Irimura, T. MUC1 mucin expression as a marker of progression and metastasis of human colorectal carcinoma. Gastroenterology 1994, 106, 353–361. [Google Scholar] [CrossRef]
- Ajioka, Y.; Allison, L.J.; Jass, J.R. Significance of MUC1 and MUC2 mucin expression in colorectal cancer. J. Clin. Pathol. 1996, 49, 560–564. [Google Scholar] [CrossRef]
- Vlad, A.M.; Kettel, J.C.; Alajez, N.M.; Carlos, C.A.; Finn, O.J. MUC1 immunobiology: From discovery to clinical applications. Adv. Immunol. 2004, 82, 249–293. [Google Scholar] [CrossRef]
- Adsay, N.V.; Merati, K.; Andea, A.; Sarkar, F.; Hruban, R.H.; Wilentz, R.E.; Goggins, M.; Iocobuzio-Donahue, C.; Longnecker, D.S.; Klimstra, D.S. The dichotomy in the preinvasive neoplasia to invasive carcinoma sequence in the pancreas: Differential expression of MUC1 and MUC2 supports the existence of two separate pathways of carcinogenesis. Mod. Pathol. 2002, 15, 1087–1095. [Google Scholar] [CrossRef]
- Furr, A.E.; Ranganathan, S.; Finn, O.J. Aberrant expression of MUC1 mucin in pediatric inflammatory bowel disease. Pediatr. Dev. Pathol. 2010, 13, 24–31. [Google Scholar] [CrossRef]
- Ryan, S.O.; Vlad, A.M.; Islam, K.; Gariepy, J.; Finn, O.J. Tumor-associated MUC1 glycopeptide epitopes are not subject to self-tolerance and improve responses to MUC1 peptide epitopes in MUC1 transgenic mice. Biol. Chem. 2009, 390, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Vlad, A.M.; Muller, S.; Cudic, M.; Paulsen, H.; Otvos, L., Jr.; Hanisch, F.G.; Finn, O.J. Complex carbohydrates are not removed during processing of glycoproteins by dendritic cells: Processing of tumor antigen MUC1 glycopeptides for presentation to major histocompatibility complex class II-restricted T cells. J. Exp. Med. 2002, 196, 1435–1446. [Google Scholar] [CrossRef]
- Nath, S.; Mukherjee, P. MUC1: A multifaceted oncoprotein with a key role in cancer progression. Trends Mol. Med. 2014, 20, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Beatty, P.L.; Narayanan, S.; Gariepy, J.; Ranganathan, S.; Finn, O.J. Vaccine against MUC1 antigen expressed in inflammatory bowel disease and cancer lessens colonic inflammation and prevents progression to colitis-associated colon cancer. Cancer Prev. Res. 2010, 3, 438–446. [Google Scholar] [CrossRef]
- Mukherjee, P.; Basu, G.D.; Tinder, T.L.; Subramani, D.B.; Bradley, J.M.; Arefayene, M.; Skaar, T.; De Petris, G. Progression of pancreatic adenocarcinoma is significantly impeded with a combination of vaccine and COX-2 inhibition. J. Immunol. 2009, 182, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Taylor-Papadimitriou, J.; Burchell, J.M.; Graham, R.; Beatson, R. Latest developments in MUC1 immunotherapy. Biochem. Soc. Trans. 2018, 46, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; McKolanis, J.R.; Dzubinski, L.A.; Islam, K.; Potter, D.M.; Salazar, A.M.; Schoen, R.E.; Finn, O.J. MUC1 vaccine for individuals with advanced adenoma of the colon: A cancer immunoprevention feasibility study. Cancer Prev. Res. 2013, 6, 18–26. [Google Scholar] [CrossRef]
- Zhang, S.; Graeber, L.A.; Helling, F.; Ragupathi, G.; Adluri, S.; Lloyd, K.O.; Livingston, P.O. Augmenting the immunogenicity of synthetic MUC1 peptide vaccines in mice. Cancer Res. 1996, 56, 3315–3319. [Google Scholar] [PubMed]
- Gong, J.; Chen, L.; Chen, D.; Kashiwaba, M.; Manome, Y.; Tanaka, T.; Kufe, D. Induction of antigen-specific antitumor immunity with adenovirus-transduced dendritic cells. Gene Ther. 1997, 4, 1023–1028. [Google Scholar] [CrossRef]
- Wang, Q.; Cao, W.; Yang, Z.G.; Zhao, G.F. DC targeting DNA vaccines induce protective and therapeutic antitumor immunity in mice. Int. J. Clin. Exp. Med. 2015, 8, 17565–17577. [Google Scholar]
- Glaffig, M.; Stergiou, N.; Hartmann, S.; Schmitt, E.; Kunz, H. A Synthetic MUC1 Anticancer Vaccine Containing Mannose Ligands for Targeting Macrophages and Dendritic Cells. ChemMedChem 2018, 13, 25–29. [Google Scholar] [CrossRef]
- Lees, C.J.; Apostolopoulos, V.; Acres, B.; Ong, C.S.; Popovski, V.; McKenzie, I.F. The effect of T1 and T2 cytokines on the cytotoxic T cell response to mannan-MUC1. Cancer Immunol. Immunother. 2000, 48, 644–652. [Google Scholar] [CrossRef]
- Graham, R.A.; Burchell, J.M.; Beverley, P.; Taylor-Papadimitriou, J. Intramuscular immunisation with MUC1 cDNA can protect C57 mice challenged with MUC1-expressing syngeneic mouse tumour cells. Int. J. Cancer 1996, 65, 664–670. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, H.; Shi, H.; Yu, X.; Kong, W.; Li, W. Induction of immune response and anti-tumor activities in mice with a DNA vaccine encoding human mucin 1 variable-number tandem repeats. Hum. Immunol. 2008, 69, 250–258. [Google Scholar] [CrossRef]
- Mukherjee, P.; Pathangey, L.B.; Bradley, J.B.; Tinder, T.L.; Basu, G.D.; Akporiaye, E.T.; Gendler, S.J. MUC1-specific immune therapy generates a strong anti-tumor response in a MUC1-tolerant colon cancer model. Vaccine 2007, 25, 1607–1618. [Google Scholar] [CrossRef]
- Stergiou, N.; Glaffig, M.; Jonuleit, H.; Schmitt, E.; Kunz, H. Immunization with a Synthetic Human MUC1 Glycopeptide Vaccine against Tumor-Associated MUC1 Breaks Tolerance in Human MUC1 Transgenic Mice. ChemMedChem 2017, 12, 1424–1428. [Google Scholar] [CrossRef]
- Shi, F.F.; Gunn, G.R.; Snyder, L.A.; Goletz, T.J. Intradermal vaccination of MUC1 transgenic mice with MUC1/IL-18 plasmid DNA suppresses experimental pulmonary metastases. Vaccine 2007, 25, 3338–3346. [Google Scholar] [CrossRef]
- Snyder, L.A.; Goletz, T.J.; Gunn, G.R.; Shi, F.F.; Harris, M.C.; Cochlin, K.; McCauley, C.; McCarthy, S.G.; Branigan, P.J.; Knight, D.M. A MUC1/IL-18 DNA vaccine induces anti-tumor immunity and increased survival in MUC1 transgenic mice. Vaccine 2006, 24, 3340–3352. [Google Scholar] [CrossRef]
- Apostolopoulos, V.; Pietersz, G.A.; Tsibanis, A.; Tsikkinis, A.; Drakaki, H.; Loveland, B.E.; Piddlesden, S.J.; Plebanski, M.; Pouniotis, D.S.; Alexis, M.N.; et al. Pilot phase III immunotherapy study in early-stage breast cancer patients using oxidized mannan-MUC1 [ISRCTN71711835]. Breast Cancer Res. 2006, 8, R27. [Google Scholar] [CrossRef]
- Vassilaros, S.; Tsibanis, A.; Tsikkinis, A.; Pietersz, G.A.; McKenzie, I.F.; Apostolopoulos, V. Up to 15-year clinical follow-up of a pilot Phase III immunotherapy study in stage II breast cancer patients using oxidized mannan-MUC1. Immunotherapy 2013, 5, 1177–1182. [Google Scholar] [CrossRef]
- Kamata, M.; Denda-Nagai, K.; Kubota, N.; Aida, S.; Takeda, K.; Irimura, T. Vaccination of mice with MUC1 cDNA suppresses the development of lung metastases. Clin. Exp. Metastasis 2002, 19, 689–696. [Google Scholar] [CrossRef]
- Sugiura, D.; Aida, S.; Denda-Nagai, K.; Takeda, K.; Kamata-Sakurai, M.; Yagita, H.; Irimura, T. Differential effector mechanisms induced by vaccination with MUC1 DNA in the rejection of colon carcinoma growth at orthotopic sites and metastases. Cancer Sci. 2008, 99, 2477–2484. [Google Scholar] [CrossRef] [PubMed]
- Rowse, G.J.; Tempero, R.M.; VanLith, M.L.; Hollingsworth, M.A.; Gendler, S.J. Tolerance and immunity to MUC1 in a human MUC1 transgenic murine model. Cancer Res. 1998, 58, 315–321. [Google Scholar] [PubMed]
- Neufert, C.; Becker, C.; Neurath, M.F. An inducible mouse model of colon carcinogenesis for the analysis of sporadic and inflammation-driven tumor progression. Nat. Protoc. 2007, 2, 1998. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Kohno, H.; Suzuki, R.; Yamada, Y.; Sugie, S.; Mori, H. A novel inflammation-related mouse colon carcinogenesis model induced by azoxymethane and dextran sodium sulfate. Cancer Sci. 2003, 94, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Wirtz, S.; Neufert, C.; Weigmann, B.; Neurath, M.F. Chemically induced mouse models of intestinal inflammation. Nat. Protoc. 2007, 2, 541–546. [Google Scholar] [CrossRef]
- Banchereau, J.; Steinman, R.M. Dendritic cells and the control of immunity. Nature 1998, 392, 245–252. [Google Scholar] [CrossRef]
- Wculek, S.K.; Cueto, F.J.; Mujal, A.M.; Melero, I.; Krummel, M.F.; Sancho, D. Dendritic cells in cancer immunology and immunotherapy. Nat. Rev. Immunol. 2020, 20, 7–24. [Google Scholar] [CrossRef]
- Apostolopoulos, V.; Pietersz, G.A.; Tsibanis, A.; Tsikkinis, A.; Stojanovska, L.; McKenzie, I.F.; Vassilaros, S. Dendritic cell immunotherapy: Clinical outcomes. Clin. Transl. Immunol. 2014, 3, e21. [Google Scholar] [CrossRef]
- Denda-Nagai, K.; Fujita, K.; Fujime, M.; Nakatsugawa, S.; Ishigaki, T.; Irimura, T. Absence of correlation of MUC1 expression to malignant behavior of renal cell carcinoma in experimental systems. Clin. Exp. Metastasis 2000, 18, 77–81. [Google Scholar] [CrossRef]
- Inaba, K.; Inaba, M.; Romani, N.; Aya, H.; Deguchi, M.; Ikehara, S.; Muramatsu, S.; Steinman, R.M. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 1992, 176, 1693–1702. [Google Scholar] [CrossRef]
- Boivin, G.P.; Washington, K.; Yang, K.; Ward, J.M.; Pretlow, T.P.; Russell, R.; Besselsen, D.G.; Godfrey, V.L.; Doetschman, T.; Dove, W.F.; et al. Pathology of mouse models of intestinal cancer: Consensus report and recommendations. Gastroenterology 2003, 124, 762–777. [Google Scholar] [CrossRef]
- Reeve, D.R. Squamous metaplasia in the healing of chronic colonic ulcers of the rat. J. Pathol. 1975, 117, 15–22. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: http://www.R-project.org/ (accessed on 25 January 2023).
- GraphPad Software. Available online: www.graphpad.com (accessed on 25 January 2023).
- Cao, Y.; Schlag, P.M.; Karsten, U. Immunodetection of epithelial mucin (MUC1, MUC3) and mucin-associated glycotopes (TF, Tn, and sialosyl-Tn) in benign and malignant lesions of colonic epithelium: Apolar localization corresponds to malignant transformation. Virchows Arch. 1997, 431, 159–166. [Google Scholar] [CrossRef]
- Higuchi, T.; Xin, P.; Buckley, M.S.; Erickson, D.R.; Bhavanandan, V.P. Characterization of the rabbit homolog of human MUC1 glycoprotein isolated from bladder by affinity chromatography on immobilized jacalin. Glycobiology 2000, 10, 659–667. [Google Scholar] [CrossRef]
- Takeuchi, H.; Kato, K.; Denda-Nagai, K.; Hanisch, F.G.; Clausen, H.; Irimura, T. The epitope recognized by the unique anti-MUC1 monoclonal antibody MY.1E12 involves sialyl alpha 2-3galactosyl beta 1-3N-acetylgalactosaminide linked to a distinct threonine residue in the MUC1 tandem repeat. J. Immunol. Methods 2002, 270, 199–209. [Google Scholar] [CrossRef]
- Yoshimura, Y.; Denda-Nagai, K.; Takahashi, Y.; Nagashima, I.; Shimizu, H.; Kishimoto, T.; Noji, M.; Shichino, S.; Chiba, Y.; Irimura, T. Products of Chemoenzymatic Synthesis Representing MUC1 Tandem Repeat Unit with T-, ST- or STn-antigen Revealed Distinct Specificities of Anti-MUC1 Antibodies. Sci. Rep. 2019, 9, 16641. [Google Scholar] [CrossRef]
- Sugiura, D.; Denda-Nagai, K.; Takashima, M.; Murakami, R.; Nagai, S.; Takeda, K.; Irimura, T. Local effects of regulatory T cells in MUC1 transgenic mice potentiate growth of MUC1 expressing tumor cells in vivo. PLoS ONE 2012, 7, e44770. [Google Scholar] [CrossRef]
- Maecker, H.T.; Umetsu, D.T.; DeKruyff, R.H.; Levy, S. Cytotoxic T cell responses to DNA vaccination: Dependence on antigen presentation via class II MHC. J. Immunol. 1998, 161, 6532–6536. [Google Scholar] [CrossRef]
- Liu, M.A. DNA vaccines: A review. J. Intern. Med. 2003, 253, 402–410. [Google Scholar] [CrossRef]
- Kontani, K.; Taguchi, O.; Ozaki, Y.; Hanaoka, J.; Tezuka, N.; Sawai, S.; Inoue, S.; Fujino, S.; Maeda, T.; Itoh, Y.; et al. Novel vaccination protocol consisting of injecting MUC1 DNA and nonprimed dendritic cells at the same region greatly enhanced MUC1-specific antitumor immunity in a murine model. Cancer Gene Ther. 2002, 9, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Jager, E.; Ringhoffer, M.; Altmannsberger, M.; Arand, M.; Karbach, J.; Jager, D.; Oesch, F.; Knuth, A. Immunoselection in vivo: Independent loss of MHC class I and melanocyte differentiation antigen expression in metastatic melanoma. Int. J. Cancer 1997, 71, 142–147. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murwanti, R.; Denda-Nagai, K.; Sugiura, D.; Mogushi, K.; Gendler, S.J.; Irimura, T. Prevention of Inflammation-Driven Colon Carcinogenesis in Human MUC1 Transgenic Mice by Vaccination with MUC1 DNA and Dendritic Cells. Cancers 2023, 15, 1920. https://doi.org/10.3390/cancers15061920
Murwanti R, Denda-Nagai K, Sugiura D, Mogushi K, Gendler SJ, Irimura T. Prevention of Inflammation-Driven Colon Carcinogenesis in Human MUC1 Transgenic Mice by Vaccination with MUC1 DNA and Dendritic Cells. Cancers. 2023; 15(6):1920. https://doi.org/10.3390/cancers15061920
Chicago/Turabian StyleMurwanti, Retno, Kaori Denda-Nagai, Daisuke Sugiura, Kaoru Mogushi, Sandra J. Gendler, and Tatsuro Irimura. 2023. "Prevention of Inflammation-Driven Colon Carcinogenesis in Human MUC1 Transgenic Mice by Vaccination with MUC1 DNA and Dendritic Cells" Cancers 15, no. 6: 1920. https://doi.org/10.3390/cancers15061920
APA StyleMurwanti, R., Denda-Nagai, K., Sugiura, D., Mogushi, K., Gendler, S. J., & Irimura, T. (2023). Prevention of Inflammation-Driven Colon Carcinogenesis in Human MUC1 Transgenic Mice by Vaccination with MUC1 DNA and Dendritic Cells. Cancers, 15(6), 1920. https://doi.org/10.3390/cancers15061920