Clinical Determinants of Extraurinary Tract Recurrence and Survival after Radical Surgery for pT2 Upper Tract Urothelial Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
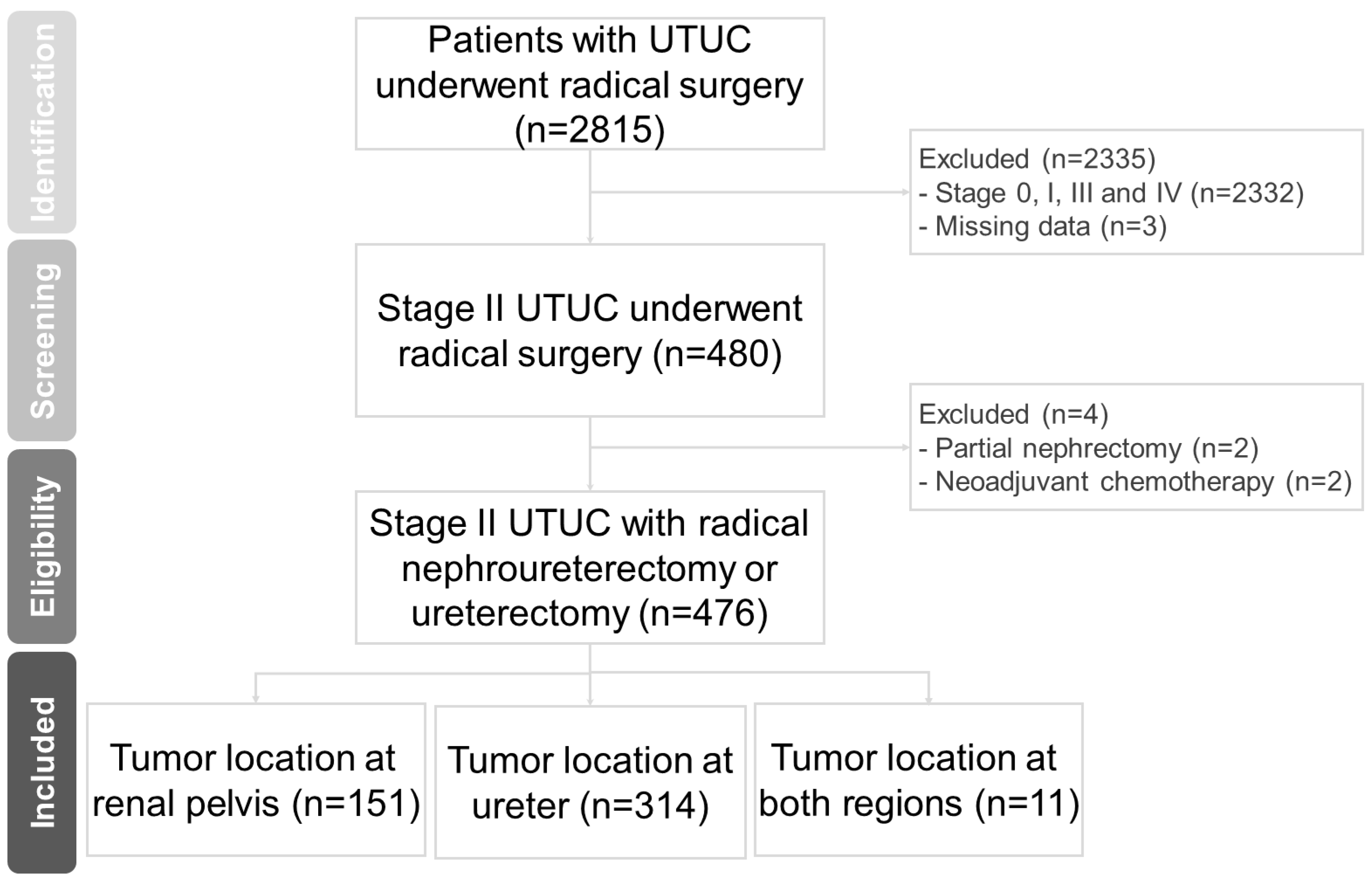
2.1. Patient Selection
2.2. Pathological Evaluation
2.3. Outcome Measures
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Recurrence and Survival
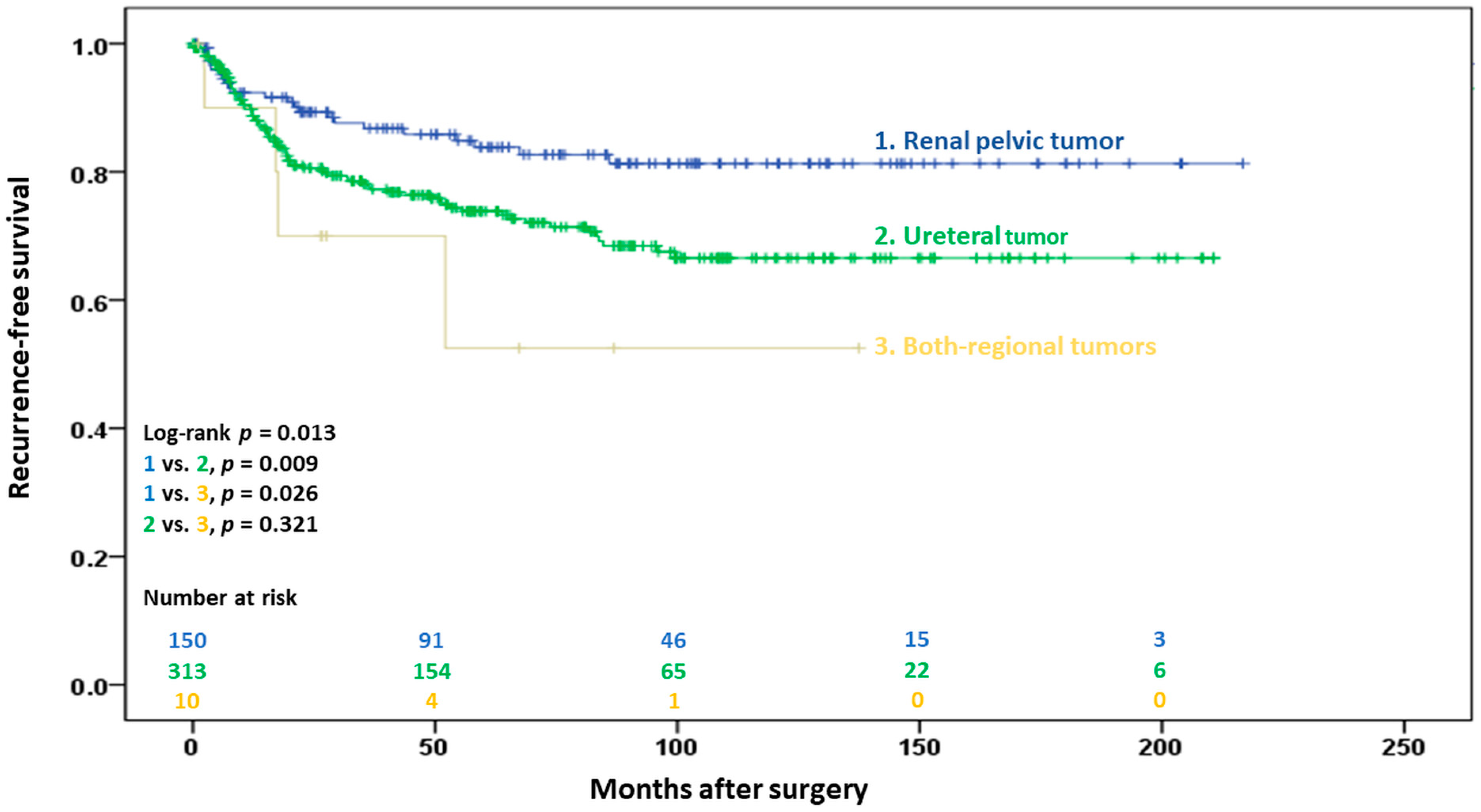
3.2.1. Extraurinary Tract Recurrence
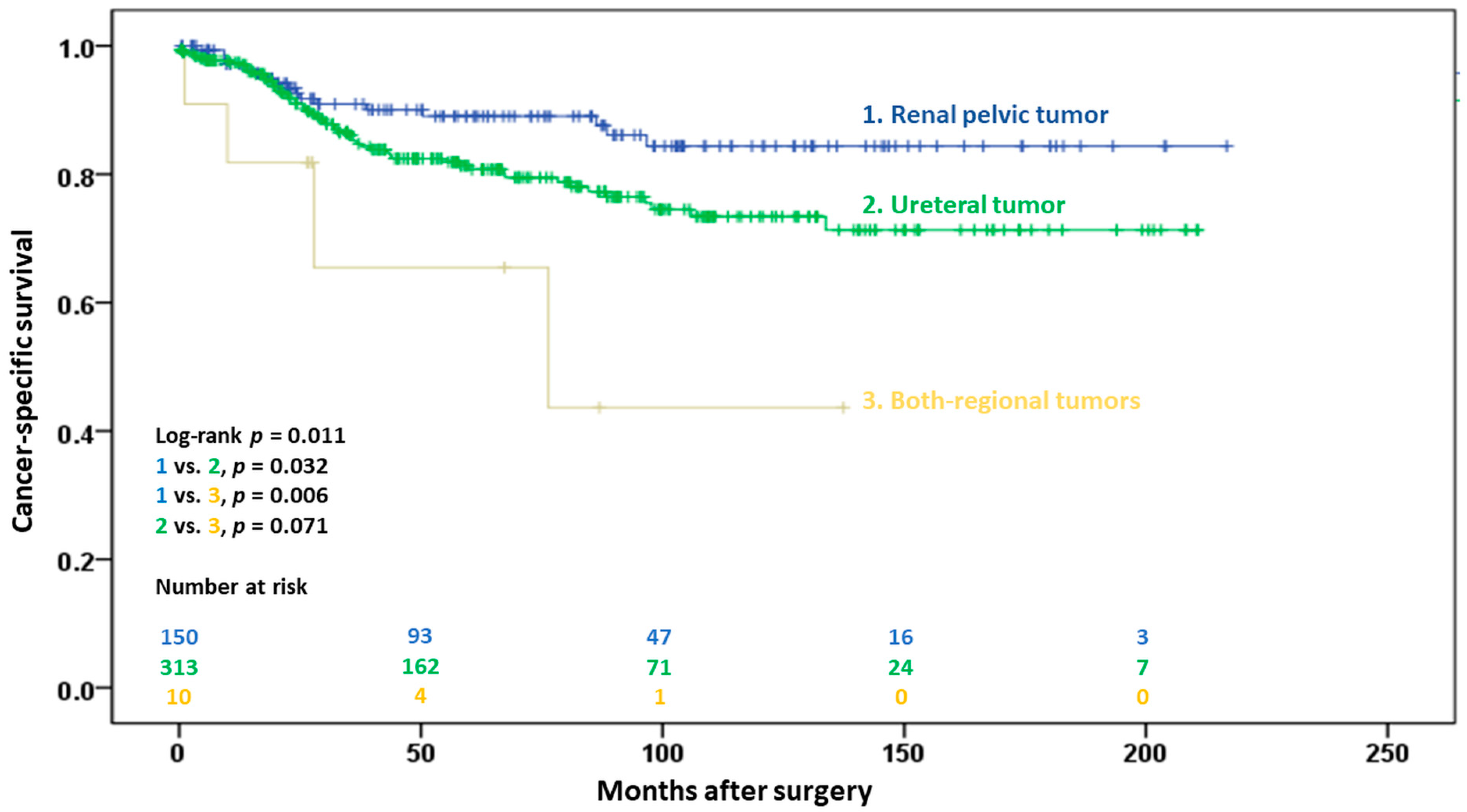
3.2.2. Cancer-Specific Survival
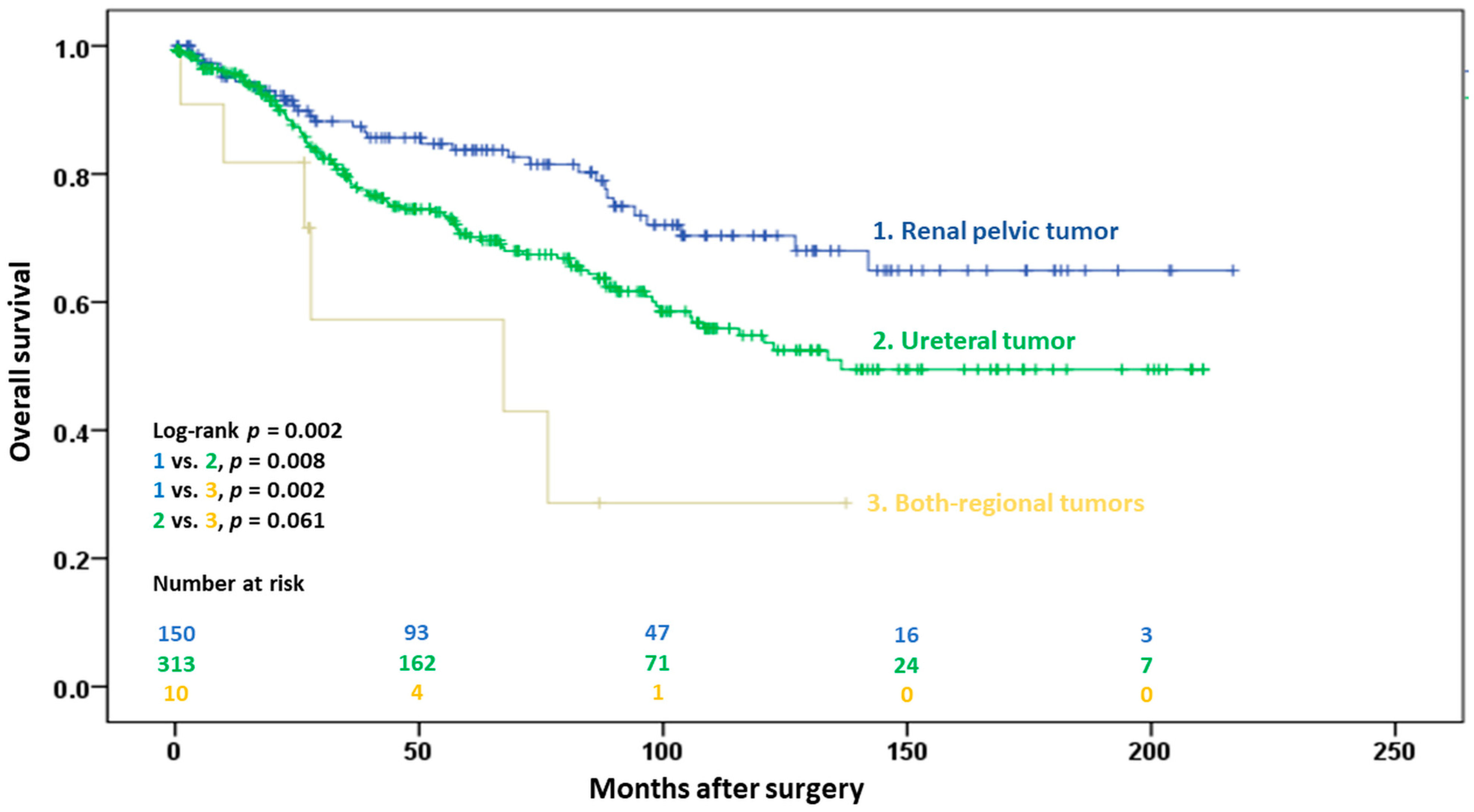
3.2.3. Overall Survival
3.3. Subset Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Soria, F.; Shariat, S.F.; Lerner, S.P.; Fritsche, H.M.; Rink, M.; Kassouf, W.; Spiess, P.E.; Lotan, Y.; Ye, D.; Fernandez, M.I.; et al. Epidemiology, diagnosis, preoperative evaluation and prognostic assessment of upper-tract urothelial carcinoma (UTUC). World J. Urol. 2017, 35, 379–387. [Google Scholar] [CrossRef]
- Catto, J.W.; Yates, D.R.; Rehman, I.; Azzouzi, A.R.; Patterson, J.; Sibony, M.; Cussenot, O.; Hamdy, F.C. Behavior of urothelial carcinoma with respect to anatomical location. J. Urol. 2007, 177, 1715–1720. [Google Scholar] [CrossRef]
- Roupret, M.; Babjuk, M.; Burger, M.; Capoun, O.; Cohen, D.; Comperat, E.M.; Cowan, N.C.; Dominguez-Escrig, J.L.; Gontero, P.; Hugh Mostafid, A.; et al. European Association of Urology Guidelines on Upper Urinary Tract Urothelial Carcinoma: 2020 Update. Eur. Urol. 2021, 79, 62–79. [Google Scholar] [CrossRef]
- Margulis, V.; Shariat, S.F.; Matin, S.F.; Kamat, A.M.; Zigeuner, R.; Kikuchi, E.; Lotan, Y.; Weizer, A.; Raman, J.D.; Wood, C.G.; et al. Outcomes of radical nephroureterectomy: A series from the Upper Tract Urothelial Carcinoma Collaboration. Cancer 2009, 115, 1224–1233. [Google Scholar] [CrossRef] [PubMed]
- Flaig, T.W.; Spiess, P.E.; Agarwal, N.; Bangs, R.; Boorjian, S.A.; Buyyounouski, M.K.; Chang, S.; Downs, T.M.; Efstathiou, J.A.; Friedlander, T.; et al. Bladder Cancer, Version 3.2020, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2020, 18, 329–354. [Google Scholar] [CrossRef] [PubMed]
- Birtle, A.; Johnson, M.; Chester, J.; Jones, R.; Dolling, D.; Bryan, R.T.; Harris, C.; Winterbottom, A.; Blacker, A.; Catto, J.W.F.; et al. Adjuvant chemotherapy in upper tract urothelial carcinoma (the POUT trial): A phase 3, open-label, randomised controlled trial. Lancet 2020, 395, 1268–1277. [Google Scholar] [CrossRef] [PubMed]
- Leow, J.J.; Chong, Y.L.; Chang, S.L.; Valderrama, B.P.; Powles, T.; Bellmunt, J. Neoadjuvant and Adjuvant Chemotherapy for Upper Tract Urothelial Carcinoma: A 2020 Systematic Review and Meta-analysis, and Future Perspectives on Systemic Therapy. Eur. Urol. 2021, 79, 635–654. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.S.; Lin, M.H.; Lee, C.P.; Yang, Y.H.; Chen, W.C.; Chang, G.H.; Tsai, Y.T.; Chen, P.C.; Tsai, Y.H. Chang Gung Research Database: A multi-institutional database consisting of original medical records. Biomed. J. 2017, 40, 263–269. [Google Scholar] [CrossRef]
- Ito, Y.; Kikuchi, E.; Tanaka, N.; Miyajima, A.; Mikami, S.; Jinzaki, M.; Oya, M. Preoperative hydronephrosis grade independently predicts worse pathological outcomes in patients undergoing nephroureterectomy for upper tract urothelial carcinoma. J. Urol. 2011, 185, 1621–1626. [Google Scholar] [CrossRef]
- Inker, L.A.; Eneanya, N.D.; Coresh, J.; Tighiouart, H.; Wang, D.; Sang, Y.; Crews, D.C.; Doria, A.; Estrella, M.M.; Froissart, M.; et al. New Creatinine- and Cystatin C-Based Equations to Estimate GFR without Race. N. Engl. J. Med. 2021, 385, 1737–1749. [Google Scholar] [CrossRef] [PubMed]
- Delgado, C.; Baweja, M.; Crews, D.C.; Eneanya, N.D.; Gadegbeku, C.A.; Inker, L.A.; Mendu, M.L.; Miller, W.G.; Moxey-Mims, M.M.; Roberts, G.V.; et al. A Unifying Approach for GFR Estimation: Recommendations of the NKF-ASN Task Force on Reassessing the Inclusion of Race in Diagnosing Kidney Disease. Am. J. Kidney Dis. 2022, 79, 268–288.e1. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.C.; Liu, Y.L.; Chen, M.F.; Chen, C.S.; Wu, C.T. Treatment Strategy for Dialysis Patient with Urothelial Carcinoma. Diagnostics 2021, 11, 1966. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.L.; Wu, C.T.; Hsu, Y.C.; Chen, M.F.; Chen, C.S.; Shi, C.S.; Huang, Y.C. Perioperative Complications and Oncologic Outcomes after Radical Cystectomy in End-Stage Renal Disease Patients with Bladder Cancer Obtained Using a Standardized Reporting System. Cancers 2022, 14, 3512. [Google Scholar] [CrossRef] [PubMed]
- Rink, M.; Ehdaie, B.; Cha, E.K.; Green, D.A.; Karakiewicz, P.I.; Babjuk, M.; Margulis, V.; Raman, J.D.; Svatek, R.S.; Fajkovic, H.; et al. Stage-specific impact of tumor location on oncologic outcomes in patients with upper and lower tract urothelial carcinoma following radical surgery. Eur. Urol. 2012, 62, 677–684. [Google Scholar] [CrossRef]
- Hakimi, K.; Carbonara, U.; Djaladat, H.; Mehrazin, R.; Eun, D.; Reese, A.; Gonzalgo, M.L.; Margulis, V.; Uzzo, R.G.; Porter, J.; et al. Outcomes of Lymph Node Dissection in Nephroureterectomy in the Treatment of Upper Tract Urothelial Carcinoma: Analysis of the ROBUUST Registry. J. Urol. 2022, 208, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Raman, J.D.; Ng, C.K.; Scherr, D.S.; Margulis, V.; Lotan, Y.; Bensalah, K.; Patard, J.J.; Kikuchi, E.; Montorsi, F.; Zigeuner, R.; et al. Impact of tumor location on prognosis for patients with upper tract urothelial carcinoma managed by radical nephroureterectomy. Eur. Urol. 2010, 57, 1072–1079. [Google Scholar] [CrossRef]
- Isbarn, H.; Jeldres, C.; Shariat, S.F.; Liberman, D.; Sun, M.; Lughezzani, G.; Widmer, H.; Arjane, P.; Pharand, D.; Fisch, M.; et al. Location of the primary tumor is not an independent predictor of cancer specific mortality in patients with upper urinary tract urothelial carcinoma. J. Urol. 2009, 182, 2177–2181. [Google Scholar] [CrossRef]
- Ouzzane, A.; Colin, P.; Xylinas, E.; Pignot, G.; Ariane, M.M.; Saint, F.; Hoarau, N.; Adam, E.; Azemar, M.D.; Bensadoun, H.; et al. Ureteral and multifocal tumours have worse prognosis than renal pelvic tumours in urothelial carcinoma of the upper urinary tract treated by nephroureterectomy. Eur. Urol. 2011, 60, 1258–1265. [Google Scholar] [CrossRef]
- Waseda, Y.; Saito, K.; Ishioka, J.; Matsuoka, Y.; Numao, N.; Fujii, Y.; Sakai, Y.; Koga, F.; Okuno, T.; Arisawa, C.; et al. Ureteral Involvement Is Associated with Poor Prognosis in Upper Urinary Tract Urothelial Carcinoma Patients Treated by Nephroureterectomy: A Multicenter Database Study. Eur. Urol. Focus. 2016, 2, 296–302. [Google Scholar] [CrossRef]
- Hashimoto, T.; Ohno, Y.; Nakashima, J.; Gondo, T.; Nakagami, Y.; Namiki, K.; Horiguchi, Y.; Yoshioka, K.; Ohori, M.; Tachibana, M. Prediction of renal function after nephroureterectomy in patients with upper tract urothelial carcinoma. Jpn. J. Clin. Oncol. 2015, 45, 1064–1068. [Google Scholar] [CrossRef] [PubMed]
- Youssef, R.F.; Shariat, S.F.; Lotan, Y.; Wood, C.G.; Sagalowsky, A.I.; Zigeuner, R.; Langner, C.; Montorsi, F.; Bolenz, C.; Margulis, V. Prognostic effect of urinary bladder carcinoma in situ on clinical outcome of subsequent upper tract urothelial carcinoma. Urology 2011, 77, 861–866. [Google Scholar] [CrossRef] [PubMed]
- Nuhn, P.; Novara, G.; Seitz, C.; Gupta, A.; Matsumoto, K.; Kassouf, W.; Walton, T.J.; Fritsche, H.M.; Tritschler, S.; Martinez-Salamanca, J.I.; et al. Prognostic value of prior history of urothelial carcinoma of the bladder in patients with upper urinary tract urothelial carcinoma: Results from a retrospective multicenter study. World J. Urol. 2015, 33, 1005–1013. [Google Scholar] [CrossRef]
- Sikic, D.; Wach, S.; Taubert, H.; Richterstetter, M.; Kunath, F.; Goebell, P.J.; Schick, S.; Olbert, P.; Huber, J.; Wullich, B.; et al. Female Gender Is an Age-dependent Negative Prognostic Factor for Patients with Upper Tract Urothelial Carcinoma. Anticancer. Res. 2015, 35, 4277–4281. [Google Scholar] [PubMed]
- Milojevic, B.; Dzamic, Z.; Grozdic Milojevic, I.; Bumbasirevic, U.; Santric, V.; Kajmakovic, B.; Janicic, A.; Durutovic, O.; Dragicevic, D.; Bojanic, N.; et al. Prognostic value of Balkan endemic nephropathy and gender on upper tract urothelial carcinoma outcomes after radical nephroureterectomy: A cohort study. Urol. Oncol. 2021, 39, 786.e9–786.e16. [Google Scholar] [CrossRef]
- Wu, Y.T.; Luo, H.L.; Wang, H.J.; Chen, Y.T.; Cheng, Y.T.; Chiang, P.H. Gender effect on the oncologic outcomes of upper urinary tract urothelial carcinoma in Taiwan. Int. Urol. Nephrol. 2020, 52, 1043–1048. [Google Scholar] [CrossRef]
- Mori, K.; Mostafaei, H.; Enikeev, D.V.; Lysenko, I.; Quhal, F.; Kimura, S.; Karakiewicz, P.I.; Egawa, S.; Shariat, S.F. Differential Effect of Sex on Outcomes after Radical Surgery for Upper Tract and Bladder Urothelial Carcinoma: A Systematic Review and Meta-Analysis. J. Urol. 2020, 204, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Yap, S.A.; Schupp, C.W.; Chamie, K.; Evans, C.P.; Koppie, T.M. Effect of age on transitional cell carcinoma of the upper urinary tract: Presentation, treatment, and outcomes. Urology 2011, 78, 87–92. [Google Scholar] [CrossRef]
- Shariat, S.F.; Godoy, G.; Lotan, Y.; Droller, M.; Karakiewicz, P.I.; Raman, J.D.; Isbarn, H.; Weizer, A.; Remzi, M.; Roscigno, M.; et al. Advanced patient age is associated with inferior cancer-specific survival after radical nephroureterectomy. BJU Int. 2010, 105, 1672–1677. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.L.; Chen, Y.T.; Cheng, Y.T.; Chiang, P.H. Re: Thomas Seisen, Benoit Peyronnet, Jose Luis Dominguez-Escrig; et al. Oncologic Outcomes of Kidney-sparing Surgery Versus Radical Nephroureterectomy for Upper Tract Urothelial Carcinoma: A Systematic Review by the EAU Non-muscle Invasive Bladder Cancer Guidelines Panel. Eur Urol 2016;70:1052-68: Preoperative Bladder Cancer History and Chronic Kidney Disease Are Associated with Occult Renal Pelvis Cancer in Preoperative Solitary Ureteral Cancer. Eur. Urol. 2017, 71, e109–e110. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Matsumoto, K.; Hirayama, T.; Koguchi, D.; Murakami, Y.; Matsuda, D.; Okuno, N.; Utsunomiya, T.; Taoka, Y.; Irie, A.; et al. Oncologic Outcomes of Salvage Chemotherapy in Patients with Recurrent or Metastatic Lesions after Radical Nephroureterectomy: A Multi-Institutional Retrospective Study. Chemotherapy 2020, 65, 134–140. [Google Scholar] [CrossRef] [PubMed]
| Main Tumor Location | |||||
|---|---|---|---|---|---|
| Total (n = 476) | Renal Pelvis (n = 151) | Ureter (n = 314) | Both Regions (n = 11) | p Value | |
| Gender | 0.236 | ||||
| Female | 252 (52.9) | 87 (57.6) | 161 (51.3) | 4 (36.4) | |
| Male | 224 (47.1) | 64 (42.4) | 153 (48.7) | 7 (63.6) | |
| Age, years, median (IQR) | 70.7 (62.4–77.0) | 69.2 (61.2–76.1) | 70.8 (62.7–77.5) | 68.9 (58.5–74.0) | 0.224 |
| <60 | 94 (19.7) | 34 (22.5) | 56 (17.8) | 4 (36.4) | 0.439 |
| 60–70 | 133 (27.9) | 44 (29.1) | 87 (27.7) | 2 (18.2) | |
| 70–80 | 180 (37.8) | 51 (33.8) | 124 (39.5) | 5 (45.5) | |
| >80 | 69 (14.5) | 22 (14.6) | 47 (15.0) | 0 (0) | |
| Contralateral UTUC history | 0.370 | ||||
| Previous | 23 (4.8) | 10 (6.6) | 13 (4.1) | 0 (0) | |
| Synchronous | 7 (1.5) | 2 (1.3) | 4 (1.3) | 1 (9.1) | |
| Metachronous | 32 (6.7) | 10 (6.6) | 21 (6.7) | 1 (9.1) | |
| Bladder cancer history | |||||
| Previous | 58 (12.2) | 16 (10.6) | 39 (12.4) | 3 (27.3) | 0.258 |
| Synchronous | 93 (19.5) | 18 (11.9) | 70 (22.3) | 5 (45.5) | 0.003 |
| Metachronous | 158 (33.2) | 37 (24.5) | 114 (36.3) | 7 (63.6) | 0.004 |
| Hydronephrosis grade | <0.001 | ||||
| 0 | 41 (8.6) | 25 (16.6) | 15 (4.8) | 1 (9.1) | |
| 1 | 59 (12.4) | 38 (25.2) | 19 (6.1) | 2 (18.2) | |
| 2 | 114 (23.9) | 39 (25.8) | 71 (22.6) | 4 (36.4) | |
| 3 | 121 (25.4) | 25 (16.6) | 96 (30.6) | 0 (0) | |
| 4 | 130 (27.3) | 21 (13.9) | 105 (33.4) | 4 (36.4) | |
| Unknown | 11 (2.3) | 3 (2.0) | 8 (2.5) | 0 (0) | |
| ASA score, median (IQR) | 3 (2–3) | 3 (2–3) | 3 (2–3) | 3 (2–3) | 0.542 |
| ≤2 | 189 (39.7) | 64 (42.4) | 122 (38.9) | 3 (27.3) | 0.533 |
| ≥3 | 287 (60.3) | 87 (57.6) | 192 (61.1) | 8 (72.7) | |
| Diagnostic ureteroscopy | 0.001 | ||||
| Ureteroscopic biopsy | 125 (26.3) | 22 (14.6) | 100 (31.8) | 3 (27.3) | |
| Ureteroscopy without biopsy | 100 (21.0) | 31 (20.5) | 68 (21.7) | 1 (9.1) | |
| Surgical approach | 0.608 | ||||
| Open | 247 (51.9) | 76 (50.3) | 164 (52.2) | 7 (63.6) | |
| Laparoscopic | 213 (44.7) | 72 (47.7) | 137 (43.6) | 4 (36.4) | |
| Robotic | 16 (3.4) | 3 (2.0) | 13 (4.1) | 0 (0) | |
| Surgical procedure | <0.001 | ||||
| Nephroureterectomy | 440 (92.4) | 151 (100.0) | 278 (88.5) | 11 (100.0) | |
| Ureterectomy | 36 (7.6) | 0 (0) | 36 (11.5) | 0 (0) | |
| Tumor grade | 0.307 | ||||
| Low | 25 (5.3) | 11 (7.3) | 13 (4.1) | 1 (9.1) | |
| High | 451 (94.7) | 140 (92.7) | 301 (95.9) | 10 (90.9) | |
| Multifocal disease | 141 (29.6) | 38 (25.2) | 92 (29.3) | 11 (100.0) | <0.001 |
| Carcinoma in situ | 125 (26.3) | 35 (23.2) | 85 (27.1) | 5 (45.5) | 0.230 |
| Lymphovascular invasion | 54 (11.3) | 26 (17.2) | 26 (8.3) | 2 (18.2) | 0.013 |
| Positive surgical margin | 18 (3.8) | 2 (1.3) | 16 (5.1) | 0 (0) | 0.109 |
| eGFR, mL/min/1.73 m2, median (IQR) | 44.9 (24.3–57.0) | 43.3 (20.8–56.8) | 46.2 (27.2–57.1) | 35.3 (0–53.9) | 0.156 |
| <60 | 362 (76.1) | 117 (77.5) | 235 (74.8) | 10 (90.9) | 0.315 |
| ≥60 | 97 (20.4) | 26 (17.2) | 70 (22.3) | 1 (9.1) | |
| Unknown | 17 (3.6) | 8 (5.3) | 9 (2.9) | 0 (0) | |
| Recurrence | 107 (22.5) | 23 (15.2) | 80 (25.5) | 4 (36.4) | 0.025 |
| Locoregional failure | 42 (8.8) | 7 (4.6) | 33 (10.5) | 2 (18.2) | 0.111 |
| Distant metastasis | 51 (10.7) | 14 (9.3) | 36 (11.5) | 1 (9.1%) | |
| Locoregional + distant metastasis | 14 (2.9) | 2 (1.3) | 11 (3.5) | 1 (9.1) | |
| Recurrence-Free Survival | ||||
|---|---|---|---|---|
| Univariate | Multivariate | |||
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Male gender (referent: female) | 1.54 (1.05–2.26) | 0.026 | 1.41 (0.96–2.08) | 0.080 |
| Age (referent: <60 years) | 0.085 | 0.056 | ||
| 60–70 years | 1.84 (1.02–3.34) | 0.044 | 2.12 (1.16–3.88) | 0.015 |
| 70–80 years | 1.70 (0.95–3.04) | 0.073 | 1.86 (1.03–3.37) | 0.040 |
| >80 | 2.38 (1.20–4.74) | 0.013 | 2.42 (1.21–4.87) | 0.013 |
| Contralateral UTUC (referent: absent) | 0.766 | |||
| Previous | 1.37 (0.64–2.96) | 0.418 | ||
| Synchronous | 0.69 (0.10–4.96) | 0.713 | ||
| Metachronous | 0.81 (0.38–1.75) | 0.592 | ||
| Bladder cancer (referent: absent) | ||||
| Previous | 2.20 (1.39–3.50) | 0.001 | 2.12 (1.31–3.42) | 0.002 |
| Synchronous | 1.28 (0.81–2.01) | 0.289 | ||
| Metachronous | 1.44 (0.98–2.11) | 0.062 | ||
| Hydronephrosis grade (referent: grade 0) | 0.209 | |||
| 1 | 0.42 (0.16–1.11) | 0.081 | ||
| 2 | 0.98 (0.47–2.01) | 0.945 | ||
| 3 | 1.14 (0.56–2.32) | 0.715 | ||
| 4 | 0.88 (0.43–1.81) | 0.725 | ||
| ASA score ≥ 3 (referent: ASA ≤ 2) | 0.97 (0.67–1.43) | 0.892 | ||
| Diagnostic ureteroscopy (referent: no) | 0.453 | |||
| Ureteroscopic biopsy | 1.27 (0.82–1.97) | 0.282 | ||
| Ureteroscopy without biopsy | 0.94 (0.57–1.54) | 0.793 | ||
| Surgical approach (referent: open) | 0.173 | |||
| Laparoscopic | 1.11 (0.75–1.64) | 0.610 | ||
| Robotic | 2.24 (0.96–5.22) | 0.062 | ||
| Ureterectomy procedure (referent: NU) | 1.70 (0.93–3.10) | 0.083 | ||
| Tumor location (referent: renal pelvis) | 0.015 | 0.022 | ||
| Ureter | 1.85 (1.16–2.94) | 0.010 | 1.77 (1.11–2.83) | 0.016 |
| Synchronous renal pelvis and ureter | 3.09 (1.07–8.94) | 0.038 | 3.18 (1.07–9.41) | 0.037 |
| Tumor grade (referent: low grade) | 1.45 (0.53–3.94) | 0.466 | ||
| Multifocal disease (referent: absent) | 1.12 (0.75–1.69) | 0.579 | ||
| Carcinoma in situ (referent: absent) | 0.82 (0.52–1.28) | 0.374 | ||
| Lymphovascular invasion (referent: absent) | 1.50 (0.88–2.55) | 0.135 | ||
| Positive surgical margin (referent: absent) | 4.48 (2.33–8.61) | <0.001 | 3.79 (1.95–7.35) | <0.001 |
| Chronic kidney disease a (referent: absent) | 0.86 (0.54–1.35) | 0.499 | ||
| Cancer-Specific Survival | ||||
|---|---|---|---|---|
| Univariate | Multivariate | |||
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Male gender (referent: female) | 1.72 (1.10–2.69) | 0.017 | 1.64 (1.04–2.59) | 0.034 |
| Age (referent: <60 years) | 0.001 | <0.001 | ||
| 60–70 years | 3.87 (1.69–8.85) | 0.001 | 4.89 (2.10–11.4) | <0.001 |
| 70–80 years | 2.96 (1.29–6.78) | 0.010 | 3.66 (1.57–8.52) | 0.003 |
| >80 | 5.86 (2.37–14.5) | <0.001 | 6.78 (2.69–17.1) | <0.001 |
| Contralateral UTUC (referent: absent) | 0.929 | |||
| Previous | 1.35 (0.54–3.34) | 0.523 | ||
| Synchronous | 0.93 (0.13–6.67) | 0.938 | ||
| Metachronous | 1.10 (0.50–2.39) | 0.817 | ||
| Bladder cancer (referent: absent) | ||||
| Previous | 2.54 (1.50–4.32) | 0.001 | 2.49 (1.44–4.32) | 0.001 |
| Synchronous | 1.64 (1.0–2.71) | 0.051 | ||
| Metachronous | 1.30 (0.83–2.03) | 0.249 | ||
| Hydronephrosis grade (referent: grade 0) | 0.350 | |||
| 1 | 0.555 | |||
| 2 | 0.72 (0.24–2.14) | 0.776 | ||
| 3 | 0.87 (0.33–2.27) | 0.474 | ||
| 4 | 1.39 (0.57–3.39) | 0.472 | ||
| ASA score ≥ 3 (referent: ASA ≤ 2) | 1.41 (0.89–2.22) | 0.146 | ||
| Diagnostic ureteroscopy (referent: no) | 0.511 | |||
| Ureteroscopic biopsy | 1.19 (0.71–1.98) | 0.510 | ||
| Ureteroscopy without biopsy | 0.81 (0.45–1.46) | 0.483 | ||
| Surgical approach (referent: open) | 0.365 | |||
| Laparoscopic | 0.95 (0.60–1.49) | 0.809 | ||
| Robotic | 2.01 (0.72–5.60) | 0.185 | ||
| Ureterectomy procedure (referent: NU) | 1.10 (0.48–2.53) | 0.823 | ||
| Tumor location (referent: renal pelvis) | 0.015 | 0.008 | ||
| Ureter | 1.79 (1.04–3.08) | 0.035 | 1.75 (1.01–3.02) | 0.045 |
| Synchronous renal pelvis and ureter | 4.35 (1.46–13.0) | 0.008 | 5.39 (1.76–16.5) | 0.003 |
| Tumor grade (referent: low grade) | 1.36 (0.43–4.33) | 0.598 | ||
| Multifocal disease (referent: absent) | 1.34 (0.84–2.13) | 0.221 | ||
| Carcinoma in situ (referent: absent) | 0.97 (0.59–1.60) | 0.971 | ||
| Lymphovascular invasion (referent: absent) | 1.57 (0.85–2.90) | 0.151 | ||
| Positive surgical margin (referent: absent) | 3.48 (1.51–8.03) | 0.004 | 2.64 (1.13–6.17) | 0.026 |
| Chronic kidney disease a (referent: absent) | 0.81 (0.49–1.35) | 0.424 | ||
| Overall Survival | ||||
|---|---|---|---|---|
| Univariate | Multivariate | |||
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Male gender (referent: female) | 1.27 (0.91–1.76) | 0.162 | ||
| Age (referent: <60 years) | <0.001 | <0.001 | ||
| 60–70 years | 2.38 (1.35–4.21) | 0.003 | 2.63 (1.48–4.68) | 0.001 |
| 70–80 years | 2.88 (1.68–4.94) | <0.001 | 3.0 (1.72–5.23) | <0.001 |
| >80 | 4.32 (2.29–8.16) | <0.001 | 4.04 (2.10–7.80) | <0.001 |
| Contralateral UTUC (referent: absent) | 0.860 | |||
| Previous | 1.23 (0.60–2.52) | 0.567 | ||
| Synchronous | 1.47 (0.47–4.64) | 0.507 | ||
| Metachronous | 1.06 (0.60–1.89) | 0.839 | ||
| Bladder cancer (referent: absent) | ||||
| Previous | 2.24 (1.46–3.41) | <0.001 | 2.18 (1.40–3.40) | 0.001 |
| Synchronous | 1.58 (1.08–2.31) | 0.017 | 1.22 (0.81–1.83) | 0.338 |
| Metachronous | 1.00 (0.71–1.41) | 0.981 | ||
| Hydronephrosis grade (referent: grade 0) | 0.280 | |||
| 1 | 0.60 (0.27–1.33) | 0.210 | ||
| 2 | 1.02 (0.53–1.98) | 0.954 | ||
| 3 | 1.21 (0.64–2.30) | 0.563 | ||
| 4 | 1.17 (0.61–2.21) | 0.640 | ||
| ASA score ≥ 3 (referent: ASA ≤ 2) | 1.89 (1.33–2.70) | <0.001 | 1.40 (0.96–2.04) | 0.084 |
| Diagnostic ureteroscopy (referent: no) | 0.301 | |||
| Ureteroscopic biopsy | 1.16 (0.79–1.71) | 0.447 | ||
| Ureteroscopy without biopsy | 0.79 (0.51–1.23) | 0.290 | ||
| Surgical approach (referent: open) | 0.806 | |||
| Laparoscopic | 0.91 (0.65–1.28) | 0.596 | ||
| Robotic | 1.17 (0.43–3.21) | 0.757 | ||
| Ureterectomy procedure (referent: NU) | 1.30 (0.72–2.35) | 0.389 | ||
| Tumor location (referent: renal pelvis) | 0.003 | 0.005 | ||
| Ureter | 1.69 (1.14–2.50) | 0.009 | 1.62 (1.09–2.40) | 0.018 |
| Synchronous renal pelvis and ureter | 3.67 (1.53–8.78) | 0.003 | 3.84 (1.56–9.45) | 0.003 |
| Tumor grade (referent: low grade) | 1.19 (0.52–2.69) | 0.682 | ||
| Multifocal disease (referent: absent) | 1.29 (0.91–1.83) | 0.151 | ||
| Carcinoma in situ (referent: absent) | 1.21 (0.85–1.72) | 0.298 | ||
| Lymphovascular invasion (referent: absent) | 1.33 (0.81–2.18) | 0.263 | ||
| Positive surgical margin (referent: absent) | 3.13 (1.59–6.17) | 0.001 | 2.59 (1.27–5.25) | 0.009 |
| Chronic kidney disease a (referent: absent) | 1.15 (0.76–1.75) | 0.504 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.-C.; Liu, J.-M.; Liu, H.-Y.; Chang, Y.-L.; Chen, C.-S.; Ho, D.-R.; Wu, C.-T.; Chen, M.-F.; Wang, H.-J.; Luo, H.-L. Clinical Determinants of Extraurinary Tract Recurrence and Survival after Radical Surgery for pT2 Upper Tract Urothelial Carcinoma. Cancers 2023, 15, 1858. https://doi.org/10.3390/cancers15061858
Huang Y-C, Liu J-M, Liu H-Y, Chang Y-L, Chen C-S, Ho D-R, Wu C-T, Chen M-F, Wang H-J, Luo H-L. Clinical Determinants of Extraurinary Tract Recurrence and Survival after Radical Surgery for pT2 Upper Tract Urothelial Carcinoma. Cancers. 2023; 15(6):1858. https://doi.org/10.3390/cancers15061858
Chicago/Turabian StyleHuang, Yun-Ching, Jui-Ming Liu, Hui-Ying Liu, Yin-Lun Chang, Chih-Shou Chen, Dong-Ru Ho, Chun-Te Wu, Miao-Fen Chen, Hung-Jen Wang, and Hao-Lun Luo. 2023. "Clinical Determinants of Extraurinary Tract Recurrence and Survival after Radical Surgery for pT2 Upper Tract Urothelial Carcinoma" Cancers 15, no. 6: 1858. https://doi.org/10.3390/cancers15061858
APA StyleHuang, Y.-C., Liu, J.-M., Liu, H.-Y., Chang, Y.-L., Chen, C.-S., Ho, D.-R., Wu, C.-T., Chen, M.-F., Wang, H.-J., & Luo, H.-L. (2023). Clinical Determinants of Extraurinary Tract Recurrence and Survival after Radical Surgery for pT2 Upper Tract Urothelial Carcinoma. Cancers, 15(6), 1858. https://doi.org/10.3390/cancers15061858







