Modern Kidney-Sparing Management of Upper Tract Urothelial Carcinoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
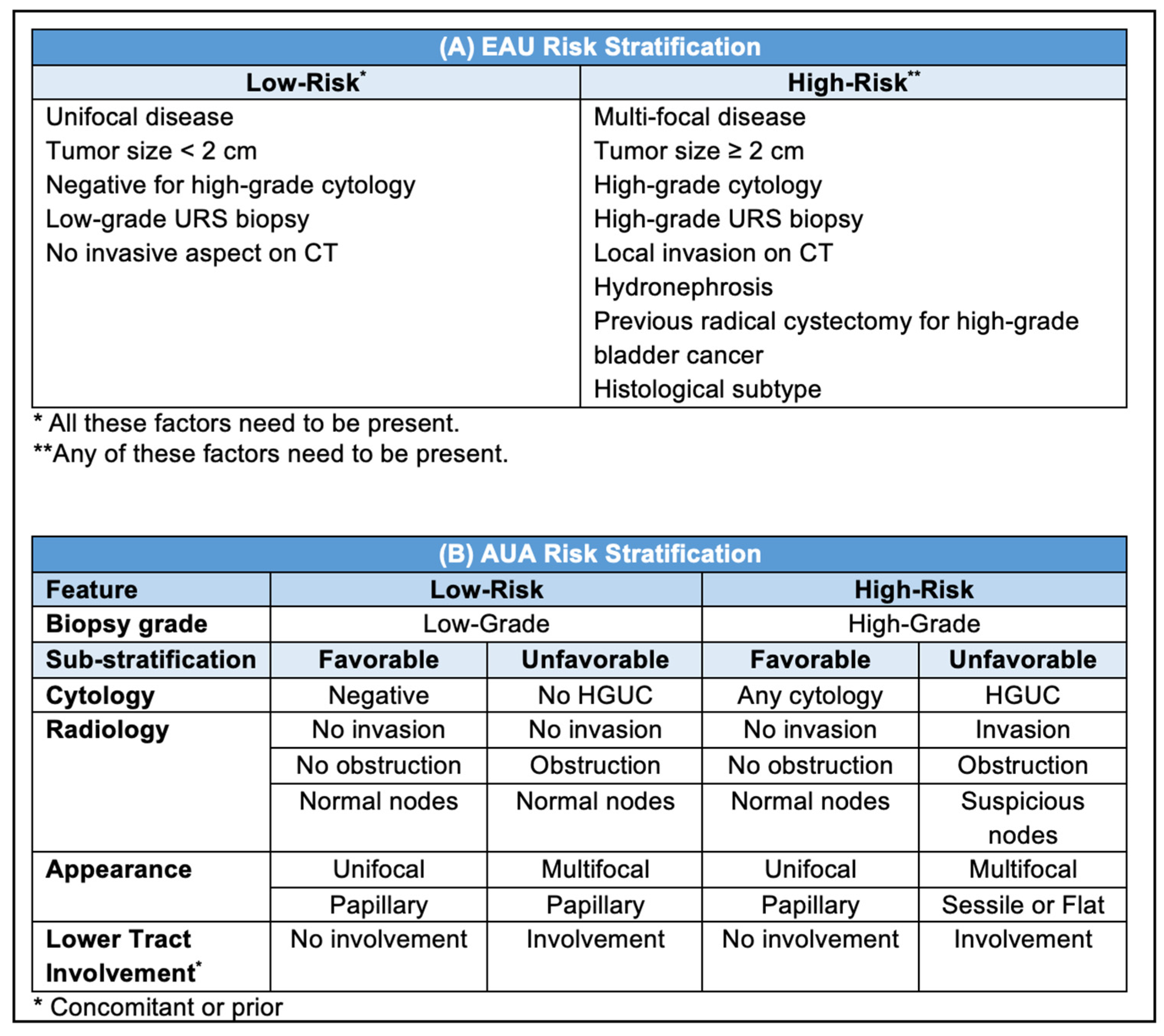
3. Indications
4. Endoscopic Ablation
4.1. Techniques
4.2. Adjuvant Instillation
4.3. Follow-Up
4.4. Outcomes
5. Segmental Ureterectomy
5.1. Technical Considerations
5.2. Outcomes
6. Novel Chemoablation Therapies and Ongoing Trials
7. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Soria, F.; Shariat, S.F.; Lerner, S.P.; Fritsche, H.M.; Rink, M.; Kassouf, W.; Spiess, P.E.; Lotan, Y.; Ye, D.; Fernández, M.I.; et al. Epidemiology, diagnosis, preoperative evaluation and prognostic assessment of upper-tract urothelial carcinoma (UTUC). World J. Urol. 2017, 35, 379–387. [Google Scholar] [CrossRef]
- Almås, B.; Halvorsen, O.J.; Johannesen, T.B.; Beisland, C. Higher than expected and significantly increasing incidence of upper tract urothelial carcinoma. A population based study. World J. Urol. 2021, 39, 3385–3391. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.A.; Clark, P.E.; Bixler, B.R.; Buckley, D.I.; Chang, S.S.; Chou, R.; Hoffman-Censits, J.; Kulkarni, G.S.; Matin, S.F.; Pierorazio, P.M.; et al. Diagnosis and Management of Non-Metastatic Upper Tract Urothelial Carcinoma: AUA/SUO Guideline. J. Urol. 2023, 209, 1071–1081. [Google Scholar] [CrossRef] [PubMed]
- Rouprêt, M.; Seisen, T.; Birtle, A.J.; Capoun, O.; Compérat, E.M.; Dominguez-Escrig, J.L.; Gürses Andersson, I.; Liedberg, F.; Mariappan, P.; Hugh Mostafid, A.; et al. European Association of Urology Guidelines on Upper Urinary Tract Urothelial Carcinoma: 2023 Update. Eur. Urol. 2023, 84, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Seisen, T.; Peyronnet, B.; Dominguez-Escrig, J.L.; Bruins, H.M.; Yuan, C.Y.; Babjuk, M.; Böhle, A.; Burger, M.; Compérat, E.M.; Cowan, N.C.; et al. Oncologic Outcomes of Kidney-sparing Surgery Versus Radical Nephroureterectomy for Upper Tract Urothelial Carcinoma: A Systematic Review by the EAU Non-muscle Invasive Bladder Cancer Guidelines Panel. Eur. Urol. 2016, 70, 1052–1068. [Google Scholar] [CrossRef]
- Foerster, B.; Abufaraj, M.; Matin, S.F.; Azizi, M.; Gupta, M.; Li, W.M.; Seisen, T.; Clinton, T.; Xylinas, E.; Mir, M.C.; et al. Pretreatment Risk Stratification for Endoscopic Kidney-sparing Surgery in Upper Tract Urothelial Carcinoma: An International Collaborative Study. Eur. Urol. 2021, 80, 507–515. [Google Scholar] [CrossRef]
- Marcq, G.; Foerster, B.; Abufaraj, M.; Matin, S.F.; Azizi, M.; Gupta, M.; Li, W.M.; Seisen, T.; Clinton, T.; Xylinas, E.; et al. Novel Classification for Upper Tract Urothelial Carcinoma to Better Risk-stratify Patients Eligible for Kidney-sparing Strategies: An International Collaborative Study. Eur. Urol. Focus. 2022, 8, 491–497. [Google Scholar] [CrossRef]
- Favaretto, R.L.; Shariat, S.F.; Savage, C.; Godoy, G.; Chade, D.C.; Kaag, M.; Bochner, B.H.; Coleman, J.; Dalbagni, G. Combining imaging and ureteroscopy variables in a preoperative multivariable model for prediction of muscle-invasive and non-organ confined disease in patients with upper tract urothelial carcinoma. BJU Int. 2012, 109, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Brien, J.C.; Shariat, S.F.; Herman, M.P.; Ng, C.K.; Scherr, D.S.; Scoll, B.; Uzzo, R.G.; Wille, M.; Eggener, S.E.; Terrell, J.D.; et al. Preoperative hydronephrosis, ureteroscopic biopsy grade and urinary cytology can improve prediction of advanced upper tract urothelial carcinoma. J. Urol. 2010, 184, 69–73. [Google Scholar] [CrossRef]
- Chromecki, T.F.; Cha, E.K.; Fajkovic, H.; Margulis, V.; Novara, G.; Scherr, D.S.; Lotan, Y.; Raman, J.D.; Kassouf, W.; Bensalah, K.; et al. The impact of tumor multifocality on outcomes in patients treated with radical nephroureterectomy. Eur. Urol. 2012, 61, 245–253. [Google Scholar] [CrossRef]
- Lucca, I.; Klatte, T.; Rouprêt, M.; Shariat, S.F. Kidney-sparing surgery for upper tract urothelial cancer. Curr. Opin. Urol. 2015, 25, 100–104. [Google Scholar] [CrossRef]
- Keller, E.X.; Doizi, S.; Villa, L.; Traxer, O. Which flexible ureteroscope is the best for upper tract urothelial carcinoma treatment? World J. Urol. 2019, 37, 2325–2333. [Google Scholar] [CrossRef]
- Douglawi, A.; Ghoreifi, A.; Lee, R.; Yip, W.; Seyedian, S.S.L.; Ahmadi, H.; Cai, J.; Miranda, G.; Yu, W.; Bhanvadia, S.; et al. Bladder Recurrence Following Diagnostic Ureteroscopy in Patients Undergoing Nephroureterectomy for Upper Tract Urothelial Cancer: Is Ureteral Access Sheath Protective? Urology. 2022, 160, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Motamedinia, P.; Keheila, M.; Leavitt, D.A.; Rastinehad, A.R.; Okeke, Z.; Smith, A.D. The Expanded Use of Percutaneous Resection for Upper Tract Urothelial Carcinoma: A 30-Year Comprehensive Experience. J. Endourol. 2016, 30, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Laukhtina, E.; Kawada, T.; Quhal, F.; Yanagisawa, T.; Rajwa, P.; von Deimling, M.; Pallauf, M.; Bianchi, A.; Majdoub, M.; Enikeev, D.; et al. Oncologic and Safety Outcomes for Retrograde and Antegrade Endoscopic Surgeries for Upper Tract Urothelial Carcinoma: A Systematic Review and Meta-analysis. Eur. Urol. Focus. 2023, 9, 258–263. [Google Scholar] [CrossRef]
- Shvero, A.; Zilberman, D.E.; Dotan, Z.A.; Laufer, M.; Fridman, E.; Winkler, H.; Kleinmann, N. Endoscopic management of upper tract urothelial carcinoma-tips and tricks. Transl. Androl. Urol. 2020, 9, 1815–1820. [Google Scholar] [CrossRef] [PubMed]
- Proietti, S.; Rodríguez-Socarrás, M.E.; Eisner, B.H.; Lucianò, R.; Basulto Martinez, M.J.; Yeow, Y.; Rapallo, I.; Saitta, G.; Scarfò, F.; Gaboardi, F.; et al. Thulium:YAG Versus Holmium:YAG Laser Effect on Upper Urinary Tract Soft Tissue: Evidence from an Ex Vivo Experimental Study. J. Endourol. 2021, 35, 544–551. [Google Scholar] [CrossRef]
- Doizi, S.; Germain, T.; Panthier, F.; Compérat, E.; Traxer, O.; Berthe, L. Comparison of Holmium:YAG and Thulium Fiber Lasers on Soft Tissue: An Ex Vivo Study. J. Endourol. 2022, 36, 251–258. [Google Scholar] [CrossRef]
- Tada, Y.; Yokomizo, A.; Koga, H.; Seki, N.; Kuroiwa, K.; Tatsugami, K.; Yamaguchi, A.; Naito, S. Transurethral endoscopic treatment of patients with upper tract urothelial carcinomas using neodymium-YAG and/or holmium-YAG laser ablation. BJU Int. 2010, 106, 362–366. [Google Scholar] [CrossRef]
- Korn, S.M.; Hübner, N.A.; Seitz, C.; Shariat, S.F.; Fajkovic, H. Role of lasers in urology. Photochem. Photobiol. Sci. 2019, 18, 295–303. [Google Scholar] [CrossRef]
- Candela, L.; Ventimiglia, E.; Solano, C.; Chicaud, M.; Kutchukian, S.; Panthier, F.; Corrales, M.; Villa, L.; Briganti, A.; Montorsi, F.; et al. Endoscopic Conservative Treatment of Upper Urinary Tract Urothelial Carcinoma with a Thulium Laser: A Systematic Review. J. Clin. Med. 2023, 12, 4907. [Google Scholar] [CrossRef] [PubMed]
- Shoen, E.; Zollinger, B.; Gresham, T.; Rezaei, K.M.; Whalen, M. Use of the T-1470 LiteTouch™ Laser in the En Bloc Resection of an Upper Tract Urothelial Cancer. Case Rep. Urol. 2021, 2021, 6623326. [Google Scholar] [CrossRef] [PubMed]
- Jue, J.S.; Armenakas, N.A. Upper Tract Tumor En Bloc Enucleation: A Novel Approach to the Diagnosis and Management of Upper Tract Urothelial Carcinoma. Urology. 2023, 174, 196–200. [Google Scholar] [CrossRef]
- Foerster, B.; D’Andrea, D.; Abufaraj, M.; Broenimann, S.; Karakiewicz, P.I.; Rouprêt, M.; Gontero, P.; Lerner, S.P.; Shariat, S.F.; Soria, F. Endocavitary treatment for upper tract urothelial carcinoma: A meta-analysis of the current literature. Urol. Oncol. 2019, 37, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Gallioli, A.; Boissier, R.; Territo, A.; Vila Reyes, H.; Sanguedolce, F.; Gaya, J.M.; Regis, F.; Subiela, J.D.; Palou, J.; Breda, A. Adjuvant Single-Dose Upper Urinary Tract Instillation of Mitomycin C After Therapeutic Ureteroscopy for Upper Tract Urothelial Carcinoma: A Single-Centre Prospective Non-Randomized Trial. J. Endourol. 2020, 34, 573–580. [Google Scholar] [CrossRef]
- Labbate, C.; Woldu, S.; Murray, K.; Rose, K.; Sexton, W.; Tachibana, I.; Kaimakliotis, H.; Jacob, J.; Dickstein, R.; Linehan, J.; et al. Efficacy and Safety of Mitomycin Gel (UGN-101) as an Adjuvant Therapy After Complete Endoscopic Management of Upper Tract Urothelial Carcinoma. J. Urol. 2023, 209, 872–881. [Google Scholar] [CrossRef]
- Villa, L.; Cloutier, J.; Letendre, J.; Ploumidis, A.; Salonia, A.; Cornu, J.N.; Montorsi, F.; Traxer, O. Early repeated ureteroscopy within 6–8 weeks after a primary endoscopic treatment in patients with upper tract urothelial cell carcinoma: Preliminary findings. World J. Urol. 2016, 34, 1201–1206. [Google Scholar] [CrossRef]
- Lucas, S.M.; Svatek, R.S.; Olgin, G.; Arriaga, Y.; Kabbani, W.; Sagalowsky, A.I.; Lotan, Y. Conservative management in selected patients with upper tract urothelial carcinoma compares favourably with early radical surgery. BJU Int. 2008, 102, 172–176. [Google Scholar] [CrossRef]
- Cutress, M.L.; Stewart, G.D.; Tudor, E.C.; Egong, E.A.; Wells-Cole, S.; Phipps, S.; Thomas, B.G.; Riddick, A.C.; McNeill, S.A.; Tolley, D.A. Endoscopic versus laparoscopic management of noninvasive upper tract urothelial carcinoma: 20-year single center experience. J. Urol. 2013, 189, 2054–2060. [Google Scholar] [CrossRef]
- Fajkovic, H.; Klatte, T.; Nagele, U.; Dunzinger, M.; Zigeuner, R.; Hübner, W.; Remzi, M. Results and outcomes after endoscopic treatment of upper urinary tract carcinoma: The Austrian experience. World J. Urol. 2013, 31, 37–44. [Google Scholar] [CrossRef]
- Seisen, T.; Nison, L.; Remzi, M.; Klatte, T.; Mathieu, R.; Lucca, I.; Bozzini, G.; Capitanio, U.; Novara, G.; Cussenot, O.; et al. Oncologic Outcomes of Kidney Sparing Surgery versus Radical Nephroureterectomy for the Elective Treatment of Clinically Organ Confined Upper Tract Urothelial Carcinoma of the Distal Ureter. J. Urol. 2016, 195, 1354–1361. [Google Scholar] [CrossRef]
- Vemana, G.; Kim, E.H.; Bhayani, S.B.; Vetter, J.M.; Strope, S.A. Survival Comparison Between Endoscopic and Surgical Management for Patients With Upper Tract Urothelial Cancer: A Matched Propensity Score Analysis Using Surveillance, Epidemiology and End Results-Medicare Data. Urology. 2016, 95, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.T.; Yu, C.C.; Yeh, H.C.; Lee, H.Y.; Jiang, Y.H.; Lee, Y.K.; Kuei, C.H.; Wu, C.C.; Huang, C.Y.; Lin, W.Y.; et al. Endoscopic management versus radical nephroureterectomy for localized upper tract urothelial carcinoma in a high endemic region. Sci. Rep. 2021, 11, 4040. [Google Scholar] [CrossRef] [PubMed]
- Shenhar, C.; Veredgorn, Y.; Bulis, S.; Aviv, T.; Darawsha, A.E.; Gilad, R.; Baniel, J.; Ehrlich, Y.; Lifshitz, D. Endoscopic Management of Low-Grade Upper Tract Urothelial Carcinoma: Characterizing the Long-term Burden of Care in Comparison to Radical Nephroureterectomy. Urology. 2022, 159, 152–159. [Google Scholar] [CrossRef]
- Shen, C.Y.; Jou, Y.C.; Kan, W.C.; Tzai, T.S.; Tsai, Y.S. Outcome of Non-Muscle Invasive Upper Tract Urothelial Carcinoma Receiving Endoscopic Ablation: An Inverse Probability of Treatment Weighting Analysis. J. Clin. Med. 2022, 11, 1307. [Google Scholar] [CrossRef]
- Rouprêt, M.; Hupertan, V.; Traxer, O.; Loison, G.; Chartier-Kastler, E.; Conort, P.; Bitker, M.O.; Gattegno, B.; Richard, F.; Cussenot, O. Comparison of open nephroureterectomy and ureteroscopic and percutaneous management of upper urinary tract transitional cell carcinoma. Urology. 2006, 67, 1181–1187. [Google Scholar] [CrossRef]
- Gadzinski, A.J.; Roberts, W.W.; Faerber, G.J.; Wolf, J.S., Jr. Long-term outcomes of nephroureterectomy versus endoscopic management for upper tract urothelial carcinoma. J. Urol. 2010, 183, 2148–2153. [Google Scholar] [CrossRef] [PubMed]
- Grasso, M.; Fishman, A.I.; Cohen, J.; Alexander, B. Ureteroscopic and extirpative treatment of upper urinary tract urothelial carcinoma: A 15-year comprehensive review of 160 consecutive patients. BJU Int. 2012, 110, 1618–1626. [Google Scholar] [CrossRef]
- Kawada, T.; Laukhtina, E.; Quhal, F.; Yanagisawa, T.; Rajwa, P.; Pallauf, M.; von Deimling, M.; Bianchi, A.; Pradere, B.; Fajkovic, H.; et al. Oncologic and Safety Outcomes for Endoscopic Surgery Versus Radical Nephroureterectomy for Upper Tract Urothelial Carcinoma: An Updated Systematic Review and Meta-analysis. Eur. Urol. Focus. 2023, 9, 236–240. [Google Scholar] [CrossRef]
- Knoedler, J.J. Outcomes of endoscopic management of upper tract urothelial carcinoma. Transl. Androl. Urol. 2020, 9, 1821–1830. [Google Scholar] [CrossRef]
- Chen, Y.T.; Yeh, H.C.; Lee, H.Y.; Hsieh, P.F.; Chou, E.C.; Tsai, Y.C.; Hong, J.H.; Huang, C.Y.; Jiang, Y.H.; Lee, Y.K.; et al. Endoscopic management of upper tract urothelial cancer in a highly endemic area: A Taiwan nationwide collaborative study. Asian J. Surg. 2023, 46, 3058–3065. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.; Yossepowitch, O.; Erlich, Y.; Holland, R.; Lifshitz, D. Oncologic results of nephron sparing endoscopic approach for upper tract low grade transitional cell carcinoma in comparison to nephroureterectomy—A case control study. BMC Urol. 2014, 14, 97. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Ji, Z.G.; Li, H.Z. Treatment of upper tract urothelial carcinoma with ureteroscopy and thulium laser: A retrospective single center study. BMC Cancer. 2018, 18, 196. [Google Scholar] [CrossRef]
- Ou, Y.C.; Hu, C.Y.; Cheng, H.L.; Yang, W.H. Long-term outcomes of total ureterectomy with ileal-ureteral substitution treatment for ureteral cancer: A single-center experience. BMC Urol. 2018, 18, 73. [Google Scholar] [CrossRef]
- Wei, W.; Liu, J.; Wang, L.; Duan, X.; Ding, D. Segmental ureterectomy for high-risk ureteral carcinoma: A preliminary report. BMC Urol. 2023, 23, 103. [Google Scholar] [CrossRef]
- Jia, Z.; Gong, Y.Q.; Zhang, C.J.; Bao, Z.Q.; Li, X.S.; Hao, H.; Xiong, G.Y.; Zhang, L.; Fang, D.; He, Z.S.; et al. Segmental ureterectomy can be performed safely in patients with urothelial carcinoma of distal ureter. Can. Urol. Assoc. J. 2019, 13, E202–E209. [Google Scholar] [CrossRef]
- Campi, R.; Cotte, J.; Sessa, F.; Seisen, T.; Tellini, R.; Amparore, D.; Mormile, N.; Gobert, A.; Mari, A.; Porpiglia, F.; et al. Robotic radical nephroureterectomy and segmental ureterectomy for upper tract urothelial carcinoma: A multi-institutional experience. World J. Urol. 2019, 37, 2303–2311. [Google Scholar] [CrossRef]
- Dalpiaz, O.; Ehrlich, G.; Quehenberger, F.; Pummer, K.; Zigeuner, R. Distal ureterectomy is a safe surgical option in patients with urothelial carcinoma of the distal ureter. Urol. Oncol. 2014, 32, e1–e8. [Google Scholar] [CrossRef] [PubMed]
- Abrate, A.; Sessa, F.; Sebastianelli, A.; Preto, M.; Olivero, A.; Varca, V.; Benelli, A.; Campi, R.; Sessa, M.; Pavone, C.; et al. Segmental resection of distal ureter with termino-terminal ureteric anastomosis vs bladder cuff removal and ureteric re-implantation for upper tract urothelial carcinoma: Results of a multicentre study. BJU Int. 2019, 124, 116–123. [Google Scholar] [CrossRef]
- Matin, S.F.; Sfakianos, J.P.; Espiritu, P.N.; Coleman, J.A.; Spiess, P.E. Patterns of Lymphatic Metastases in Upper Tract Urothelial Carcinoma and Proposed Dissection Templates. J. Urol. 2015, 194, 1567–1574. [Google Scholar] [CrossRef]
- Campi, R.; Minervini, A.; Mari, A.; Hatzichristodoulou, G.; Sessa, F.; Lapini, A.; Sessa, M.; Gschwend, J.E.; Serni, S.; Roscigno, M.; et al. Anatomical templates of lymph node dissection for upper tract urothelial carcinoma: A systematic review of the literature. Expert. Rev. Anticancer. Ther. 2017, 17, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Fang, D.; Seisen, T.; Yang, K.; Liu, P.; Fan, X.; Singla, N.; Xiong, G.; Zhang, L.; Li, X.; Zhou, L. A systematic review and meta-analysis of oncological and renal function outcomes obtained after segmental ureterectomy versus radical nephroureterectomy for upper tract urothelial carcinoma. Eur. J. Surg. Oncol. 2016, 42, 1625–1635. [Google Scholar] [CrossRef] [PubMed]
- Veccia, A.; Antonelli, A.; Checcucci, E.; Falagario, U.; Carrieri, G.; Guruli, G.; De Sio, M.; Simeone, C.; Porpiglia, F.; Autorino, R. Segmental Ureterectomy for Upper Tract Urothelial Carcinoma: A Systematic Review and Meta-analysis of Comparative Studies. Clin Genitourin Cancer. 2020, 18, e10–e20. [Google Scholar] [CrossRef]
- Paciotti, M.; Alkhatib, K.Y.; Nguyen, D.D.; Yim, K.; Lipsitz, S.R.; Mossanen, M.; Casale, P.; Pierorazio, P.M.; Kibel, A.S.; Trinh, Q.D.; et al. Is Segmental Ureterectomy Associated with Inferior Survival for Localized Upper-Tract Urothelial Carcinoma of the Ureter Compared to Radical Nephroureterectomy? Cancers. 2023, 15, 1373. [Google Scholar] [CrossRef]
- Kokorovic, A.; Matin, S.F. UGN-101 (mitomycin gel): A novel treatment for low-grade upper tract urothelial carcinoma. Ther. Adv. Med. Oncol. 2020, 12, 1758835920937950. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-mitomycin-low-grade-upper-tract-urothelial-cancer (accessed on 25 June 2023).
- Kleinmann, N.; Matin, S.F.; Pierorazio, P.M.; Gore, J.L.; Shabsigh, A.; Hu, B.; Chamie, K.; Godoy, G.; Hubosky, S.; Rivera, M.; et al. Primary chemoablation of low-grade upper tract urothelial carcinoma using UGN-101, a mitomycin-containing reverse thermal gel (OLYMPUS): An open-label, single-arm, phase 3 trial. Lancet Oncol. 2020, 21, 776–785. [Google Scholar] [CrossRef]
- Matin, S.F.; Pierorazio, P.M.; Kleinmann, N.; Gore, J.L.; Shabsigh, A.; Hu, B.; Chamie, K.; Godoy, G.; Hubosky, S.G.; Rivera, M.; et al. Durability of Response to Primary Chemoablation of Low-Grade Upper Tract Urothelial Carcinoma Using UGN-101, a Mitomycin-Containing Reverse Thermal Gel: OLYMPUS Trial Final Report. J. Urol. 2022, 207, 779–788. [Google Scholar] [CrossRef]
- Rose, K.M.; Murray, K.S.; Labbate, C.; Woldu, S.; Linehan, J.; Jacob, J.; Kaimakliotis, H.; Dickstein, R.; Feldman, A.; Matin, S.F.; et al. Mitomycin Gel (UGN-101) as a Kidney-sparing Treatment for Upper Tract Urothelial Carcinoma in Patients with Imperative Indications and High-grade Disease. Eur. Urol. Focus 2023, S2405-4569(23)00085-8, Online ahead of print. [Google Scholar] [CrossRef]
- Rosen, G.H.; Nallani, A.; Muzzey, C.; Murray, K.S. Antegrade Instillation of UGN-101 (Mitomycin for Pyelocalyceal Solution) for Low-Grade Upper Tract Urothelial Carcinoma: Initial Clinical Experience. J. Urol. 2022, 207, 1302–1311. [Google Scholar] [CrossRef]
- Linehan, J.; Gottlieb, J.; Woldu, S.L.; Labbate, C.; Rose, K.; Sexton, W.; Kaimakliotis, H.; Jacob, J.; Dickstein, R.; Nieder, A.; et al. Route of Administration for UGN-101 and Impact on Oncological and Safety Outcomes. Eur. Urol. Focus 2023, S2405-4569(23)00123-2, Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Yip, W.; Sjoberg, D.D.; Nogueira, L.M.; Tracey, A.T.; Alvim, R.G.; Reisz, P.A.; Demac, Q.; Benfante, N.E.; Vanden Berg, R.W.; Kim, K.; et al. Final Results of a Phase I Trial of WST-11 (TOOKAD Soluble) Vascular-targeted Photodynamic Therapy for Upper Tract Urothelial Carcinoma. J. Urol. 2023, 209, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://classic.clinicaltrials.gov/ct2/show/NCT04620239 (accessed on 25 June 2023).
- Ghoreifi, A.; Ladi-Seyedian, S.S.; Piatti, P.; Chew, Y.C.; Jara, B.; Sanossian, L.; Bhasin, J.M.; Yamada, T.; Fuchs, G.; Bhanvadia, S.; et al. A Urine-based DNA Methylation Marker Test to Detect Upper Tract Urothelial Carcinoma: A Prospective Cohort Study. J. Urol. 2023, 209, 854–862. [Google Scholar] [CrossRef]
- Shishido, S.N.; Ghoreifi, A.; Sayeed, S.; Courcoubetis, G.; Huang, A.; Ye, B.; Mrutyunjaya, S.; Gill, I.S.; Kuhn, P.; Mason, J.; et al. Liquid Biopsy Landscape in Patients with Primary Upper Tract Urothelial Carcinoma. Cancers. 2022, 14, 3007. [Google Scholar] [CrossRef]
| Study (yr) [Ref] | Patients (n) | Bladder Recurrence (%) | 5 yr OS (%) | 5 yr CSS (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| EA | RNU | EA | RNU | EA | RNU | p Value | EA | RNU | p Value | |
| Lucas et al. (2008) [28] | 39 | 77 | 5 | 8 | 62 | 72 | 0.36 | 82 | 83 | 0.98 |
| Cutress et al. (2012) [29] | 59 | 70 | 42 | 33 | 64 | 75 | 0.02 | 85 | 92.1 | 0.21 |
| Fajkovic et al. (2012) [30] | 20 | 178 | 15 | 36 | 45 | 76 | 0.001 | 67 | 91 | 0.36 |
| Seisen et al. (2016) [31] | 42 | 128 | NA | NA | 74 | 73 | 0.06 | 83 | 87 | 0.18 |
| Vemana et al. (2016) [32] | 151 | 302 | NA | NA | NA | NA | NA | 88 | 92 | NA |
| Chen et al. (2021) [33] | 84 | 272 | 23 | 34 | 85 | 75 | 0.19 | 89 | 90 | 0.49 |
| Shenhar et al. (2021) [34] | 24 | 37 | NA | NA | 85 | 84 | 0.71 | 89 | 92 | 0.96 |
| Shen et al. (2022) [35] | 23 | 42 | 30 | 33 | 95 | 95 | 0.99 | NA | NA | NA |
| Study (yr) [Ref] | Patients (n) | Garde | 5 yr OS (%) | 5 yr CSS (%) | 5 yr MFS (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EA | RNU | EA | RNU | p Value | EA | RNU | p Value | EA | RNU | p Value | ||
| Rouprêt et al. (2006) [36] | 43 | 54 | Low | NA | NA | NA | 81 | 84 | 0.89 | NA | NA | NA |
| Lucas et al. (2008) [28] | 39 | 77 | Low | 75 | 66 | 0.28 | 86 | 87 | 0.91 | NA | NA | NA |
| High | 45 | 72 | 0.08 | 69 | 75 | 0.53 | ||||||
| Gadzinski et al. (2010) [37] | 34 | 62 | Low | 75 | 72 | 0.30 | 100 | 89 | 0.63 | 94 | 88 | 0.25 |
| High | 25 | 48 | 0.62 | 86 | 72 | 0.94 | 86 | 64 | 0.79 | |||
| Cutress et al. (2012) [29] | 59 | 70 | G1 | 75 | 86 | 0.62 | 100 | 100 | 0.65 | NA | NA | NA |
| G2 | 56 | 73 | 0.08 | 62 | 92 | 0.03 | ||||||
| G3 | 33 | 75 | 0.001 | 83 | 89 | 0.26 | ||||||
| Grasso et al. (2012) [39] | 80 | 80 | Low | 74 | 88 | NA | 87 | 93 | NA | 84 | 95 | NA |
| High | 0 | 68 | NA | 0 | 78 | NA | 0 | 61 | NA | |||
| Study (yr) [Ref] | Patients (n) | Renal Function | ||||
|---|---|---|---|---|---|---|
| EA | RNU | Variable | EA | RNU | p Value | |
| Fajkovic et al. (2013) [30] | 20 | 178 | Preoperative Cr (mg%) Postoperative Cr (mg%) | 1.46 ± 0.52 1.3 ± 0.47 | 1.53 ± 1.2 1.64 ± 0.79 | 0.82 0.048 |
| Hoffman et al. (2014) [42] | 25 | 22 | Preoperative eGFR Postoperative eGFR | 66 62 | 68 58 | >0.05 >0.05 |
| Wen et al. (2018) [43] | 32 | 107 | Cr level POD1 (umol/L) | 89 ± 7.5 | 123 ± 9.4 | <0.01 |
| Chen et al. (2021) [33] | 84 | 272 | Preoperative Cr (mg/dL) Postoperative Cr (1 mo) Postoperative Cr (final) ESRD | 2.1 ± 1.9 3.57 ± 10.5 3.34 ± 3.01 29% | 1.33 ± 2.82 1.61 ± 2.49 1.80 ± 2.73 27% | 0.90 0.38 0.74 0.31 |
| Shenhar et al. (2022) [34] | 24 | 37 | eGFR (mL/min/1.73 m2) # CKD (GFR < 60) Severe CKD (GFR < 30) | 58.7 ± 21.5 45% 9% | 49.2 ± 22.1 70% 16% | 0.12 0.59 0.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghoreifi, A.; Sari Motlagh, R.; Fuchs, G. Modern Kidney-Sparing Management of Upper Tract Urothelial Carcinoma. Cancers 2023, 15, 4495. https://doi.org/10.3390/cancers15184495
Ghoreifi A, Sari Motlagh R, Fuchs G. Modern Kidney-Sparing Management of Upper Tract Urothelial Carcinoma. Cancers. 2023; 15(18):4495. https://doi.org/10.3390/cancers15184495
Chicago/Turabian StyleGhoreifi, Alireza, Reza Sari Motlagh, and Gerhard Fuchs. 2023. "Modern Kidney-Sparing Management of Upper Tract Urothelial Carcinoma" Cancers 15, no. 18: 4495. https://doi.org/10.3390/cancers15184495
APA StyleGhoreifi, A., Sari Motlagh, R., & Fuchs, G. (2023). Modern Kidney-Sparing Management of Upper Tract Urothelial Carcinoma. Cancers, 15(18), 4495. https://doi.org/10.3390/cancers15184495







