Prognostic Value of Skeletal Muscle Loss in Patients with Hepatocellular Carcinoma Treated with Hepatic Arterial Infusion Chemotherapy
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
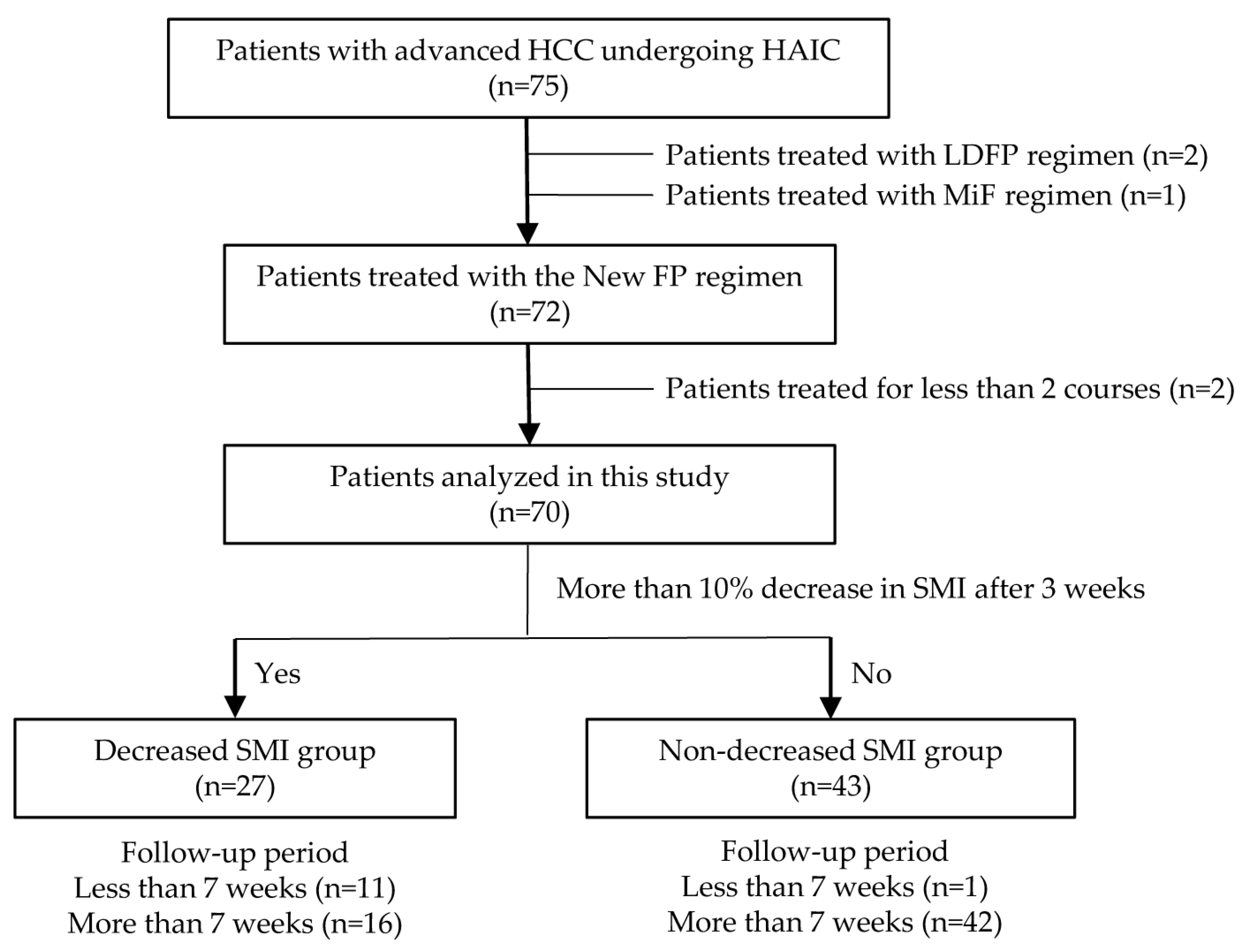
2.1. Study Design and Protocol
2.2. Treatment Protocol
2.3. Evaluation of Parameters
2.4. Statistical Analysis
2.5. Ethical Approval
3. Results
3.1. Patient Characteristics
3.2. Skeletal Muscle Mass and Related Factors
3.3. Therapeutic Effects
3.4. AEs Due to HAIC
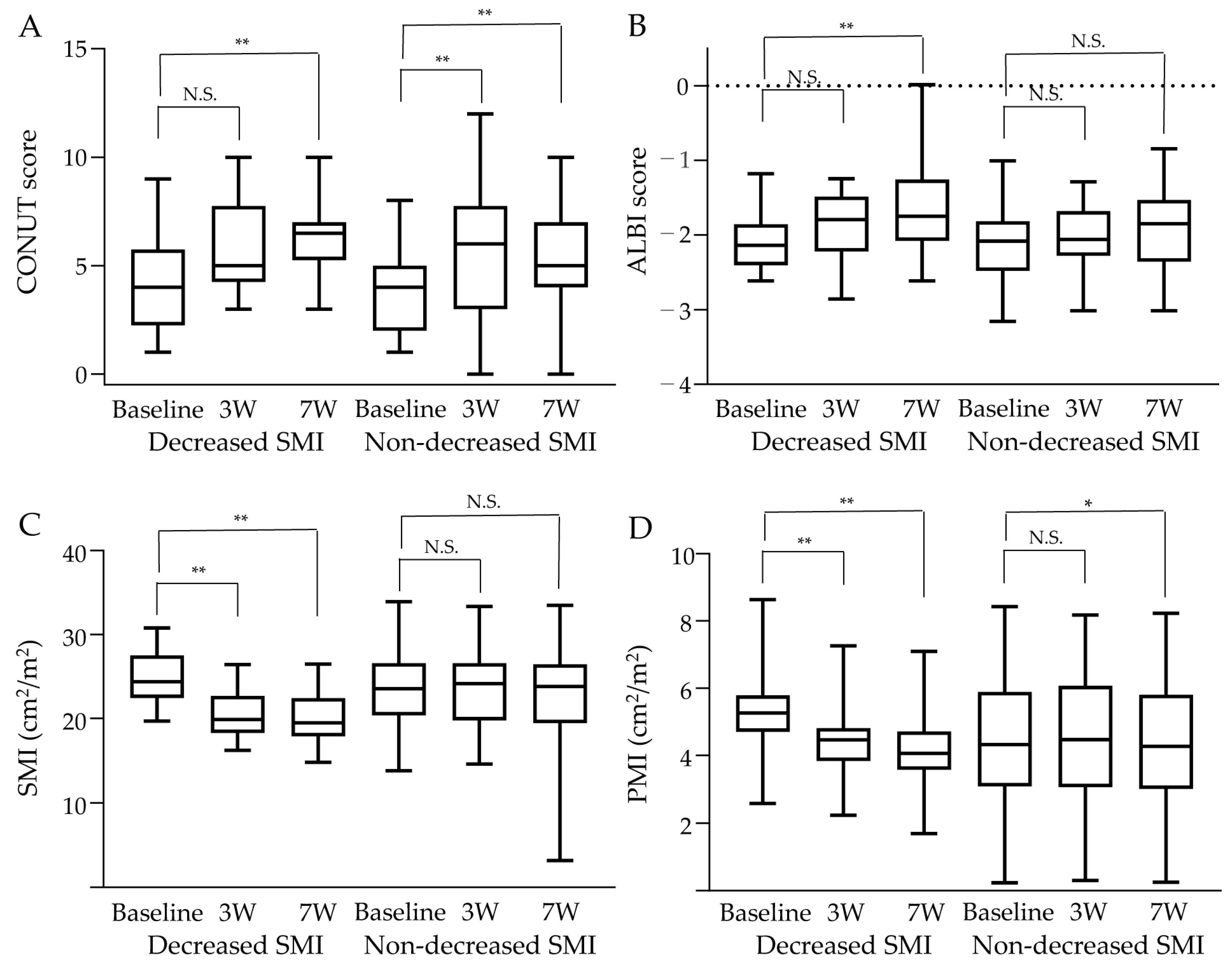
3.5. Changes in Skeletal Muscle Mass and Related Factors
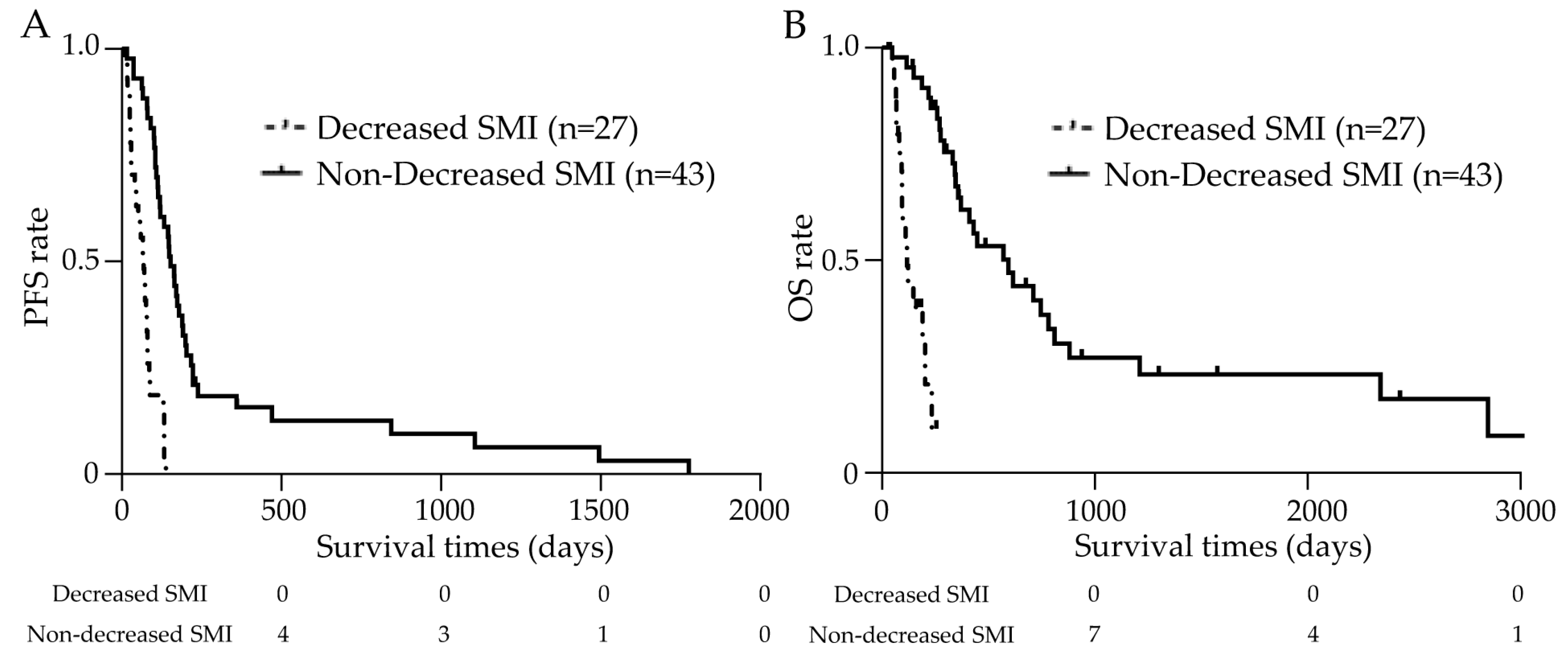
3.6. Prognosis Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2021, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Jemal, A.; Ward, E.M.; Johnson, C.J.; Cronin, K.A.; Ma, J.; Ryerson, B.; Mariotto, A.; Lake, A.J.; Wilson, R.; Sherman, R.L.; et al. Annual Report to the Nation on the Status of Cancer, 1975-2014, Featuring Survival. J. Natl. Cancer Inst. 2017, 109, djx030. [Google Scholar] [CrossRef] [PubMed]
- Forner, A.; Reig, M.; Bruix, J. Hepatocellular carcinoma. Lancet 2018, 391, 1301–1314. [Google Scholar] [CrossRef]
- Sun, J.X.; Shi, J.; Li, N.; Guo, W.X.; Wu, M.C.; Lau, W.Y.; Cheng, S.Q. Portal vein tumor thrombus is a bottleneck in the treatment of hepatocellular carcinoma. Cancer Biol. Med. 2016, 13, 452–458. [Google Scholar]
- Ueshima, K.; Komemushi, A.; Aramaki, T.; Iwamoto, H.; Obi, S.; Sato, Y.; Tanaka, T.; Matsueda, K.; Moriguchi, M.; Saito, H.; et al. Clinical Practice Guidelines for Hepatic Arterial Infusion Chemotherapy with a Port System Proposed by the Japanese Society of Interventional Radiology and Japanese Society of Implantable Port Assisted Treatment. Liver Cancer 2022, 11, 407–425. [Google Scholar] [CrossRef]
- Ueshima, K.; Ogasawara, S.; Ikeda, M.; Yasui, Y.; Terashima, T.; Yamashita, T.; Obi, S.; Sato, S.; Aikata, H.; Ohmura, T.; et al. Hepatic Arterial Infusion Chemotherapy versus Sorafenib in Patients with Advanced Hepatocellular Carcinoma. Liver Cancer 2020, 9, 583–595. [Google Scholar] [CrossRef]
- Kodama, K.; Kawaoka, T.; Aikata, H.; Uchikawa, S.; Inagaki, Y.; Hatooka, M.; Morio, K.; Nakahara, T.; Murakami, E.; Tsuge, M.; et al. Comparison of clinical outcome of hepatic arterial infusion chemotherapy and sorafenib for advanced hepatocellular carcinoma according to macrovascular invasion and transcatheter arterial chemoembolization refractory status. J. Gastroenterol. Hepatol. 2018, 33, 1780–1786. [Google Scholar] [CrossRef]
- Kawaoka, T.; Aikata, H.; Hyogo, H.; Morio, R.; Morio, K.; Hatooka, M.; Fukuhara, T.; Kobayashi, T.; Naeshiro, N.; Miyaki, D.; et al. Comparison of hepatic arterial infusion chemotherapy versus sorafenib monotherapy in patients with advanced hepatocellular carcinoma. J. Dig. Dis. 2015, 16, 505–512. [Google Scholar] [CrossRef]
- Iwamoto, H.; Niizeki, T.; Nagamatsu, H.; Ueshima, K.; Tani, J.; Kuzuya, T.; Kasai, K.; Kooka, Y.; Hiraoka, A.; Sugimoto, R.; et al. The Clinical Impact of Hepatic Arterial Infusion Chemotherapy New-FP for Hepatocellular Carcinoma with Preserved Liver Function. Cancers 2022, 14, 4873. [Google Scholar] [CrossRef]
- Nomura, T.; Tani, J.; Deguchi, A.; Nakahara, M.; Oura, K.; Tadokoro, T.; Fujita, K.; Mimura, S.; Sakamoto, T.; Morishita, A.; et al. Efficacy of combined modality therapy with sorafenib following hepatic arterial injection chemotherapy and three-dimensional conformal radiotherapy for advanced hepatocellular carcinoma with major vascular invasion. Mol. Clin. Oncol. 2019, 11, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Landi, F.; Schneider, S.M.; Zuniga, C.; Arai, H.; Boirie, Y.; Chen, L.K.; Fielding, R.A.; Martin, F.C.; Michel, J.P.; et al. Prevalence of and interventions for sarcopenia in ageing adults: A systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing 2014, 43, 748–759. [Google Scholar] [CrossRef] [PubMed]
- Perisetti, A.; Goyal, H.; Yendala, R.; Chandan, S.; Tharian, B.; Thandassery, R.B. Sarcopenia in hepatocellular carcinoma: Current knowledge and future directions. World J. Gastroenterol. 2022, 28, 432–448. [Google Scholar] [CrossRef] [PubMed]
- Marasco, G.; Serenari, M.; Renzulli, M.; Alemanni, L.V.; Rossini, B.; Pettinari, I.; Dajti, E.; Ravaioli, F.; Golfieri, R.; Cescon, M.; et al. Clinical impact of sarcopenia assessment in patients with hepatocellular carcinoma undergoing treatments. J. Gastroenterol. 2020, 55, 927–943. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyere, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Nishikawa, H.; Shiraki, M.; Hiramatsu, A.; Hara, N.; Moriya, K.; Hino, K.; Koike, K. Reduced handgrip strength predicts poorer survival in chronic liver diseases: A large multicenter study in Japan. Hepatol. Res. 2021, 51, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Begini, P.; Gigante, E.; Antonelli, G.; Carbonetti, F.; Iannicelli, E.; Anania, G.; Imperatrice, B.; Pellicelli, A.M.; Fave, G.D.; Marignani, M. Sarcopenia predicts reduced survival in patients with hepatocellular carcinoma at first diagnosis. Ann. Hepatol. 2017, 16, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Chien, T.P.; Huang, S.F.; Chan, W.H.; Pan, K.T.; Yu, M.C.; Lee, W.C.; Tsai, H.I.; Lin, P.T.; Chen, H.Y.; Chen, J.H.; et al. The combination of sarcopenia and biochemical factors can predict the survival of hepatocellular carcinoma patients receiving transarterial chemoembolization. Front. Oncol. 2022, 12, 1005571. [Google Scholar] [CrossRef]
- Nishikawa, H.; Nishijima, N.; Enomoto, H.; Sakamoto, A.; Nasu, A.; Komekado, H.; Nishimura, T.; Kita, R.; Kimura, T.; Iijima, H.; et al. Prognostic significance of sarcopenia in patients with hepatocellular carcinoma undergoing sorafenib therapy. Oncol. Lett. 2017, 14, 1637–1647. [Google Scholar] [CrossRef]
- Takada, H.; Kurosaki, M.; Nakanishi, H.; Takahashi, Y.; Itakura, J.; Tsuchiya, K.; Yasui, Y.; Tamaki, N.; Takaura, K.; Komiyama, Y.; et al. Impact of pre-sarcopenia in sorafenib treatment for advanced hepatocellular carcinoma. PLoS ONE 2018, 13, e0198812. [Google Scholar] [CrossRef]
- Antonelli, G.; Gigante, E.; Iavarone, M.; Begini, P.; Sangiovanni, A.; Iannicelli, E.; Biondetti, P.; Pellicelli, A.M.; Miglioresi, L.; Marchetti, P.; et al. Sarcopenia is associated with reduced survival in patients with advanced hepatocellular carcinoma undergoing sorafenib treatment. United Eur. Gastroenterol. J. 2018, 6, 1039–1048. [Google Scholar] [CrossRef] [PubMed]
- Uojima, H.; Chuma, M.; Tanaka, Y.; Hidaka, H.; Nakazawa, T.; Iwabuchi, S.; Kobayashi, S.; Hattori, N.; Ogushi, K.; Morimoto, M.; et al. Skeletal Muscle Mass Influences Tolerability and Prognosis in Hepatocellular Carcinoma Patients Treated with Lenvatinib. Liver Cancer 2020, 9, 193–206. [Google Scholar] [CrossRef]
- Hiraoka, A.; Kumada, T.; Kariyama, K.; Tada, T.; Tani, J.; Fukunishi, S.; Atsukawa, M.; Hirooka, M.; Tsuji, K.; Ishikawa, T.; et al. Clinical importance of muscle volume in lenvatinib treatment for hepatocellular carcinoma: Analysis adjusted with inverse probability weighting. J. Gastroenterol. Hepatol. 2021, 36, 1812–1819. [Google Scholar] [CrossRef] [PubMed]
- Dong, D.; Shi, J.Y.; Shang, X.; Liu, B.; Xu, W.L.; Cui, G.Z.; Wang, N.Y. Prognostic significance of sarcopenia in patients with hepatocellular carcinoma treated with lenvatinib: A retrospective analysis. Medicine 2022, 101, e28680. [Google Scholar] [CrossRef] [PubMed]
- Nagamatsu, H.; Hiraki, M.; Mizukami, N.; Yoshida, H.; Iwamoto, H.; Sumie, S.; Torimura, T.; Sata, M. Intra-arterial therapy with cisplatin suspension in lipiodol and 5-fluorouracil for hepatocellular carcinoma with portal vein tumour thrombosis. Aliment. Pharmacol. Ther. 2010, 32, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Oken, M.M.; Creech, R.H.; Tormey, D.C.; Horton, J.; Davis, T.E.; McFadden, E.T.; Carbone, P.P. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. 1982, 5, 649–655. [Google Scholar] [CrossRef]
- de Ulibarri, J.I.; Gonzalez-Madrono, A.; de Villar, N.G.; Gonzalez, P.; Gonzalez, B.; Mancha, A.; Rodriguez, F.; Fernandez, G. CONUT: A tool for controlling nutritional status. First validation in a hospital population. Nutr. Hosp. 2005, 20, 38–45. [Google Scholar]
- Johnson, P.J.; Berhane, S.; Kagebayashi, C.; Satomura, S.; Teng, M.; Reeves, H.L.; O’Beirne, J.; Fox, R.; Skowronska, A.; Palmer, D.; et al. Assessment of liver function in patients with hepatocellular carcinoma: A new evidence-based approach-the ALBI grade. J. Clin. Oncol. 2015, 33, 550–558. [Google Scholar] [CrossRef]
- Hiraoka, A.; Michitaka, K.; Kumada, T.; Izumi, N.; Kadoya, M.; Kokudo, N.; Kubo, S.; Matsuyama, Y.; Nakashima, O.; Sakamoto, M.; et al. Validation and Potential of Albumin-Bilirubin Grade and Prognostication in a Nationwide Survey of 46,681 Hepatocellular Carcinoma Patients in Japan: The Need for a More Detailed Evaluation of Hepatic Function. Liver Cancer 2017, 6, 325–336. [Google Scholar] [CrossRef]
- Kudo, M.; Kitano, M.; Sakurai, T.; Nishida, N. General Rules for the Clinical and Pathological Study of Primary Liver Cancer, Nationwide Follow-Up Survey and Clinical Practice Guidelines: The Outstanding Achievements of the Liver Cancer Study Group of Japan. Dig. Dis. 2015, 33, 765–770. [Google Scholar] [CrossRef]
- Lencioni, R.; Llovet, J.M. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin. Liver Dis. 2010, 30, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Gallo, P.; Silletta, M.; De Vincentis, A.; Lo Prinzi, F.; Terracciani, F.; Di Fazio, G.; Flagiello, V.; Vespasiani Gentilucci, U.; Antonelli Incalzi, R.; Picardi, A. Sarcopenia in Hepatocellular Carcinoma: Pathogenesis and Management. Chemotherapy 2022, 67, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Yabusaki, N.; Fujii, T.; Yamada, S.; Suzuki, K.; Sugimoto, H.; Kanda, M.; Nakayama, G.; Koike, M.; Fujiwara, M.; Kodera, Y. Adverse impact of low skeletal muscle index on the prognosis of hepatocellular carcinoma after hepatic resection. Int. J. Surg. 2016, 30, 136–142. [Google Scholar] [CrossRef]
- Xu, L.; Jing, Y.; Zhao, C.; Zhang, Q.; Zhao, X.; Yang, J.; Wu, L.; Yang, Y. Preoperative computed tomography-assessed skeletal muscle index is a novel prognostic factor in patients with hepatocellular carcinoma following hepatectomy: A meta-analysis. J. Gastrointest. Oncol. 2020, 11, 1040–1053. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, N.; Nakagawa, H.; Kudo, Y.; Tateishi, R.; Taguri, M.; Watadani, T.; Nakagomi, R.; Kondo, M.; Nakatsuka, T.; Minami, T.; et al. Sarcopenia, intramuscular fat deposition, and visceral adiposity independently predict the outcomes of hepatocellular carcinoma. J. Hepatol. 2015, 63, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Yeh, W.S.; Chiang, P.L.; Kee, K.M.; Chang, C.D.; Lu, S.N.; Chen, C.H.; Wang, J.H. Pre-sarcopenia is the prognostic factor of overall survival in early-stage hepatoma patients undergoing radiofrequency ablation. Medicine 2020, 99, e20455. [Google Scholar] [CrossRef]
- Imai, K.; Takai, K.; Hanai, T.; Ideta, T.; Miyazaki, T.; Kochi, T.; Suetsugu, A.; Shiraki, M.; Shimizu, M. Skeletal muscle depletion predicts the prognosis of patients with hepatocellular carcinoma treated with sorafenib. Int. J. Mol. Sci. 2015, 16, 9612–9624. [Google Scholar] [CrossRef]
- Fujita, M.; Abe, K.; Kuroda, H.; Oikawa, T.; Ninomiya, M.; Masamune, A.; Okumoto, K.; Katsumi, T.; Sato, W.; Iijima, K.; et al. Influence of skeletal muscle volume loss during lenvatinib treatment on prognosis in unresectable hepatocellular carcinoma: A multicenter study in Tohoku, Japan. Sci. Rep. 2022, 12, 6479. [Google Scholar] [CrossRef]
- Saeki, I.; Yamasaki, T.; Maeda, M.; Hisanaga, T.; Iwamoto, T.; Matsumoto, T.; Hidaka, I.; Ishikawa, T.; Takami, T.; Sakaida, I. Effect of body composition on survival benefit of hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma: A comparison with sorafenib therapy. PLoS ONE 2019, 14, e0218136. [Google Scholar] [CrossRef]
- Granito, A.; Facciorusso, A.; Sacco, R.; Bartalena, L.; Mosconi, C.; Cea, U.V.; Cappelli, A.; Antonino, M.; Modestino, F.; Brandi, N.; et al. TRANS-TACE: Prognostic Role of the Transient Hypertransaminasemia after Conventional Chemoembolization for Hepatocellular Carcinoma. J. Pers. Med. 2021, 11, 1041. [Google Scholar] [CrossRef]
- Hamaguchi, Y.; Kaido, T.; Okumura, S.; Kobayashi, A.; Hammad, A.; Tamai, Y.; Inagaki, N.; Uemoto, S. Proposal for new diagnostic criteria for low skeletal muscle mass based on computed tomography imaging in Asian adults. Nutrition 2016, 32, 1200–1205. [Google Scholar] [CrossRef] [PubMed]


| Decreased SMI | Non-Decreased SMI | ||
|---|---|---|---|
| Characteristics | n = 27 | n = 43 | p Value |
| Age (years): median (range) | 67 (37–87) | 69 (41–89) | 0.38 |
| Sex (n): Male/Female (%) | 24 (88.9)/3 (11.1) | 33 (76.7)/10 (23.3) | 0.34 |
| Etiology (n): HBV/HCV/Both/NBNC (%) | 6 (22.2)/14 (51.9)/1 (3.7)/6 (22.2) | 8 (18.6)/25 (58.1)/0 (0.0)/10 (23.3) | 0.61 |
| Height (m): median (range) | 1.63 (1.44–1.82) | 1.61 (1.34–1.71) | 0.30 |
| Body weight (kg): median (range) | 61.5 (47.4–77.0) | 61.3 (40.4–81.5) | 0.56 |
| BMI (kg/m2): median (range) | 23.6 (18.5–28.1) | 23.8 (16.6–29.3) | 0.77 |
| Baseline SMI (kg/m2): median (range) | Male: 49.11 (31.20–65.05) Female: 46.56 (37.93–47.20) | Male: 48.93 (28.72–67.80) Female: 41.15 (27.57–49.25) | 0.47 |
| Above SMI cutoff (n): Yes/No (%) | 22 (81.5)/5 (18.5) | 33 (76.7)/10 (23.3) | 0.77 |
| Performance status (n): 0/1/2/3 (%) | 14 (51.9)/8 (29.6)/4 (14.8)/1 (3.7) | 32 (74.4)/9 (20.9)/2 (4.7)/0 (0.0) | 0.15 |
| CONUT score (n): 0–1/2–4/5–8/ >8 (%) | 1 (3.7)/13 (48.1)/10 (37.0)/3 (11.1) | 4 (9.3)/24 (55.8)/15 (34.9)/0 (0.0) | 0.13 |
| Recurrence (n): Yes/No (%) | 8 (29.6)/19 (70.4) | 16 (37.2)/27 (62.8) | 0.43 |
| Combined radiotherapy for MVI (n): Yes/No (%) | 15 (55.6)/12 (44.4) | 30 (69.8)/13 (30.2) | 0.23 |
| Post-MTA treatment (n): Yes/No (%) | 8 (29.6)/19 (70.4) | 28 (65.1)/15 (34.9) | <0.01 |
| Child–Pugh score (n): 5/6/7/≥8 (%) | 8 (29.6)/6 (22.2)/8 (29.6)/5 (18.5) | 15 (34.9)/19 (44.2)/5 (11.6)/4 (9.3) | 0.10 |
| mALBI grade (n): 1/2a/2b/3 (%) | 1 (3.7)/6 (22.2)/17 (63.0)/3 (11.1) | 9 (20.9)/5 (11.6)/27 (62.8)/2 (4.7) | 0.13 |
| AFP (ng/mL): median (range) | 3528 (3–202,847) | 1942 (2–337,408) | 0.07 |
| DCP (mAU/mL): median (range) | 12,346 (13–901,588) | 4348 (9–1,114,735) | 0.19 |
| Maximum tumor size (n): <3 cm/≥3 cm (%) | 2 (7.4)/25 (18.5) | 7 (16.3)/36 (83.7) | 0.47 |
| Number of tumors (n): ≤3/≥4 (%) | 1 (3.7)/26 (96.3) | 7 (16.3)/36 (83.7) | 0.14 |
| Major vascular invasion (n): Yes/No (%) | 23 (85.2)/4 (14.8) | 31 (72.1)/12 (27.9) | 0.25 |
| Extrahepatic metastasis (n): Yes/No (%) | 5 (18.5)/22 (81.5) | 5 (11.6)/38 (88.4) | 0.49 |
| TMN staging LCSGJ 6th (n): III/IVa/IVb (%) | 3 (11.1)/19 (70.4)/5 (18.5) | 13 (30.2)/26 (60.5)/4 (9.3) | 0.14 |
| Decreased SMI | Non-Decreased SMI | ||
|---|---|---|---|
| Therapeutic Effect | n = 27 | n = 43 | p Value |
| ORR, n (%) | 4 (14.8) | 30 (69.8) | <0.01 |
| DCR, n (%) | 15 (55.6) | 38 (88.4) | <0.01 |
| CR, n (%) | 0 (0.0) | 7 (16.3) | |
| PR, n (%) | 4 (14.8) | 23 (53.5) | |
| SD, n (%) | 11 (40.7) | 8 (18.6) | |
| PD, n (%) | 12 (44.4) | 5 (11.6) |
| Decreased SMI | Non-Decreased SMI | ||
|---|---|---|---|
| n = 27 | n = 43 | p Value | |
| Any adverse events, n (%) | 26 (96.3) | 34 (79.1) | 0.08 |
| Grade 1 | 3 (11.1) | 3 (6.98) | |
| Grade 2 | 9 (33.3) | 14 (32.6) | |
| Grade 3 | 9 (33.3) | 16 (37.2) | |
| Grade 4 | 5 (18.5) | 1 (2.3) | |
| Grade 5 | 0 (0.0) | 0 (0.0) | |
| Major adverse events, n (%) | |||
| Platelet count decreased | 11 (40.7) | 25 (58.1) | 0.16 |
| AST increased | 9 (33.3) | 9 (20.9) | 0.25 |
| Anorexia | 8 (29.6) | 6 (14.0) | 0.11 |
| Vomiting | 5 (18.5) | 2 (4.7) | 0.10 |
| Esophageal variceal hemorrhage | 4 (14.8) | 0 (0.0) | <0.05 |
| WBCs decreased | 2 (7.4) | 4 (9.3) | >0.99 |
| Anemia | 2 (7.4) | 7 (16.3) | 0.47 |
| Upper gastrointestinal hemorrhage | 0 (0.0) | 3 (7.0) | 0.28 |
| Severe adverse events of grade ≥ 3, n (%) | 14 (51.9) | 17 (39.5) | 0.31 |
| Platelet count decreased | 4 (14.8) | 9 (20.9) | |
| Anorexia | 3 (11.1) | 0 (0.0) | |
| Esophageal variceal hemorrhage | 3 (11.1) | 0 (0.0) | |
| Diarrhea | 3 (11.1) | 0 (0.0) | |
| WBCs decreased | 2 (7.4) | 3 (7.0) | |
| AST increased | 2 (7.4) | 0 (0.0) | |
| Wound infection | 2 (7.4) | 0 (0.0) | |
| Anemia | 1 (3.7) | 3 (7.0) | |
| Vomiting | 1 (3.7) | 1 (2.3) | |
| Tumor lysis syndrome | 1 (3.7) | 0 (0.0) | |
| Urinary tract infection | 1 (3.7) | 0 (0.0) | |
| Cholecystitis | 1 (3.7) | 0 (0.0) | |
| Creatinine increased | 1 (3.7) | 0 (0.0) | |
| Hepatic failure | 1 (3.7) | 0 (0.0) | |
| Upper gastrointestinal hemorrhage | 0 (0.0) | 3 (7.0) | |
| Fracture | 0 (0.0) | 1 (2.3) | |
| Hepatic infection | 0 (0.0) | 1 (2.3) |
| Variable | n = 70 | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|---|
| HR | 95%CI | p Value | HR | 95%CI | p Value | ||
| Sex | Male | 1.56 | 0.86–3.07 | 0.17 | |||
| Age | ≥75 years | 0.83 | 0.48–1.38 | 0.48 | |||
| BMI | <23 kg/m2 | 0.73 | 0.42–1.23 | 0.24 | |||
| Baseline SMI | Male <42 cm2/m2 or Female <38 cm2/m2 | 0.61 | 0.32–1.09 | 0.11 | |||
| Decreased SMI at 3 weeks | ≥10% | 5.65 | 3.06–10.66 | <0.01 | 5.59 | 3.00–10.69 | <0.01 |
| PS | ≥1 | 1.23 | 0.71–2.05 | 0.44 | |||
| CONUT score | ≥2 | 1.81 | 0.79–5.21 | 0.21 | |||
| Etiology | HBV | 1.36 | 0.74–2.39 | 0.30 | |||
| Previous treatment | Yes | 0.81 | 0.50–1.32 | 0.39 | |||
| Child–Pugh score | ≥7 | 2.33 | 1.34–3.95 | <0.01 | 2.21 | 1.25–3.82 | <0.01 |
| mALBI grade | 2a, 2b, or 3 | 1.21 | 0.63–2.63 | 0.59 | |||
| AFP | >300 ng/mL | 1.12 | 0.67–1.95 | 0.69 | |||
| DCP | >700 mAU/mL | 1.30 | 0.78–2.27 | 0.33 | |||
| Maximum size of tumor | ≥50 mm | 1.66 | 1.00–2.84 | 0.06 | |||
| Number of tumors | ≥4 | 2.03 | 0.97–4.96 | 0.09 | |||
| Major vascular invasion | Yes | 0.63 | 0.36–1.16 | 0.12 | |||
| Extrahepatic metastasis | Yes | 1.01 | 0.48–1.91 | 0.98 | |||
| Variable | n = 70 | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|---|
| HR | 95%CI | p Value | HR | 95%CI | p Value | ||
| Sex | Male | 1.10 | 0.54–2.56 | 0.80 | |||
| Age | ≥70 years | 0.50 | 0.25–0.95 | <0.05 | 0.98 | 0.95–1.01 | 0.17 |
| BMI | <23 kg/m2 | 0.73 | 0.38–1.35 | 0.33 | |||
| Baseline SMI | Male <42 cm2/m2 or Female <38 cm2/m2 | 0.40 | 0.15–1.01 | 0.06 | |||
| Decreased SMI at 3 weeks | ≥10% | 12.86 | 5.00–37.78 | <0.01 | 13.10 | 4.91–39.70 | <0.01 |
| PS | ≥1 | 1.37 | 0.71–2.53 | 0.32 | |||
| CONUT score | ≥5 | 1.53 | 0.83–2.77 | 0.16 | |||
| Etiology | NBNC | 1.48 | 0.73–2.83 | 0.25 | |||
| Previous treatment | Yes | 0.92 | 0.51–1.68 | 0.79 | |||
| Child–Pugh score | ≥7 | 1.93 | 0.99–3.60 | <0.05 | 1.86 | 0.93–3.54 | 0.07 |
| mALBI grade | 2a, 2b, or 3 | 2.57 | 1.03–8.58 | 0.07 | |||
| AFP | >300 ng/mL | 1.24 | 0.66–2.52 | 0.52 | |||
| DCP | >700 mAU/mL | 1.83 | 0.96–3.79 | 0.08 | |||
| Maximum size of tumor | ≥50 mm | 1.70 | 0.93–3.21 | 0.09 | |||
| Number of tumors | ≥4 | 1.93 | 0.82–5.68 | 0.17 | |||
| Major vascular invasion | Yes | 1.82 | 0.86–4.48 | 0.15 | |||
| Extrahepatic metastasis | Yes | 0.83 | 0.31–1.85 | 0.68 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oura, K.; Morishita, A.; Tani, J.; Nomura, T.; Manabe, T.; Takuma, K.; Nakahara, M.; Tadokoro, T.; Fujita, K.; Mimura, S.; et al. Prognostic Value of Skeletal Muscle Loss in Patients with Hepatocellular Carcinoma Treated with Hepatic Arterial Infusion Chemotherapy. Cancers 2023, 15, 1834. https://doi.org/10.3390/cancers15061834
Oura K, Morishita A, Tani J, Nomura T, Manabe T, Takuma K, Nakahara M, Tadokoro T, Fujita K, Mimura S, et al. Prognostic Value of Skeletal Muscle Loss in Patients with Hepatocellular Carcinoma Treated with Hepatic Arterial Infusion Chemotherapy. Cancers. 2023; 15(6):1834. https://doi.org/10.3390/cancers15061834
Chicago/Turabian StyleOura, Kyoko, Asahiro Morishita, Joji Tani, Takako Nomura, Takushi Manabe, Kei Takuma, Mai Nakahara, Tomoko Tadokoro, Koji Fujita, Shima Mimura, and et al. 2023. "Prognostic Value of Skeletal Muscle Loss in Patients with Hepatocellular Carcinoma Treated with Hepatic Arterial Infusion Chemotherapy" Cancers 15, no. 6: 1834. https://doi.org/10.3390/cancers15061834
APA StyleOura, K., Morishita, A., Tani, J., Nomura, T., Manabe, T., Takuma, K., Nakahara, M., Tadokoro, T., Fujita, K., Mimura, S., Sanomura, T., Nishiyama, Y., & Masaki, T. (2023). Prognostic Value of Skeletal Muscle Loss in Patients with Hepatocellular Carcinoma Treated with Hepatic Arterial Infusion Chemotherapy. Cancers, 15(6), 1834. https://doi.org/10.3390/cancers15061834





