Is Pediatric Melanoma Really That Different from Adult Melanoma? A Multicenter Epidemiological, Clinical and Dermoscopic Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Garbe, C.; Amaral, T.; Peris, K.; Hauschild, A.; Arenberger, P.; Bastholt, L.; Fargnoli, M.C.; Grob, J.J.; Höller, C.; Kaufmann, R.; et al. European Dermatology Forum (EDF), the European Association of Dermato-Oncology (EADO), and the European Organization for Research and Treatment of Cancer (EORTC). European consensus-based interdisciplinary guideline for melanoma. Part 1: Diagnostics—Update 2019. Eur. J. Cancer 2020, 126, 141–158. [Google Scholar] [CrossRef]
- Austin, M.T.; Xing, Y.; Hayes-Jordan, A.A.; Lally, K.P.; Cormier, J.N. Melanoma incidence rises for children and adolescents: An epidemiologic review of pediatric melanoma in the United States. J. Pediatr. Surg. 2013, 48, 2207–2213. [Google Scholar] [CrossRef]
- Sreeraman Kumar, R.; Messina, J.L.; Reed, D.; Navid, F.; Sondak, V.K. Pediatric Melanoma and Atypical Melanocytic Neoplasms. Cancer Treat. Res. 2016, 167, 331–369. [Google Scholar] [CrossRef] [PubMed]
- Merkel, E.A.; Mohan, L.S.; Shi, K.; Panah, E.; Zhang, B.; Gerami, P. Paediatric melanoma: Clinical update, genetic basis, and advances in diagnosis. Lancet Child Adolesc. Health 2019, 3, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, A.; Barnhill, R.L. Pathology and genomics of pediatric melanoma: A critical reexamination and new insights. Pediatr. Blood. Cancer. 2018, 65, e26792. [Google Scholar] [CrossRef] [PubMed]
- Aldrink, J.H.; Polites, S.; Lautz, T.B.; Malek, M.M.; Rhee, D.; Bruny, J.; Christison-Lagay, E.R.; Tracy, E.T.; Abdessalam, S.; Ehrlich, P.F.; et al. What’s new in pediatric melanoma: An update from the APSA cancer committee. J. Pediatr. Surg. 2020, 55, 1714–1721. [Google Scholar] [CrossRef]
- Kim, D.J.; Yuan, T.A.; Chen, P.C.; Liu-Smith, F.; Koh, S.S.; Mesinkovska, N.A.; Sarpa, H.G. Pediatric melanoma in the Hispanic population: An analysis of institutional and national data. Pediatr. Dermatol. 2021, 38, 1102–1110. [Google Scholar] [CrossRef]
- Faghihi, G.; Radan, M. Xeroderma pigmentosum and lentigo maligna in identical twins. J. Dermatolog. Treat. 2006, 17, 241–243. [Google Scholar] [CrossRef]
- Cordoro, K.M.; Gupta, D.; Frieden, I.J.; McCalmont, T.; Kashani-Sabet, M. Pediatric melanoma: Results of a large cohort. study and proposal for modified ABCD detection criteria for children. J. Am. Acad. Dermatol. 2013, 68, 913–925. [Google Scholar] [CrossRef]
- Ferrari, A.; Bono, A.; Baldi, M.; Collini, P.; Casanova, M.; Pennacchioli, E.; Terenziani, M.; Marcon, I.; Santinami, M.; Bartoli, C. Does melanoma behave differently in younger children than in adults? A retrospective study of 33 cases of childhood melanoma from a single institution. Pediatrics 2005, 115, 649–654. [Google Scholar] [CrossRef]
- Bailey, K.M.; Durham, A.B.; Zhao, L.; Fullen, D.; Geiger, J.; Bradford, C.; Opipari, V.; Johnson, T.; Mody, R. Pediatric melanoma and aggressive Spitz tumors: A retrospective diagnostic, exposure and outcome analysis. Transl. Pediatr. 2018, 7, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Carrera, C.; Scope, A.; Dusza, S.W.; Argenziano, G.; Nazzaro, G.; Phan, A.; Tromme, I.; Rubegni, P.; Malvehy, J.; Puig, S.; et al. Clinical and dermoscopic characterization of pediatric and adolescent melanomas: Multicenter study of 52 cases. J. Am. Acad. Dermatol. 2018, 78, 278–288. [Google Scholar] [CrossRef]
- Livestro, D.P.; Kaine, E.M.; Michaelson, J.S.; Mihm, M.C.; Haluska, F.G.; Muzikansky, A.; Sober, A.J.; Tanabe, K.K. Melanoma in the young: Differences and similarities with adult melanoma: A case-matched controlled analysis. Cancer 2007, 110, 614–624. [Google Scholar] [CrossRef]
- Carli, P.; Nardini, P.; Crocetti, E.; De Giorgi, V.; Giannotti, B. Frequency and characteristics of melanomas missed at a pigmented lesion clinic: A registry-based study. Melanoma Res. 2004, 14, 403–407. [Google Scholar] [CrossRef]
- Parent, A.S.; Teilmann, G.; Juul, A.; Skakkebaek, N.E.; Toppari, J.; Bourguignon, J.P. The timing of normal puberty and the age limits of sexual precocity: Variations around the world, secular trends, and changes after migration. Endocr. Rev. 2003, 24, 668–693. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, K.; Mouritsen, A.; Aksglaede, L.; Hagen, C.P.; Mogensen, S.S.; Juul, A. Recent secular trends in pubertal timing: Implications for evaluation and diagnosis of precocious puberty. Horm. Res. Paediatr. 2012, 77, 137–145. [Google Scholar] [CrossRef]
- Elder, D.E.; Massi, D.; Scolyer, R.A.; Willemze, R. WHO Classification of Skin Tumours, 4th ed.; World Health Organization Classification of Tu-mours; IARC: Lyon, France, 2018; Volume 11, pp. 66–71. [Google Scholar]
- Soura, E.; Eliades, P.J.; Shannon, K.; Stratigos, A.J.; Tsao, H. Hereditary melanoma: Update on syndromes and management: Genetics of familial atypical multiple mole melanoma syndrome. J. Am. Acad. Dermatol. 2016, 74, 395–407. [Google Scholar] [CrossRef]
- El Sharouni, M.A.; Rawson, R.V.; Potter, A.J.; Paver, E.C.; Wilmott, J.S.; Witkamp, A.J.; Sigurdsson, V.; van Diest, P.J.; Scolyer, R.A.; Thompson, J.F.; et al. Melanomas in children and adolescents: Clinicopathologic features and survival outcomes. J. Am. Acad. Dermatol. 2022, 88, 609–616. [Google Scholar] [CrossRef]
- De Giorgi, V.; Gori, A.; Gandini, S.; Papi, F.; Grazzini, M.; Rossari, S.; Simoni, A.; Maio, V.; Massi, D. Oestrogen receptor beta and melanoma: A comparative study. Br. J. Dermatol. 2013, 168, 513–519. [Google Scholar] [CrossRef] [PubMed]
- De Giorgi, V.; Gori, A.; Grazzini, M.; Rossari, S.; Scarfì, F.; Corciova, S.; Verdelli, A.; Lotti, T.; Massi, D. Estrogens, estrogen receptors and melanoma. Expert. Rev. Anticancer Ther. 2011, 11, 739–747. [Google Scholar] [CrossRef] [PubMed]
- De Giorgi, V.; Gori, A.; Alfaioli, B.; Papi, F.; Grazzini, M.; Rossari, S.; Lotti, T.; Massi, D. Influence of sex hormones on melanoma. J. ClinOncol. 2011, 29, e94–e95. [Google Scholar] [CrossRef] [PubMed]
- Dika, E.; Patrizi, A.; Lambertini, M.; Manuelpillai, N.; Fiorentino, M.; Altimari, A.; Ferracin, M.; Lauriola, M.; Fabbri, E.; Campione, E.; et al. Estrogen Receptors and Melanoma: A Review. Cells 2019, 8, 1463. [Google Scholar] [CrossRef]
- De Giorgi, V.; Mavilia, C.; Massi, D.; Gozzini, A.; Aragona, P.; Tanini, A.; Sestini, S.; Paglierani, M.; Boddi, V.; Brandi, M.L.; et al. Estrogen receptor expression in cutaneous melanoma: A real-time reverse transcriptase-polymerase chain reaction and immunohistochemical study. Arch. Dermatol. 2009, 145, 30–36. [Google Scholar] [CrossRef]
- Gori, A.; Savarese, I.; D’Errico, A.; Grazzini, M.; Papi, F.; Maio, V.; Covarelli, P.; Massi, D.; Gandini, S.; De Giorgi, V. Estrogen receptor (ER)βexpression and worseoutcome from melanoma in pregnant and perimenopausal women. J. Am. Acad. Dermatol. 2016, 75, e117. [Google Scholar] [CrossRef]
- Paradela, S.; Fonseca, E.; Pita-Fernández, S.; Kantrow, S.M.; Diwan, A.H.; Herzog, C.; Prieto, V.G. Prognostic factors for melanoma in children and adolescents: A clinicopathologic, single-center study of 137 Patients. Cancer 2010, 116, 4334–4344. [Google Scholar] [CrossRef]
- Aldrink, J.H.; Selim, M.A.; Diesen, D.L.; Johnson, J.; Pruitt, S.K.; Tyler, D.S.; Seigler, H.F. Pediatric melanoma: A single-institution experience of 150 patients. J. Pediatr. Surg. 2009, 44, 1514–1521. [Google Scholar] [CrossRef]
- Falk Delgado, A.; Zommorodi, S.; Falk Delgado, A. Sentinel Lymph Node Biopsy and Complete Lymph Node Dissection for Melanoma. Curr. Oncol. Rep. 2019, 21, 54. [Google Scholar] [CrossRef] [PubMed]
- Morrison, S.; Han, D. Re-evaluation of Sentinel Lymph Node Biopsy for Melanoma. Curr. Treat. Options. Oncol. 2021, 22, 22. [Google Scholar] [CrossRef] [PubMed]
- Rathod, D.; Kroumpouzos, G.; Lallas, A.; Rao, B.; Murrell, D.F.; Apalla, Z.; Grabbe, S.; Loquai, C.; Goldust, M. Critical Review of the Sentinel Lymph Node Surgery in Malignant Melanoma. J. Drugs Dermatol. 2022, 21, 510–516. [Google Scholar] [CrossRef] [PubMed]
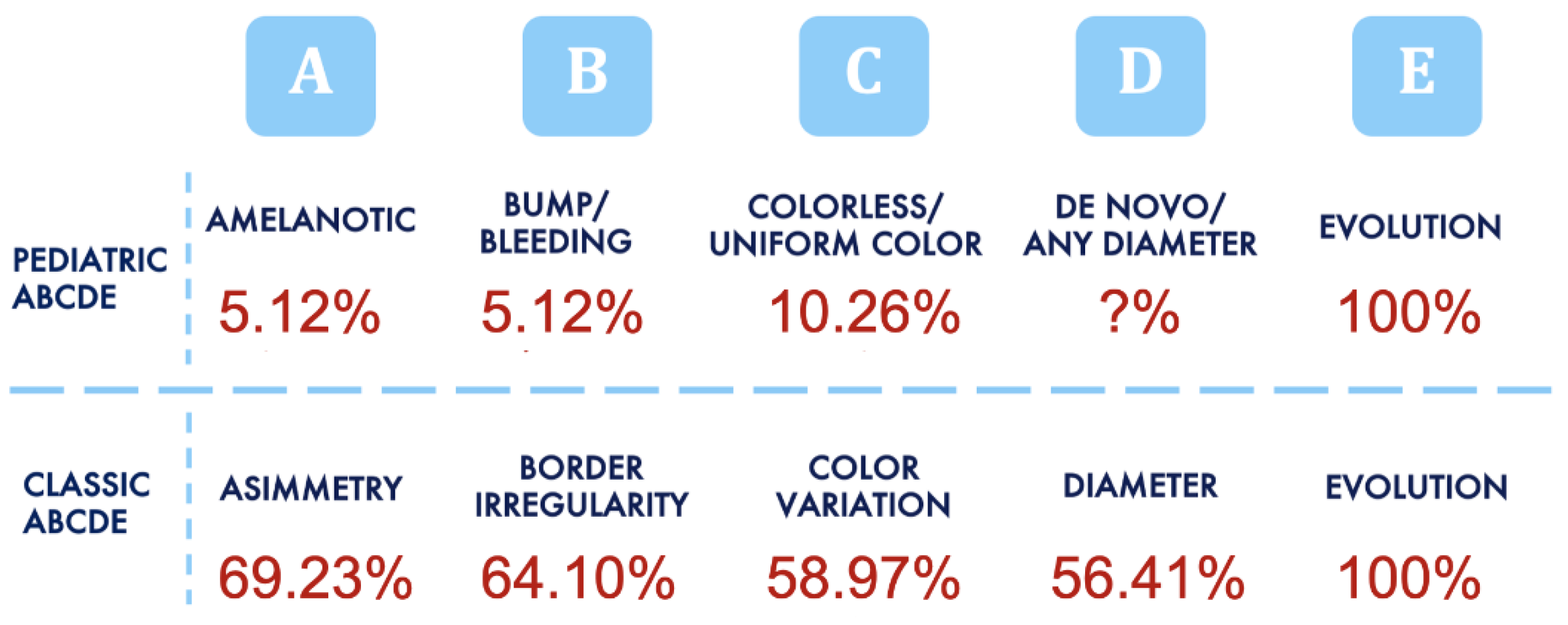
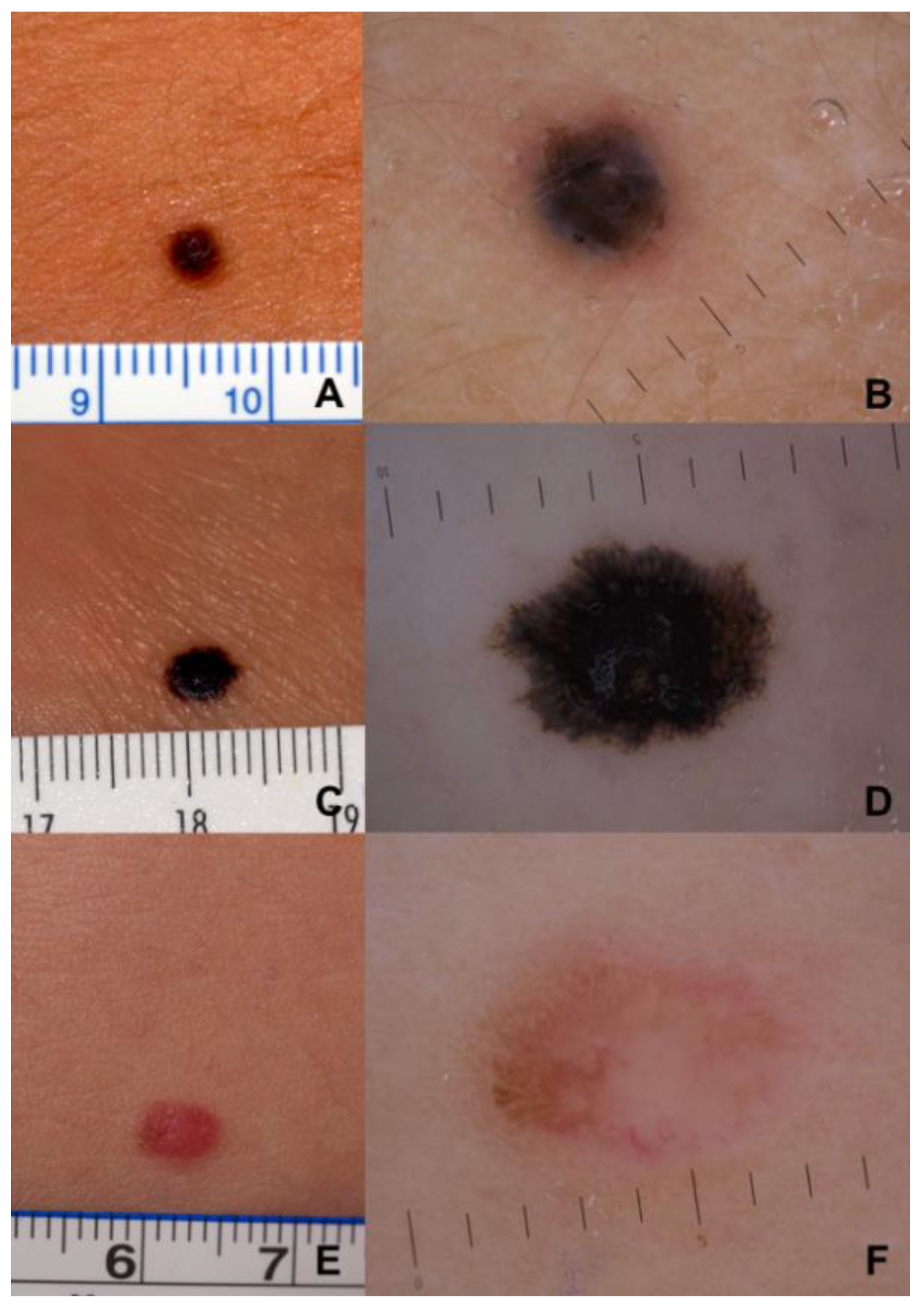
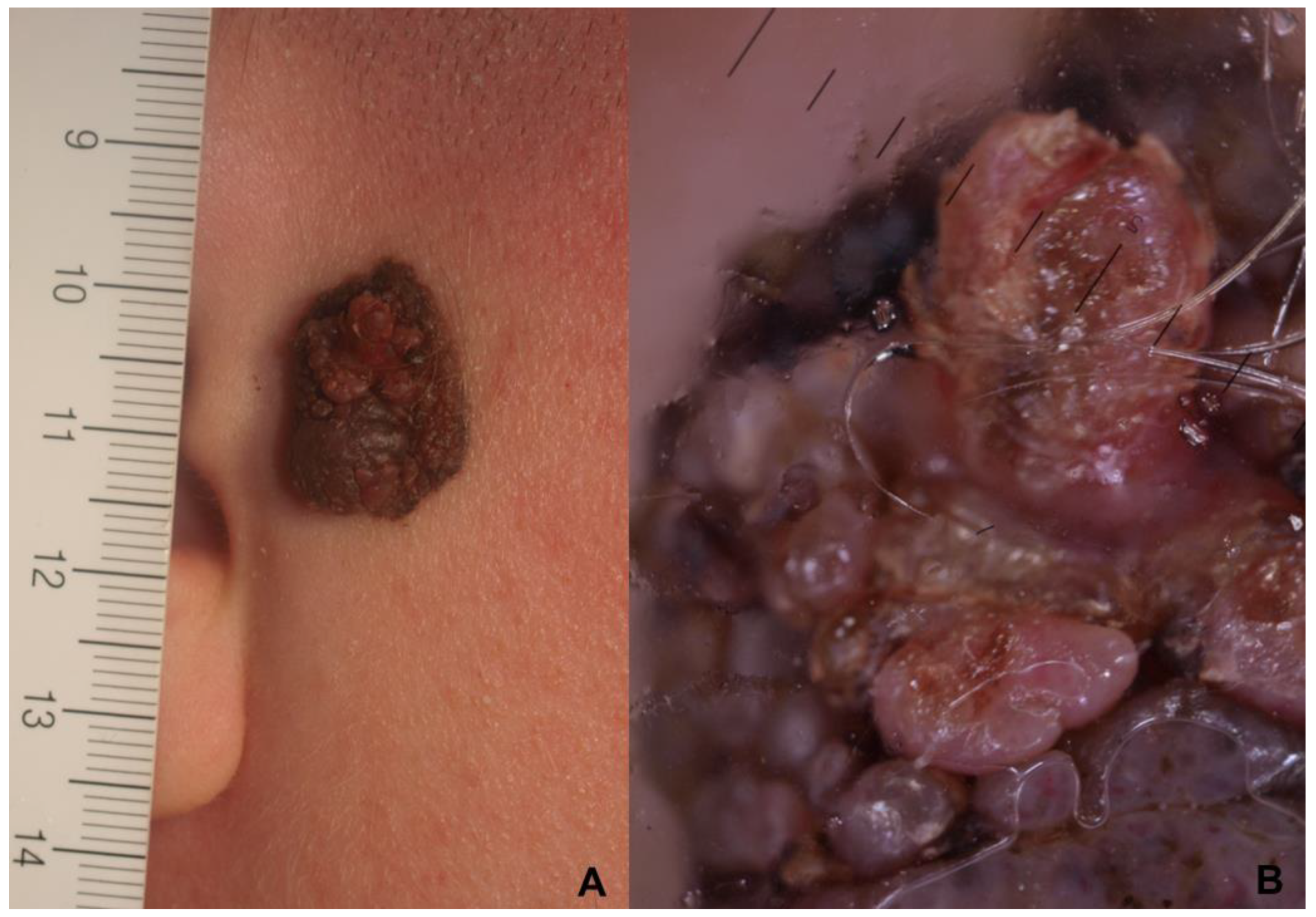
| Histopathological Parameters | 0–12 Years | 13–18 Years | Overall |
|---|---|---|---|
| Ulceration, n (%) | 1/8 (12.5) | 1/31 (3.2) | 2/39 (5.1) |
| Mean Breslow thickness, millimeters, mean (SD) | 2.02 (0.74) | 0.77 (0.17) | 1.05 (0.23) |
| Males | 0.88 (0.51) | 0.78 (0.23) | 0.80 (0.20) |
| Females | 2.36 (1.01) | 0.77 (0.25) | 1.21 (0.26) |
| In situ M, n (%) | 0/8 | 9/31 (29.0) | 9/39 (23.1) |
| Males | 3/31 (9.7) | 3/39 (7.7) | |
| Females | 6/31 (19.3) | 6/39 (15.4) | |
| Spitzoid melanoma, n (%) | 7/8 (87.5) | 8/31 (25.8) | 15 (38.5) |
| SSM, n (%) | 0/8 | 18/31 (58.1) | 18/39 (46.2) |
| Melanoma arising in congenital melanocytic Nevus, n (%) | 0/8 | 2/31 (6.5) | 2/39 (5.1) |
| Rare melanoma *, n (%) | 1/8 (12.5) | 2/31 (6.5) | 3/39 (7.8) |
| Anatomic location | 0–12 years | 13–18 years | Overall |
| Head/neck, n (%) ** | 0/7 | 3/31 (9.7) | 3/38(7.9) |
| Trunk, n (%) ** | 6/7 (85.7) | 9/31 (29.0) | 15/38 (39.5) |
| Upper limbs, n (%) ** | 0/7 | 6/31 (19.4) | 6/38 (15.8) |
| Lower limbs, n (%) ** | 1/7 (14.3) | 13/31 (41.9) | 14/38 (36.8) |
| Risk factors | 0–12 years | 13–18 years | Overall |
| Multiple primary MM, n (%) | 0/8 | 3/31 (9.7) | 3/39 (7.7) |
| Photo-type I, n (%) | 6/8 (75.0) | 13/31 (41.9) | 19/39 (48.7) |
| Familial MM, n (%) | 2/8 (25.0) | 8/31 (25.8) | 10/39 (25.6) |
| Outcome | 0–12 years | 13–18 years | Overall |
| SNB performed, n (%) | 4/8 (50.0) | 5/31 (16.1) | 9/39 (23.1) |
| Positive SNB, n (%) | 1/8 (12.5) | 3/31 (9.7) | 4/39 (10.3) |
| Metastasis, n (%) | 0/8 | 1/31 (3.2) | 1/39 (2.6) |
| Dermoscopic Features | % |
|---|---|
| Irregular streaks/pseudopods | 74.4 |
| Atypical pigment networks | 30.8 |
| Atypical globules | 30.8 |
| Regression structures | 30.8 |
| Atypical vascular pattern | 20.5 |
| Blue white veil | 15.4 |
| Inverse network | 10.3 |
| Scar-like areas | 10.3 |
| Prominent network | 10.3 |
| Amelanosis | 5.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Giorgi, V.; Magnaterra, E.; Zuccaro, B.; Magi, S.; Magliulo, M.; Medri, M.; Mazzoni, L.; Venturi, F.; Silvestri, F.; Tomassini, G.M.; et al. Is Pediatric Melanoma Really That Different from Adult Melanoma? A Multicenter Epidemiological, Clinical and Dermoscopic Study. Cancers 2023, 15, 1835. https://doi.org/10.3390/cancers15061835
De Giorgi V, Magnaterra E, Zuccaro B, Magi S, Magliulo M, Medri M, Mazzoni L, Venturi F, Silvestri F, Tomassini GM, et al. Is Pediatric Melanoma Really That Different from Adult Melanoma? A Multicenter Epidemiological, Clinical and Dermoscopic Study. Cancers. 2023; 15(6):1835. https://doi.org/10.3390/cancers15061835
Chicago/Turabian StyleDe Giorgi, Vincenzo, Elisabetta Magnaterra, Biancamaria Zuccaro, Serena Magi, Manfredi Magliulo, Matelda Medri, Laura Mazzoni, Federico Venturi, Flavia Silvestri, Gian Marco Tomassini, and et al. 2023. "Is Pediatric Melanoma Really That Different from Adult Melanoma? A Multicenter Epidemiological, Clinical and Dermoscopic Study" Cancers 15, no. 6: 1835. https://doi.org/10.3390/cancers15061835
APA StyleDe Giorgi, V., Magnaterra, E., Zuccaro, B., Magi, S., Magliulo, M., Medri, M., Mazzoni, L., Venturi, F., Silvestri, F., Tomassini, G. M., Gola, M., Tramontana, M., Berti, S., Stanganelli, I., Stingeni, L., & Covarelli, P. (2023). Is Pediatric Melanoma Really That Different from Adult Melanoma? A Multicenter Epidemiological, Clinical and Dermoscopic Study. Cancers, 15(6), 1835. https://doi.org/10.3390/cancers15061835







