Efficacy of Antibodies Targeting TfR1 in Xenograft Mouse Models of AIDS-Related Non-Hodgkin Lymphoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Recombinant Antibodies
2.3. In Vivo Efficacy Studies
2.4. Bioluminescence Imaging
3. Results
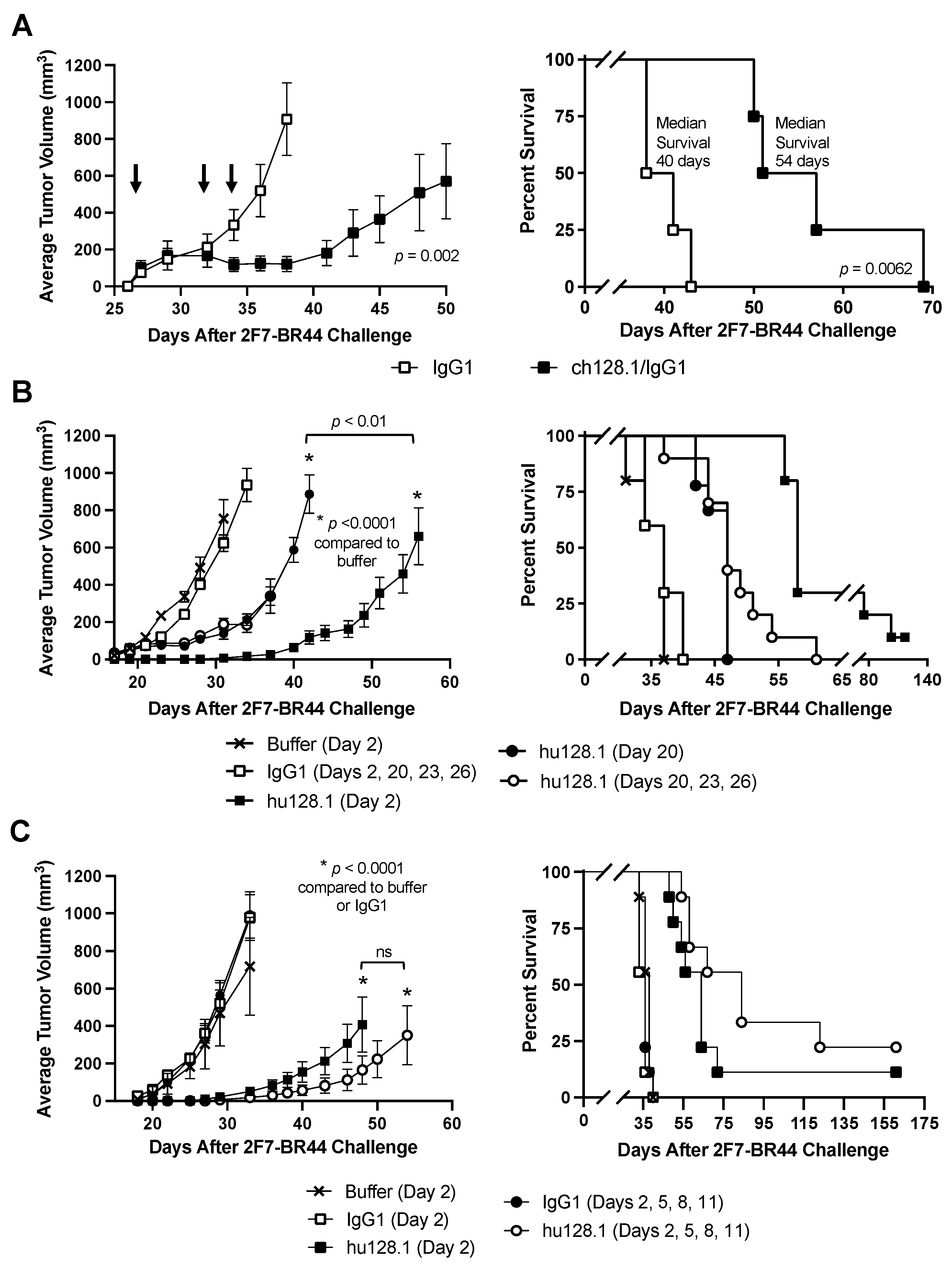
3.1. Efficacy of ch128.1/IgG1 and hu128.1 in a Local Model of AIDS-NHL Using 2F7-BR44 Cells
| Treatment | Number of Animals | Median Survival (Days) | p-Value Compared to IgG1 | p-Value Compared to Buffer | p-Value Compared to hu128.1 (Day 2) |
|---|---|---|---|---|---|
| Data presented in Figure 1B | |||||
| Buffer (Day 2) | 10 | 37 | |||
| 400 μg IgG1 (Days 2, 20, 23, 26) | 10 | 37 | 0.2094 | ||
| 400 μg hu128.1 (Day 2) | 10 | 58 | <0.0001 | <0.0001 | |
| 400 μg hu128.1 (Day 20) | 9 | 47 | <0.0001 | <0.0001 | <0.0001 |
| 400 μg hu128.1 (Days 20, 23, 26) | 10 | 47 | <0.0001 | <0.0001 | 0.0003 |
| Data presented in Figure 1C | |||||
| Buffer (Day 2) | 9 | 38 | |||
| 400 μg IgG1 (Day 2) | 9 | 36 | 0.1307 | ||
| 400 μg hu128.1 (Day 2) | 9 | 64 | <0.0001 | <0.0001 | |
| 400 μg IgG1 (Days 2, 5, 8, 11) | 9 | 36 | 0.2262 | ||
| 400 μg hu128.1 (Days 2, 5, 8, 11) | 9 | 84 | <0.0001 | <0.0001 | 0.1555 |
3.2. Efficacy of ch128.1/IgG1 and hu128.1 in a Disseminated Model of AIDS-NHL Using 2F7-BR44 Cells
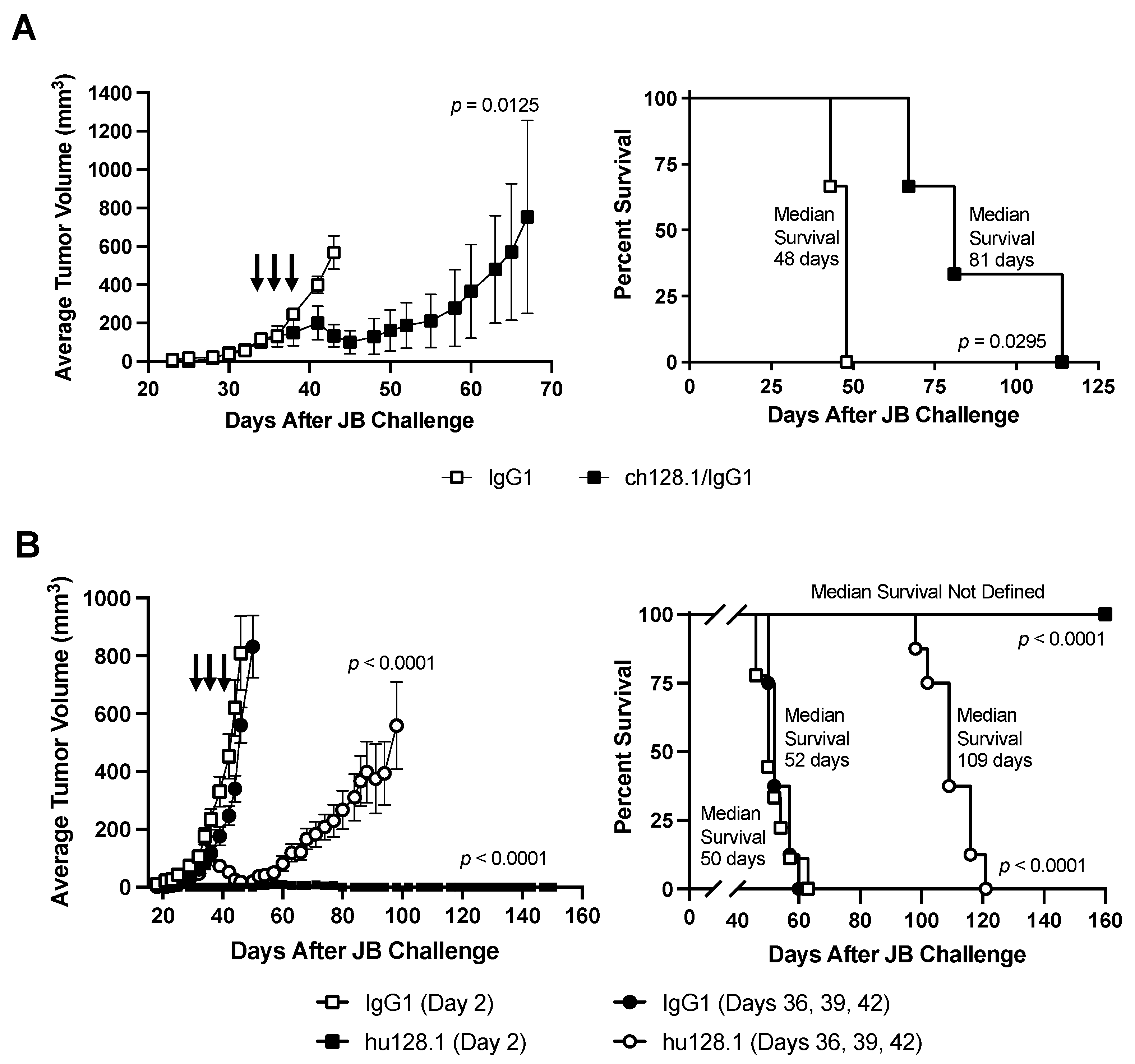
3.3. Efficacy of ch128.1/IgG1 and hu128.1 in Local and Disseminated Models of AIDS-NHL Using JB Cells
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ziegler, J.L.; Bragg, K.; Abrams, D.; Beckstead, J.; Cogan, M.; Volberding, P.; Baer, D.; Wilkinson, L.; Rosenbaum, E.; Grant, K.; et al. High-grade non-Hodgkin’s lymphoma in patients with AIDS. Ann. N. Y. Acad. Sci. 1984, 437, 412–419. [Google Scholar] [CrossRef]
- Noy, A. HIV Lymphoma and Burkitts Lymphoma. Cancer J. 2020, 26, 260–268. [Google Scholar] [CrossRef]
- Gibson, T.M.; Morton, L.M.; Shiels, M.S.; Clarke, C.A.; Engels, E.A. Risk of non-Hodgkin lymphoma subtypes in HIV-infected people during the HAART era: A population-based study. AIDS 2014, 28, 2313–2318. [Google Scholar] [CrossRef]
- Seaberg, E.C.; Wiley, D.; Martinez-Maza, O.; Chmiel, J.S.; Kingsley, L.; Tang, Y.; Margolick, J.B.; Jacobson, L.P.; Multicenter, A.C.S. Cancer incidence in the multicenter AIDS Cohort Study before and during the HAART era: 1984 to 2007. Cancer 2010, 116, 5507–5516. [Google Scholar] [CrossRef] [PubMed]
- Kimani, S.M.; Painschab, M.S.; Horner, M.J.; Muchengeti, M.; Fedoriw, Y.; Shiels, M.S.; Gopal, S. Epidemiology of haematological malignancies in people living with HIV. Lancet HIV 2020, 7, e641–e651. [Google Scholar] [CrossRef]
- Centers for Disease Control. Revision of the case definition of acquired immunodeficiency syndrome for national reporting--United States. MMWR Morb. Mortal Wkly. Rep. 1985, 34, 373–375. [Google Scholar]
- Cesarman, E. Pathology of lymphoma in HIV. Curr. Opin. Oncol. 2013, 25, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Biggar, R.J. AIDS-related cancers in the era of highly active antiretroviral therapy. Oncology 2001, 15, 439–448; discussion 448–449. [Google Scholar]
- Atallah-Yunes, S.A.; Murphy, D.J.; Noy, A. HIV-associated Burkitt lymphoma. Lancet Haematol. 2020, 7, e594–e600. [Google Scholar] [CrossRef] [PubMed]
- Nishijima, T.; Inaba, Y.; Kawasaki, Y.; Tsukada, K.; Teruya, K.; Kikuchi, Y.; Gatanaga, H.; Oka, S. Mortality and causes of death in people living with HIV in the era of combination antiretroviral therapy compared with the general population in Japan. AIDS 2020, 34, 913–921. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, F.; Balestre, E.; Thiebaut, R.; Morlat, P.; Pellegrin, J.L.; Neau, D.; Dabis, F.; Groupe d’Epidemiologie Clinique du SIDA en Aquitaine. Factors associated with the occurrence of AIDS-related non-Hodgkin lymphoma in the era of highly active antiretroviral therapy: Aquitaine Cohort, France. Clin. Infect. Dis. 2006, 42, 411–417. [Google Scholar] [CrossRef]
- Epeldegui, M.; Vendrame, E.; Martinez-Maza, O. HIV-associated immune dysfunction and viral infection: Role in the pathogenesis of AIDS-related lymphoma. Immunol. Res. 2010, 48, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Siewe, B.; Epeldegui, M.; Detels, R.; Landay, A.; Martinez-Maza, O. TLR2 activated B cells are phenotypically similar to the abnormal circulating B cells seen preceding the diagnosis of AIDS related non-Hodgkin lymphoma (NHL) diagnosis. J. Acquir. Immune Defic. Syndr. 2013, 64, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Lilly, A.J.; Fedoriw, Y. Human Immunodeficiency Virus-Associated Lymphoproliferative Disorders. Surg. Pathol. Clin. 2019, 12, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Maza, O.; Crabb, E.; Mitsuyasu, R.T.; Fahey, J.L.; Giorgi, J.V. Infection with the human immunodeficiency virus (HIV) is associated with an in vivo increase in B lymphocyte activation and immaturity. J. Immunol. 1987, 138, 3720–3724. [Google Scholar] [CrossRef] [PubMed]
- Candelaria, P.V.; Leoh, L.S.; Penichet, M.L.; Daniels-Wells, T.R. Antibodies Targeting the Transferrin Receptor 1 (TfR1) as Direct Anti-cancer Agents. Front. Immunol. 2021, 12, 607692. [Google Scholar] [CrossRef] [PubMed]
- Essaghir, A.; Demoulin, J.B. A minimal connected network of transcription factors regulated in human tumors and its application to the quest for universal cancer biomarkers. PLoS ONE 2012, 7, e39666. [Google Scholar] [CrossRef]
- Maguire, A.; Chen, X.; Wisner, L.; Malasi, S.; Ramsower, C.; Kendrick, S.; Barrett, M.T.; Glinsmann-Gibson, B.; McGrath, M.; Rimsza, L.M. Potential Alternative Survival Mechanisms in HIV-Associated Diffuse Large B-Cell Lymphoma (DLBCL) of Germinal Center (GCB) Origin. In Proceedings of the 17th International Confereence on Malignancies in HIV/AIDS, Bethesda, MD, USA, 21–22 October 2019. [Google Scholar]
- Maguire, A.; Chen, X.; Wisner, L.; Ramsower, C.; Glinsmann-Gibson, B.; Rimsza, L.M. Over-Expression of Transferrin Receptor (TFRC/CD71) and Low Expression of Innate and Adaptive Immune Cell Subsets in HIV-Associated, GCB-DLBCL By Digital Gene Expression Profiling. Blood 2019, 134, 2783. [Google Scholar] [CrossRef]
- Rodriguez, J.A.; Helguera, G.; Daniels, T.R.; Neacato, I.I.; Lopez-Valdes, H.E.; Charles, A.C.; Penichet, M.L. Binding specificity and internalization properties of an antibody-avidin fusion protein targeting the human transferrin receptor. J. Control. Release 2007, 124, 35–42. [Google Scholar] [CrossRef]
- Ng, P.P.; Helguera, G.; Daniels, T.R.; Lomas, S.Z.; Rodriguez, J.A.; Schiller, G.; Bonavida, B.; Morrison, S.L.; Penichet, M.L. Molecular events contributing to cell death in malignant human hematopoietic cells elicited by an IgG3-avidin fusion protein targeting the transferrin receptor. Blood 2006, 108, 2745–2754. [Google Scholar] [CrossRef]
- White, S.; Taetle, R.; Seligman, P.A.; Rutherford, M.; Trowbridge, I.S. Combinations of anti-transferrin receptor monoclonal antibodies inhibit human tumor cell growth in vitro and in vivo: Evidence for synergistic antiproliferative effects. Cancer Res. 1990, 50, 6295–6301. [Google Scholar]
- Daniels, T.R.; Ortiz-Sanchez, E.; Luria-Perez, R.; Quintero, R.; Helguera, G.; Bonavida, B.; Martinez-Maza, O.; Penichet, M.L. An antibody-based multifaceted approach targeting the human transferrin receptor for the treatment of B-cell malignancies. J. Immunother 2011, 34, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Leoh, L.S.; Kim, Y.K.; Candelaria, P.V.; Martinez-Maza, O.; Daniels-Wells, T.R.; Penichet, M.L. Efficacy and Mechanism of Antitumor Activity of an Antibody Targeting Transferrin Receptor 1 in Mouse Models of Human Multiple Myeloma. J. Immunol. 2018, 200, 3485–3494. [Google Scholar] [CrossRef] [PubMed]
- Daniels-Wells, T.R.; Widney, D.P.; Leoh, L.S.; Martínez-Maza, O.; Penichet, M.L. Efficacy of an Anti-transferrin Receptor 1 Antibody Against AIDS-related Non-Hodgkin Lymphoma. J. Immunother. 2015, 38, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Daniels-Wells, T.R.; Candelaria, P.V.; Leoh, L.S.; Nava, M.; Martinez-Maza, O.; Penichet, M.L. An IgG1 Version of the Anti-transferrin Receptor 1 Antibody ch128.1 Shows Significant Antitumor Activity Against Different Xenograft Models of Multiple Myeloma: A Brief Communication. J. Immunother. 2020, 43, 48–52. [Google Scholar] [CrossRef]
- Farrell, P.J. Epstein-Barr Virus and Cancer. Annu. Rev. Pathol. 2019, 14, 29–53. [Google Scholar] [CrossRef]
- Moses, A.V.; Williams, S.E.; Strussenberg, J.G.; Heneveld, M.L.; Ruhl, R.A.; Bakke, A.C.; Bagby, G.C.; Nelson, J.A. HIV-1 induction of CD40 on endothelial cells promotes the outgrowth of AIDS-associated B-cell lymphomas. Nat. Med. 1997, 3, 1242–1249. [Google Scholar] [CrossRef]
- Wen, J.; Wu, D.; Qin, M.; Liu, C.; Wang, L.; Xu, D.; Vinters, H.V.; Liu, Y.; Kranz, E.; Guan, X.; et al. Sustained delivery and molecular targeting of a therapeutic monoclonal antibody to metastases in the central nervous system of mice. Nat. Biomed. Eng. 2019, 3, 706–716. [Google Scholar] [CrossRef]
- Penichet, M.L.; Daniels-Wells, T.R.; Candelaria, P.V.; Almagro, J.C. Compositions and Methods for Transferrin Receptor 1 Targeting. International Patent Application No. PCT/US2020/059532, 6 November 2020. Publication Number: WO/2021/092482A1, Publication Date: 14 May 2021. [Google Scholar]
- Tao, M.H.; Canfield, S.M.; Morrison, S.L. The differential ability of human IgG1 and IgG4 to activate complement is determined by the COOH-terminal sequence of the CH2 domain. J. Exp. Med. 1991, 173, 1025–1028. [Google Scholar] [CrossRef]
- Hather, G.; Liu, R.; Bandi, S.; Mettetal, J.; Manfredi, M.; Shyu, W.C.; Donelan, J.; Chakravarty, A. Growth rate analysis and efficient experimental design for tumor xenograft studies. Cancer Inform. 2014, 13, 65–72. [Google Scholar] [CrossRef]
- Martinez, L.E.; Daniels-Wells, T.R.; Guo, Y.; Magpantay, L.I.; Candelaria, P.V.; Penichet, M.L.; Martinez-Maza, O.; Epeldegui, M. Targeting TfR1 with the ch128.1/IgG1 Antibody Inhibits EBV-driven Lymphomagenesis in Immunosuppressed Mice Bearing EBV(+) Human Primary B-cells. Mol. Cancer Ther. 2021, 20, 1592–1602. [Google Scholar] [CrossRef]
- Helguera, G.; Jemielity, S.; Abraham, J.; Cordo, S.M.; Martinez, M.G.; Rodriguez, J.A.; Bregni, C.; Wang, J.J.; Farzan, M.; Penichet, M.L.; et al. An antibody recognizing the apical domain of human transferrin receptor 1 efficiently inhibits the entry of all new world hemorrhagic Fever arenaviruses. J. Virol. 2012, 86, 4024–4028. [Google Scholar] [CrossRef] [PubMed]
- Carbone, A.; Gloghini, A.; Capello, D.; Gaidano, G. Genetic pathways and histogenetic models of AIDS-related lymphomas. Eur. J. Cancer 2001, 37, 1270–1275. [Google Scholar] [CrossRef]
- Barta, S.K.; Joshi, J.; Mounier, N.; Xue, X.; Wang, D.; Ribera, J.M.; Navarro, J.T.; Hoffmann, C.; Dunleavy, K.; Little, R.F.; et al. Central nervous system involvement in AIDS-related lymphomas. Br. J. Haematol. 2016, 173, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Collaboration of Observational HIV Epidemiological Research Europe (COHERE) Study Group; Bohlius, J.; Schmidlin, K.; Costagliola, D.; Fatkenheuer, G.; May, M.; Caro Murillo, A.M.; Mocroft, A.; Bonnet, F.; Clifford, G.; et al. Prognosis of HIV-associated non-Hodgkin lymphoma in patients starting combination antiretroviral therapy. AIDS 2009, 23, 2029–2037. [Google Scholar] [CrossRef] [PubMed]
- Bosma, G.C.; Custer, R.P.; Bosma, M.J. A severe combined immunodeficiency mutation in the mouse. Nature 1983, 301, 527–530. [Google Scholar] [CrossRef]
- Roder, J.C. The beige mutation in the mouse. I. A stem cell predetermined impairment in natural killer cell function. J. Immunol. 1979, 123, 2168–2173. [Google Scholar] [CrossRef]
- Roder, J.C.; Lohmann-Matthes, M.L.; Domzig, W.; Wigzell, H. The beige mutation in the mouse. II. Selectivity of the natural killer (NK) cell defect. J. Immunol. 1979, 123, 2174–2181. [Google Scholar] [CrossRef]
- Jones-Carson, J.; Vazquez-Torres, A.; Balish, E. Defective killing of Candida albicans hyphae by neutrophils from beige mice. J. Infect. Dis. 1995, 171, 1664–1667. [Google Scholar] [CrossRef]
- Leoh, L.S.; Daniels-Wells, T.R.; Martinez-Maza, O.; Penichet, M.L. Insights into the effector functions of human IgG3 in the context of an antibody targeting transferrin receptor 1. Mol. Immunol. 2015, 67, 407–415. [Google Scholar] [CrossRef]
- Hickerson, B.T.; Daniels-Wells, T.R.; Payes, C.; Clark, L.E.; Candelaria, P.V.; Bailey, K.W.; Sefing, E.J.; Zink, S.; Ziegenbein, J.; Abraham, J.; et al. Host receptor-targeted therapeutic approach to counter pathogenic New World mammarenavirus infections. Nat. Commun. 2022, 13, 558. [Google Scholar] [CrossRef]
- Richard, C.; Verdier, F. Transferrin Receptors in Erythropoiesis. Int. J. Mol. Sci. 2020, 21, 9713. [Google Scholar] [CrossRef]
- Sakamoto, S.; Kawabata, H.; Masuda, T.; Uchiyama, T.; Mizumoto, C.; Ohmori, K.; Koeffler, H.P.; Kadowaki, N.; Takaori-Kondo, A. H-Ferritin Is Preferentially Incorporated by Human Erythroid Cells through Transferrin Receptor 1 in a Threshold-Dependent Manner. PLoS ONE 2015, 10, e0139915. [Google Scholar] [CrossRef] [PubMed]
- Montemiglio, L.C.; Testi, C.; Ceci, P.; Falvo, E.; Pitea, M.; Savino, C.; Arcovito, A.; Peruzzi, G.; Baiocco, P.; Mancia, F.; et al. Cryo-EM structure of the human ferritin-transferrin receptor 1 complex. Nat. Commun. 2019, 10, 1121. [Google Scholar] [CrossRef]
- Phase I, Open-Label, Multicentre, Dose-Escalation Study to Evaluate the Safety and Pharmacokinetics of a Single Intravenous PPMX-T003 in Polycythemia Vera (Identifier: NCT05074550). Available online: ClinicalTrials.gov (accessed on 6 December 2022).
- Shimosaki, S.; Nakahata, S.; Ichikawa, T.; Kitanaka, A.; Kameda, T.; Hidaka, T.; Kubuki, Y.; Kurosawa, G.; Zhang, L.; Sudo, Y.; et al. Development of a complete human IgG monoclonal antibody to transferrin receptor 1 targeted for adult T-cell leukemia/lymphoma. Biochem. Biophys. Res. Commun. 2017, 485, 144–151. [Google Scholar] [CrossRef]
- Zhang, L.; Nomura, F.; Aikawa, Y.; Kurosawa, Y.; Morishita, K.; Sudo, Y. PPMX-T003, a fully human anti-TfR1 antibody with potent efficacy against hematologic malignancies [abstract]. Cancer Res. 2017, 77, 5586. [Google Scholar] [CrossRef]
- Ogama, Y.; Kumagai, Y.; Komatsu, N.; Araki, M.; Masubuchi, N.; Akiyoshi, H.; Matsuura, T.; Kirisako, H.; Kyoya, A.; Nomura, F.; et al. Phase 1 Clinical Trial of PPMX-T003, a Novel Human Monoclonal Antibody Specific for Transferrin Receptor 1, to Evaluate Its Safety, Pharmacokinetics, and Pharmacodynamics. Clin. Pharmacol. Drug Dev. 2022. Online ahead of print. [Google Scholar] [CrossRef]
- Saunders, K.O. Conceptual Approaches to Modulating Antibody Effector Functions and Circulation Half-Life. Front. Immunol. 2019, 10, 1296. [Google Scholar] [CrossRef]
- Lansdorp, P.M.; Dragowska, W. Long-term erythropoiesis from constant numbers of CD34+ cells in serum-free cultures initiated with highly purified progenitor cells from human bone marrow. J. Exp. Med. 1992, 175, 1501–1509. [Google Scholar] [CrossRef]
- Knaän-Shanzer, S.; van der Velde-van Dijke, I.; van de Watering, M.J.M.; de Leeuw, P.J.; Valerio, D.; van Bekkum, D.W.; de Vries, A.A.F. Phenotypic and Functional Reversal Within the Early Human Hematopoietic Compartment. Stem Cells 2008, 26, 3210–3217. [Google Scholar] [CrossRef] [PubMed]
- Gross, S.; Helm, K.; Gruntmeir, J.J.; Stillman, W.S.; Pyatt, D.W.; Irons, R.D. Characterization and phenotypic analysis of differentiating CD34+ human bone marrow cells in liquid culture. Eur. J. Haematol. 1997, 59, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Daniels-Wells, T.R.; Helguera, G.; Rodriguez, J.A.; Leoh, L.S.; Erb, M.A.; Diamante, G.; Casero, D.; Pellegrini, M.; Martinez-Maza, O.; Penichet, M.L. Insights into the mechanism of cell death induced by saporin delivered into cancer cells by an antibody fusion protein targeting the transferrin receptor 1. Toxicol. Vitr. 2013, 27, 220–231. [Google Scholar] [CrossRef] [PubMed]




| Treatment | Number of Animals | Median Survival (Days) | p-Value Compared to IgG1 | p-Value Compared to Buffer | p-Value Compared to hu128.1 (Day 2) |
|---|---|---|---|---|---|
| Buffer (Day 2) | 9 | 23 | |||
| 100 μg IgG1 (Day 2) | 10 | 24 | 0.9024 | ||
| 100 μg hu128.1 (Day 2) | 10 | 42 | <0.0001 | <0.0001 | |
| 100 μg IgG1 (Days 2, 5, 8, 11) | 10 | 25 | 0.5451 | ||
| 100 μg hu128.1 (Days 2, 5, 8, 11) | 10 | 39 | <0.0001 | <0.0001 | 0.1528 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daniels-Wells, T.R.; Candelaria, P.V.; Kranz, E.; Wen, J.; Wang, L.; Kamata, M.; Almagro, J.C.; Martínez-Maza, O.; Penichet, M.L. Efficacy of Antibodies Targeting TfR1 in Xenograft Mouse Models of AIDS-Related Non-Hodgkin Lymphoma. Cancers 2023, 15, 1816. https://doi.org/10.3390/cancers15061816
Daniels-Wells TR, Candelaria PV, Kranz E, Wen J, Wang L, Kamata M, Almagro JC, Martínez-Maza O, Penichet ML. Efficacy of Antibodies Targeting TfR1 in Xenograft Mouse Models of AIDS-Related Non-Hodgkin Lymphoma. Cancers. 2023; 15(6):1816. https://doi.org/10.3390/cancers15061816
Chicago/Turabian StyleDaniels-Wells, Tracy R., Pierre V. Candelaria, Emiko Kranz, Jing Wen, Lan Wang, Masakazu Kamata, Juan C. Almagro, Otoniel Martínez-Maza, and Manuel L. Penichet. 2023. "Efficacy of Antibodies Targeting TfR1 in Xenograft Mouse Models of AIDS-Related Non-Hodgkin Lymphoma" Cancers 15, no. 6: 1816. https://doi.org/10.3390/cancers15061816
APA StyleDaniels-Wells, T. R., Candelaria, P. V., Kranz, E., Wen, J., Wang, L., Kamata, M., Almagro, J. C., Martínez-Maza, O., & Penichet, M. L. (2023). Efficacy of Antibodies Targeting TfR1 in Xenograft Mouse Models of AIDS-Related Non-Hodgkin Lymphoma. Cancers, 15(6), 1816. https://doi.org/10.3390/cancers15061816







